Received: March 11, 2014
Accepted: September 4, 2014
Meditterr J Hematol Infect Dis 2014, 6(1): e2014063 doi: 10.4084/MJHID.2014.063
This article is available on PDF format at:

Eldad J. Dann1,2,3
1 Department of Hematology and Bone
Marrow Transplantation, 2 Blood Bank and Transfusion
Service, Rambam Health Care Campus; 3 Bruce Rappaport
Faculty of Medicine, Technion, Israel Institute of Technology

| This is an Open Access article distributed under the
terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Therapy of advanced Hodgkin
lymphoma (HL) is a rapidly changing field due to a lot of currently
emerging data. Treatment approaches are presently based on either the
Kairos principle of giving aggressive therapy upfront and considering
de-escalation of therapy if the interim PET/CT is negative or the
Chronos principle of starting with ABVD followed by escalation of
therapy for patients with positive interim PET/CT. The International
Prognostic Score (IPS) is still valid for decision-making regarding the
type of initial therapy, since patients with a high score do have an
inferior progression free survival (PFS) with ABVD compared to those
with a low score. Escalated BEACOPP administered upfront improves PFS;
however, increase in the overall survival (OS) has not been confirmed
yet, and this therapy is accompanied by elevated toxicity and fertility
impairment. Completion of ongoing and currently initiated trials could
elucidate multiple issues related to the management of HL
patients.
|
Introduction
The treatment of advanced-stage Hodgkin lymphoma with the MOPP
regimen (mechloroethamine, vincristine, procarbazine and prednisone)
pioneered the use of chemotherapeutic protocols for therapy of
malignancies. When the high rate of secondary leukemias was
established, MOPP was replaced with the ABVD (adriamycin, bleomycin,
vinblastine and dacarbazine) protocol developed by Bonadonna et al,
which has been the standard of care since early 1970s.[1]
During the
last 15 years a debate is ongoing as to whether a more intensive
protocol such as escalated BEACOPP introduced by the German Hodgkin
Study Group should be employed and for whom.[2] The
10-year freedom
from treatment failure and OS in the arm receiving 8 cycles of
escalated BEACOPP were 82% and 87%, respectively.[3]
The IPS[4] was
re-evaluated by several study groups[5,6] and proved
to be an efficient
tool in identifying the group of patients who had a reduced PFS if
treated with ABVD. Recently, a 23-gene array was reported to be
superior to the IPS and was suggested to become a potential predicting
factor.[7] Since patients with high IPS have an
increased failure rate,
the gene array could become a tool for upfront decision-making
regarding treatment strategy in patients who may do well with a
standard-dose therapy and in those who would require intensified
therapy upfront.
Some phase II studies showed that therapy could be tailored based on
interim imaging and IPS, thus saving escalated BEACOPP only to a
limited subgroup of patients and preserving fertility in 88% of female
patients.[8-10] In the last decade, interim PET/CT
performed following
2 cycles of therapy demonstrated a high negative predictive value which
enables using less intensive and therefore less toxic regimens in
patients with a negative interim scan. On the other hand, patients with
a positive interim PET/CT following 2 cycles of ABVD are in a high risk
of treatment failure, which necessitates further therapy escalation.[11]
The current ongoing studies are designed to minimize therapy for
patients with low risk of disease progression in order to reduce
toxicity and late side effects, such as secondary tumors, cardiac
toxicity and loss of fertility. Present trials try to resolve the
dispute between the Chronos principle of starting a standard low toxic
regimen like ABVD and augmenting therapy only for patients with adverse
predictive factors and the Kairos principle claiming that high
efficiency highly toxic therapy should be started to all patients and
only individuals with good prediction should have their therapy reduced.
This is a major issue, since the median age of patients with HL is 34
years and they have a life expectancy of another 50 years. Hence, joint
international efforts are required to determine the optimal therapeutic
strategies and spare these patients from late treatment-related adverse
effects.
Results of Therapy Using Current Protocols
Recently published randomized trials have demonstrated 5-year PFS of
74%-76% and OS of 88%-90% for patients with stage IIB, III and IV
disease or IA, IIA bulky mediastinal mass treated with the ABVD
regimen. Radiotherapy was administered to 41%-62% of these patients.
(Table 1). A sub-analysis of
the data according to the IPS demonstrated
PFS of 77% and OS of 91% for patients with IPS 0-2 and PFS of 67% and
OS of 84% for patients with IPS 3-7. The superiority of escalated
BEACOPP in providing a higher PFS was demonstrated in several
randomized control studies, although a statistically significant OS
benefit was not achieved. The difference between the GHSG and the four
other studies could be related to the total cohort size of 935 patients
in the four studies and 2182 patients in GHSG HD15 trial (Table
1).[12-16] Of interest, 6 cycles of escalated
BEACOPP have been shown to
result in a significantly better PFS than 8 cycles of escalated BEACOPP
and an improved OS.[16] However, the 10-15%
difference in PFS observed in
these patients, comes with impaired fertility is women and sterility in
a vast majority of men, a heavy toll for this young population. A
higher cumulative incidence ratio of secondary myelodysplastic syndrome
(MDS), or secondary acute leukemia was reported in patients receiving
≥4 cycles of escalated BEACOPP compared to less than 4 cycles or other
chemotherapy regimen (1.7%,0.7%,0.3%, respectively).[17]
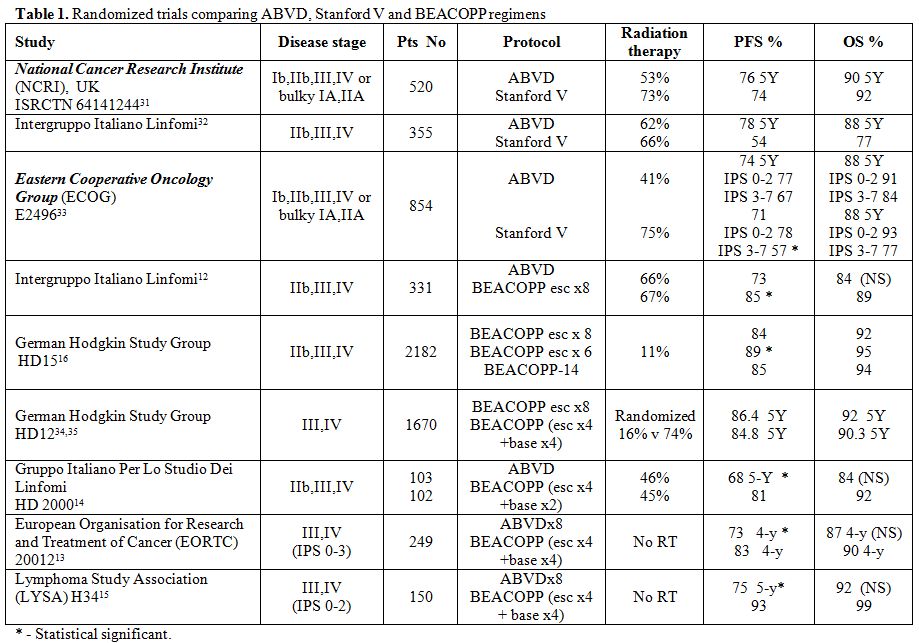 |
Table 1. Randomized trials comparing
ABVD, Stanford V and BEACOPP regimens |
Use of Interim PET/CT for Tailoring Therapy in Advanced Hodgkin Lymphoma
Gallamini et al.[11] demonstrated the capability of interim PET/CT to define the low-risk population that has a negative interim PET and carries a risk of relapse of 10% only, and the high-risk group that has a positive interim study and carries a risk of disease progression of 60-80% if treated with ABVD. Results of a retrospective international multicenter study demonstrated that negative predictive value of the interim PET-2 was around 95%, and PFS of patients with a positive interim PET was 28%.[18] At same time, it was demonstrated that BEACOPP therapy could be tailored based on IPS and interim scintigraphy (from 1998-2001 with Gallium scan and from 2001-2005 with PET) providing both high PFS and OS, with reduction in toxicity and preservation of fertility in more than 85% of women.[9,19] Several studies are currently ongoing using interim PET/CT for tailoring therapy. Of patients treated with 2 cycles of ABVD upfront, 80-85% has a negative interim PET/CT, and 15-20% has a positive scan defined as an uptake higher than in the liver. Escalation of therapy could salvage 60%-76% of these patients.[20,21] A further follow-up is needed to evaluate if a high PFS is maintained in patients with negative interim PET/CT and whether radiation therapy could be omitted in patients treated with ABVD who had a bulky disease at diagnosis and a negative interim or end-of-therapy PET/CT.
Ongoing Studies of Advanced Hodgkin lymphoma Therapy
Several large trials are ongoing or recently finished and waiting for a longer follow-up prior to publication (Table 2). These studies usually use interim PET/CT for tailoring therapy of individual patients. The RATHL (response adapted therapy in advanced Hodgkin lymphoma) study, initiated in the UK. has become a collaborative European trial. It recruited 1214 patients, 84% of whom had negative interim PET/CT and were further randomized to receive either 4 more cycles of ABVD or AVD (adriamycin, vinblastine, dacarbazine). Only 2.4% of these patients received radiation therapy. The 2-year PFS in the group with negative interim PET/CT was 86%. In the group of patients with positive interim PET (16% of patients) whose therapy was changed to escalated BEACOPP for 4 more cycles or BEACOPP-14, 76% had a negative PET/CT-3 that was performed 8-9 weeks after therapy intensification (escalated BEACOPP x3). At a 1-year follow-up, 22% of these patients had disease progression or died.[21]
| Table 2. Trials for Patients with Advanced Hodgkin's Lymphoma |
The US intergroup S-0816 study enrolled 357 patients with stage III,
IV
disease who received 2 cycles of ABVD followed by PET/CT. Eighty two
percent of patients had negative interim study (Deauville score
1-3).These patients were treated with four additional courses of ABVD.
The remaining 18% of patients had positive PET/CT (Deauville score 4-5)
and were planned for 6 cycles of escalated BEACOPP; however, in 10% of
these patients therapy was not escalated due to physician's choice. The
1-year PFS for the patients with negative and positive PET/CT was 85%
and 72% , respectively. The PFS for the whole group was 84% and OS –
98%.[22]
The Italian GITIL 0607 study registered 730 patients. Treatment of
patients with IPS 0-7 included two cycles of ABVD followed by interim
PET/CT. Patients with negative PET (82%) had a total of 6 cycles of
ABVD followed by PET/CT. If the result of this imaging was negative,
the patient was further randomized to either consolidative radiation
therapy to the bulky mediastinal mass or no radiation. Patients with
positive interim PET/CT (18%) were randomized to escalated BEACOPPx4
followed by standard BEACOPPx4 with or without rituximab. The 2-year
PFS for all patients, those with interim negative and those with
positive interim PET, was 81%, 85% and 61%, respectively.[23]
The Israeli H2 study recruited 180 patients with advanced HL. Patients
with IPS 0-2 started therapy with two cycles of ABVD and those with IPS
3-7 received two cycles of escalated BEACOPP. An interim PET was
performed following 2 cycles of treatment. If PET was negative,
patients had four additional cycles of ABVD. If interim PET was
positive, four cycles of escalated BEACOPP were administered. Eighty
five percent of interim PET/CT scans were interpreted as negative and
15% as positive. Interim PET/CT was negative in 88% of patients with
IPS 0-2 and 80% of patients with IPS 3-7. Chemotherapy was de-escalated
in 89% of patients with IPS 3-7 and at 3 years only 13% of the whole
group progressed. At a median follow-up of 26 months the 3-year PFS was
85%.[24]
The issue of early salvage therapy for patients with advanced HL who
had a positive interim PET/CT following two cycles of ABVD was assessed
by the Italian Lymphoma Study Group in the HD0801 study that enrolled
520 patients. Patients with negative interim PET/CT received a total of
6 cycles of ABVD and were further randomized to radiation therapy to
bulky mediastinal masses or to no radiation therapy. Patients with
positive interim PET/C were treated with salvage protocol including
four cycles of IGEV (ifosfamide, gemcitabine, etoposide, vinorelbine
and prednisolone). After salvage therapy a further assessment was
performed. Patients with negative PET (58% of individuals) underwent
autologous stem cell transplant with the BEAM (carmustine, etoposide,
cytarabine, and melphalan) protocol. Following four cycles of IGEV,
patients with positive PET/CT (42%) underwent tandem autologous
transplantation or autologous followed by allogeneic transplantation if
a matched related donor was available. The majority of patients with
positive interim PET could be salvaged with an early shift to high-dose
chemotherapy and stem cell rescue. The reported 2-year PFS and OS based
on PET/CT results following 2 cycles of ABVD was 75.7% and 98.6% for
patients with negative interim study and 64.1% and 86.3%, respectively
for those with positive interim scan. Eighty nine percent of patients
treated with this salvage protocol had a 2-year relapse free
survival.[25,26]
Several ongoing studies use 2 cycles of escalated BEACOPP for patients
with IPS 0-7 followed by interim PET/CT. In the HD18 trial by the
German Hodgkin Study Group, patients with negative interim PET/CT are
randomized to additional 2 or 6 cycles of escalated BEACOPP, while
patients with positive interim PET/CT are treated with 6 extra cycles
of escalated BEACOPP. In this study, radiation therapy is applied only
to patients who had residual positive uptake sites at the end of
treatment.[27]
The French LYSA AHL2011 study randomizes patients with IPS 0-7 to the
standard arm where 6 cycles of escalated BEACOPP are used regardless of
interim PET/CT performed after 2 cycles, and to the experimental arm
where patients with negative interim PET post 2 cycles of escalated
BEACOPP have their therapy de-escalated to 4 cycles of ABVD, while
patients with a positive interim scan receive additional 4 cycles of
escalated BEACOPP (a total of 6 cycles).[28]
The trials discussed above are only part of ongoing studies using
interim PET as a predictive value for further therapy of individual
patients. Recently initiated EORTC H11 is checking the predictive
accuracy of early interim PET performed following a single cycle of
escalated BEACOPP. All patients in observational arm are treated with 6
cycles of therapy, which remains unchanged irrespective of interim PET
results, while in the experimental arm therapy is de-escalated to ABVD
if interim PET is negative. This interesting study could potentially
elucidate some challenging issues associated with early use of interim
PET following escalated BEACOPP and reduction of therapy, which is
expected to maintain fertility in young female patients.[29,30]
Further
studies have been lately initiated using the anti-CD30 antibody-drug
conjugate as part of first-line chemotherapy including both ABVD type
protocols and escalated BEACOPP. These regimens have been modified to
exclude bleomycin and oncovin due to their major lung and neurotoxicity.
Discussion
The treatment of Hodgkin lymphoma is a rapidly evolving area. Many ongoing studies are designed to establish the role of upfront intensive therapy, which presents the Kairos principle, employing escalated BEACOPP, followed by de-escalation of therapy in patients with negative interim PET/CT. Other studies use the Chronos principle of starting with ABVD and further therapy escalation only for patients with positive interim PET/CT, (Deauville score 4,5) which clearly indicates their high risk for disease progression. The latter trials apply various escalation regimens ranging from escalated BEACOPP to a salvage protocol including 4 cycles of IGEV followed by stem cell collection and autologous transplantation. While all these studies are expected to provide PFS and OS rates after 4-5 years of follow-up, long-term outcomes (up to 15 years) including late side effects and problems like fertility, fatigue, secondary malignancy, ischemic and valvular heart disease, are not less important. These issues are crucial for HL patients, given their young age at diagnosis and a life expectancy of 50 years ahead of them, if appropriately treated.
Conclusion
Patients with advanced HL and IPS 0-2 can be initially treated with
ABVD; however, these patients should undergo interim PET/CT following 2
cycles of therapy. Patients with negative interim PET/CT can safely
continue with additional 4 cycles of ABVD. If an interim scan is
positive, the data from retrospective studies suggest a low PFS of
12-27% only; however, escalation of therapy led to remission in 65% of
patients. To date, only preliminary data from currently ongoing GITIL
HD0607 study are available; nevertheless, it has already been
demonstrated that patients with positive PET-2 achieved a 2-year FFS of
67% after escalation of their therapy. Moreover, preliminary data from
prospective studies like RATHL show that patients in whom therapy was
escalated based on a positive interim PET-2 achieved a complete
response at the end of chemotherapy in 76% of cases. The information
available suggests that therapy intensification is beneficial; however,
a stronger conclusion will be drawn when the results of these 2 studies
are published. Patients with advanced disease and IPS ≥3 are in a
higher risk of treatment failure if therapy is initiated with ABVD than
those in whom therapy with a more intensive regimen (escalated BEACOPP)
is started. This cohort should also undergo interim PET/CT after 2
cycles of therapy. Patients with positive interim PET/CT are in a high
risk group and need to continue with the intensified therapy. Patients
with negative interim PET/CT could do as well with reduction of therapy
to a standard regimen (ABVD) if they initiated with escalated BEACOPP
or proceed with ABVD if this was their original therapy. However,
reduction of therapy from escalated BEACOPP to ABVD in this subgroup of
patients is yet experimental awaiting confirmation by the ongoing
studies.
Radiation therapy can be omitted for patients that were treated with 6
cycles of escalated BEACOPP and have a residual mass at the end of
therapy which is PET/CT negative. This conclusion is based on the
results of the HD15 study and is the current approach applied in the
ongoing HD18, both by the German Hodgkin Study Group. In the RATHL
study, less than 5% of patients with negative interim PET received
radiation therapy. The 12-month PFS was promising, but the final
verdict will be available when the results of the study are published.
The effect of adding brentuximab vedotin to the chemotherapy regimen
for patients with positive interim PET/CT will need assessment by an
international collaborative study.
Acknowledgement
I would like to thank Sonia Kamenetsky for her devoted secretarial support.References












 .
. 



















