Brain Abscesses Caused by Nocardia paucivorans in a Multiple Myeloma Patient Treated with Lenalidomide and Dexamethasone: a Case Report and Review of Literature
Jacopo Monticelli1, Roberto Luzzati1, Cristina Maurel1, Chiara Rosin1, Romina Valentinotti1 and Claudio Farina2
1 Unit of Infectious Diseases, University Hospital, Trieste, Italy.
2 Microbiology and Virology Institute, AO "Papa Giovanni XXIII", Bergamo, Italy.
Corresponding author: Jacopo Monticelli. Unit of Infectious
Diseases, University Hospital, Trieste, Italy.; Tel. (Unit of
Infectious Diseases): +39-040-3992594; Fax: +39-0403992652. E-mail:
jacopo.mont@hotmail.it
Published: January 1, 2015
Received: October 16, 2014
Accepted: December 18, 2014
Mediterr J Hematol Infect Dis 2015, 7(1): e2015011, DOI
10.4084/MJHID.2015.011
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
We report the first case of multiple brain abscesses caused by Nocardia paucivorans in a patient suffering from multiple myeloma on treatment with lenalidomide and dexamethasone. N. paucivorans is a recently described species of the genus Nocardia,
which is supposed to have a heightened neurotropism in cases of
disseminated infection. Although nocardiosis itself is an uncommon
infectious complication in multiple myeloma so far, nocardial brain
abscess should be added to the spectrum of adverse effects due to this
novel chemotherapy regimen. |
Case Presentation
A 70-year-old male was transferred to our unit from the intensive
care unit of a nearby hospital. He had had sudden onset
clonic seizures involving the right hemisoma and the assessment of
multiple brain abscesses by computerized tomography (CT) scan. The
patient was receiving empirical treatment with metronidazole and
cefotaxime. His past medical history included a 2-year-history of IgA
myeloma (International Staging System – II). The patient was initially
treated with melphalan plus prednisone plus bortezomib (4 cycles)
according to MPV protocol. Then, he started treatment with lenalidomide
and dexamethasone having completed eleven cycles of such regimen with
partial response. During the chemotherapy regimen the patient did not
receive a trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis and, at
time of admission, we discontinued the chemotherapy regimen for
myeloma. The patient suffered from a 2-month history of subcutaneous
abscess of unknown origin on the left shoulder. The lesion was drained,
and the microbiological analysis was not conclusive because of
mixed bacterial flora. The patient was living in a small rural hamlet.
He was a construction worker, but he retired 10 years before the
present hospital admission.
At hospital presentation, body
temperature was 36.5 °C, heart rate was 92/min, blood pressure was
150/90 mmHg and Glasgow Coma Scale was 15/15. The patient was alert,
oriented and no abnormal physical findings were shown, except for a
painful hypoesthesia in the lateral and anterior sides of the left
thigh, in the lateral side of the left leg and, on the dorsal side of
the left foot. The subcutaneous abscess on the left shoulder
completely resolved with no signs of local inflammation.
Laboratory showed the following abnormalities: serum C-reactive protein
96.19 nmol/L (normal values inferior to 71.43 nmol/L), hemoglobin 90
g/L, white blood cells 3.9 x 109/L
(51% neutrophil granulocytes). In addition, the analysis of peripheral
blood lymphocytes subsets showed CD3+ 742 cells/µL, CD3+CD4+ 420
cells/µL (47%), CD3+CD8+ 241 cells/µL (27%), CD4+/CD8+ ratio 1.70,
CD19+ 72 cells/µL. Serum protein electrophoresis showed a relative
hypoalbuminemia (55.4%) according to the known monoclonal gammopathy
(serum IgA 4490 mg/L, monoclonal component 3 g/L). The chest X-ray
revealed a lower right lobe infiltrate associated with bilateral
pleural effusion. A brain CT scan (Figure 1)
with contrast medium showed 6 focal ring-enhancing lesions of which the
largest one had a maximum diameter of 16 mm (left occipital lobe). All
the lesions had a minimal perilesional oedema. The right frontal
lesions led to a mass effect on the right lateral ventricle. At that
time, the patient underwent CT-guided brain biopsy of a parietal
lesion. A bioptic sample revealed branching Gram positive nocardioform
rods. Then, cultures were incubated aerobically at 35°C for three
weeks. All whitish, chalky and wet hay-smelling colonies (Figure 2) yielded on blood agar were stained by partial acid-fast method according to Kinyoun technique modified for nocardioforms (Figure 3).
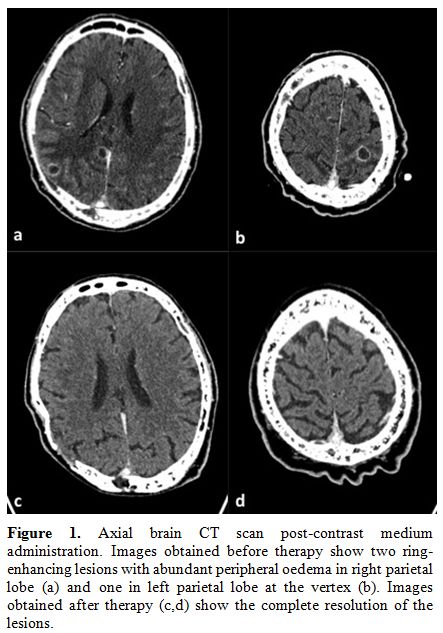 |
Figure
1. Axial brain CT scan post-contrast medium administration. Images
obtained before therapy show two ring-enhancing lesions with abundant
peripheral oedema in right parietal lobe (a) and one in left parietal
lobe at the vertex (b). Images obtained after therapy (c,d) show the
complete resolution of the lesions. |
|
|
Figure
2. Whitish chalky colonies of N. paucivorans yielded on blood agar. |
|
|
Figure
3. N. paucivorans
hyphae. Staining by partial acid-fast method according to Kinyoun
technique modified for nocardioforms. The vegetative hyphae are well
developed with irregular branches. |
The suspicion that this strain could belong to Actinomycetales genus
was based on its failure to clear casein (Biorad, France), on the
resistance to lysozime and 5-fluorouracil, and on the enzymatic
profile, by the API ZYM system (bioMérieux, France).[1-2] Identification
was completed by the evaluation of the absence of urease after growth
on Christensen urea agar and incubation at 25°C for three weeks and of
the incapacity to clear casein (Biorad, France). Finally, genotypical
identification has been performed by amplification and sequencing of a
DNA fragment coding a rRNA 16S according with the manufacturer’s
(Applied Biosystem, USA) suggestions. Sequences have been compared with
the reference ones present in the Gen Bank (NCB) database by the BLAST
program. The sequence has been deposited in the GenBank (accession
number: AY262324.1). Sequences producing significant alignments
permitted to identify the strains as N. paucivorans with 100% value of
identity with the reference strain. Minimal Inhibitory
Concentrations' (MIC) testing was performed by the Epsilometer (E test,
bioMérieux, France) testing. MICs' results were the following: amikacin
0.064 µg/mL, amoxicillin plus clavulanic acid (>256 µg/mL),
ceftriaxone (0.75 µg/mL), cefepime (6 µg/mL), ciprofloxacin (0.047
µg/mL), clarithromycin (1 µg/mL), gentamicin (8 µg/mL), imipenem (0.19
µg/mL), linezolid (0.125 µg/mL), tobramycin (0.047 µg/mL), trimethoprim
plus sulfonamide (0.002 µg/mL). Susceptibility testing was performed on
Mueller Hinton Agar (bioMérieux). The results were read after 24 h of
incubation at 37°C. Susceptibility testing was revised and interpreted
according to CLSI criteria.[3] In order to exclude concomitant pulmonary
nocardiosis, a diagnostic bronchoscopy was done but the microbiological
analysis of the bronchoalveolar lavage isolated only commensal
microbial flora. As a matter of fact, based on the initial
identification and the preliminary antimicrobial susceptibility
testing, intravenous TMP-SMX plus meropenem were administered for 24
days. Such antibiotic regimen was switched to oral ciprofloxacin for an
overall treatment period of 12 months. The patient is doing well, his
painful hypoesthesias have disappeared; no more seizure episodes and
adverse effects due to long term antibiotic therapy occurred. The brain
CT-scan monitoring showed a progressive reduction of all cerebral
lesions (Figure 1). Discussion
Nocardia (order Actinomycetales)
is a complex genus of Gram-positive and partially acid- and
alcohol-fast bacteria forming irregular branching colonies on agar,
including Nocardia cyriacigeorgica, Nocardia farcinica, Nocardia brasiliensis and Nocardia otitidiscaviarum, and other Nocardia species. N. paucivorans
is a recently described species, identified by biochemical
characteristics and 16S rDNA sequence analysis in bronchial secretions
of a patient with chronic lung disease.[4,5]Human
nocardiosis is usually recognized as a sporadic, community-acquired
infection.[6] Human nocardiosis affects both immunocompromised and
immunocompetent hosts. Within the immunocompromising conditions,
nocardiosis seems to affect primarily intravenous drug abusers,
patients on systemic corticosteroids, AIDS patients, recipients of
solid organ and bone marrow transplants, patients affected by chronic
granulomatous diseases and hematologic malignancies. Host resistance to
nocardial infection depends on cell-mediated immunity. Infection mainly
occurs through direct skin inoculation or inhalation, and possible
clinical manifestations are pulmonary nocardiosis, cutaneous and
subcutaneous nocardiosis and systemic nocardiosis, including central
nervous system (CNS) dissemination.[7-8] CNS nocardiosis can be the
result of prior pulmonary infection or can exist on its own. A recent
literature review on the nocardiosis reported that CNS involvement can
manifest mainly as cerebral abscesses but also as meningitis or both
brain abscess and meningitis.[9] The major species reported among the
patients in this literature review were Nocardia asteroides (35%), N. farcinica (19%), and N. cyriacigeorgica (6%), even if Nocardia
nomenclature dramatically changed in the last years complicating all
etiological consideration. Other less common species included Nocardia transvalensis (4%), N. brasilensis (3.6%), and N. otitidiscaviarum (2%) and others. Our patient had CNS nocardiosis due to N. paucivorans. The largest series of N. paucivorans
infections published to date included 33 cases collected in northeast
Australia over a 20-years span, with dissemination in at least 33% of
cases and CNS infection in 80% of disseminated cases. Therefore, it was
hypothesized that N. paucivorans had an enhanced neurotropism.[10-11]Nocardial
infection in multiple myeloma is quite a sporadic finding, often linked
as a complication of bone marrow transplants.[12] There is limited
literature on nocardiosis in multiple myeloma patients that did not
undergo bone marrow transplant, and there are still fewer cases of
nocardial infections that occurred during novel anti-myeloma
treatments. For instance, it was recently reported cases of brain
abscesses by N. cyriacigeorgica
in two patients treated with bortezomib.[13] A PubMed search, crossing
“Nocardia paucivorans” and “myeloma”, revealed no cases, and the N. paucivorans
infections published so far in English literature did not occur in
multiple myeloma patients.[10-11,13-15] Therefore, to the best of our
knowledge, the present case is the first infection due to N. paucivorans in a patient affected by multiple myeloma. Our
patient had multiple risk factors for nocardiosis. The myeloma-related
immunodeficiency includes mainly B cell dysfunction and cellular
immunodeficiency[12,16] with significantly reduced numbers of NK and T
cells and impaired T cells function.[17] Lenalidomide is a synthetic
derivative of thalidomide, used in multiple myeloma for its
immunomodulatory properties. The molecule induces a IL-2-mediated
primary T cell proliferation with a concomitant increase in IFN-γ
production along with anti-angiogenic, anti-proliferative, and
pro-apoptotic properties.[18] However, lenalidomide combination therapy
has shown to be prone to an increased risk of infections. In fact, a
meta-analysis of efficacy and safety of lenalidomide for multiple
myeloma showed an increased risk of infections comparing lenalidomide
regimen versus placebo (RR = 1.98; 95% CI: 1.50 to 2.62).[19]
Therefore, an antibiotic prophylaxis should be considered as part of
the treatment regimen.[19] Bortezomib induces apoptosis preferentially
in rapidly proliferating and neoplastic cells via inhibition of NF-κB
activation. However, proteasome inhibition could influence the antigen
processing and cytotoxic T-cell response and suppress essential immune
functions of human CD4+ T cells. It has been shown that bortezomib
therapy could increase the rate of opportunistic infectious
complications, mainly within eight cycles of treatment during severe
lymphocytopenia treatment-induced periods.[13,20] Corticosteroid
therapy is widely associated with nocardial infections.
Dexamethasone-based regimens in multiple myeloma increase the rate of
bacterial infections (mainly encapsulated bacteria such as
Staphylococcus aureus, and others including Enterobacteriaceae, Pseudomonas aeruginosa,
mycobacteria), viral and micotic infections.[21] An observational study
of 13 patients collected over a 13-years period in two Spanish
institutions showed that the most common risk factor for both pulmonary
and disseminated nocardiosis was corticosteroid therapy (64% in the
whole group of patients, 45.5% in disseminated nocardiosis
patients).[22] Dose and duration of steroid treatment in nocardiosis
patients receiving corticosteroids prior to diagnosis varied widely in
that study ranging from prednisone 40 mg/day for 15 days to prednisone
7.5 mg/day for 13 years. Our
patient received for the nocardiosis a combination regimen of TMP-SMX
plus meropenem that was switched to a long term oral therapy with
ciprofloxacin. Optimal antimicrobial treatment regimens have not been
firmly established for nocardiosis, and the management of the disease
must be individualized. Immunosuppressed patients and those with CNS
disease should receive at least 12 months of antimicrobial therapy with
the appropriate clinical monitoring.[7] In CNS nocardiosis, the
antibiotic regimen should include drugs with a favorable CNS
penetration, as the ones we used for our patients.In conclusion, this is the first report of multiple brain abscesses caused by N. paucivorans
in a patient suffering from multiple myeloma. In this patient the risk
factors for a nocardial infection included the hematological
disease and chemotherapy. Among the various chemotherapy
regimens, we suggest that corticosteroids and lenalidomide had a
pivotal role as risk factors for nocardiosis in the present case.
Although nocardial infection itself in multiple myeloma is an uncommon
infectious complication so far,[13] nocardial brain abscesses should be
added to the spectrum of adverse effect due to the novel anti-myeloma
drugs. We emphasize the importance in nocardial infections of obtaining
the species identification and the strain susceptibility in order to
guide the long term antibiotic therapy.Finally,
in our case etiological diagnosis was made possible by culture of brain
biopsy. Other than the nocardial infection, the causes of focal brain
infections in hematologic malignancies could include for instance
neurotoxoplasmosis, zygomycosis, aspergillosis, fusariosis.[23] Given
the risks of misdiagnosis or delayed diagnosis, we highlight the
importance of such invasive procedure, as a brain biopsy, to
obtain samples for microbiological analysis in all cases of focal brain
lesions, especially in the immunocompromised host. Contributions, Conflict of Interest and Ethical Statement
All
the authors approved the final version of the manuscript. JM
reviewed the clinical literature and drafted the first version of the
manuscript. RL edited the manuscript and provided intellectual inputs.
CF identified the strain and drafted the microbiological section of
this report. CM, CR, RV equally treated the patient, collected the
clinical data in this report and edited the manuscript. The authors
declare that they have no conflict of interest. The patient signed an
informed consent form for allowing this case report to be published for
scientific purposes.
Acknowledgements
We would like to thank doctor Gabriele Bazzocchi, Trieste (Italy), for providing the radiological imaging.
References
- Boiron P, Provost F. In-vitro susceptibility
testing of Nocardia spp. and its taxonomic implication. J Antimicrobial
Chemother 1988; 22: 623-629. http://dx.doi.org/10.1093/jac/22.5.623

- Mishra
SK, Gordon R, Barnett DA Identification of nocardiae and actinomycetes
of medical importance. J Clin Microbiol 1980; 11:
728-736 PMid:7000820 PMCid:PMC273495

- Clinical
and Laboratory Standards Institute (CLSI). Susceptibility Testing of
Mycobacteria, Nocardiae, and Other Aerobic Actinomycetes. CLSI document
M24-A2, March 2011, Wayne, Pennsylvania 19087-1898 USA, 2011.
- Yassin
AF, Rainey FA, Burghardt J, Brzezinka H, Mauch M, Schaal KP. Nocardia
paucivorans sp. nov. Int J Syst Evol Microbiol. 2000; 50 Pt 2: 803-9. http://dx.doi.org/10.1099/00207713-50-2-803 PMid:10758891

- Wauters
G, Avesani V, Charlier J, Janssens M, Veneechoutte M, Delmée M.
Distribution of Nocardia species in clinical samples and their routine
rapid identification in the laboratory. J Clin Microbiol. 2005; 43:
2624-2628. http://dx.doi.org/10.1128/JCM.43.6.2624-2628.2005 PMid:15956375 PMCid:PMC1151960

- McNeil
MM, Brown JM. The medically important aerobic actinomycetes:
epidemiology and microbiology. Clin Microbiol Rev. 1994; 7:
357-417. PMid:7923055 PMCid:PMC358331

- Wilson JW. Nocardiosis: Updates and Clinical Overview. Mayo Clin Proc. 2012; 87: 403-407. doi: 10.1016/j.mayocp.2011.11.016. http://dx.doi.org/10.1016/j.mayocp.2011.11.016

- Corti ME, Villafa-e-Fioti MF. Nocardiosis: a review. Int J Infect Dis. 2003; 7: 243-50. http://dx.doi.org/10.1016/S1201-9712(03)90102-0

- Anagnostou
T, Arvanitis M, Kourkoumpetis TK, Desalermos A, Carneiro HA, Mylonakis
E. Nocardiosis of the central nervous system: experience from a general
hospital and review of 84 cases from the literature. Medicine
(Baltimore). 2014; 93:19-32. http://dx.doi.org/10.1097/MD.0000000000000012 PMid:24378740

- Gray
TJ, Serisier DJ, Gilpin CM, Coulter C, Bowler SJ, McCormack JG.
Nocardia paucivorans --a cause of disseminated nocardiosis. J Infect.
2007; 54: e95-8. http://dx.doi.org/10.1016/j.jinf.2006.05.005 PMid:16808975

- Hammoud
M, Kraft C, Pulst-Korenberg J, Chenoweth C, Gregg KS. Disseminated
Nocardia paucivorans infection in an immunocompetent host. Infection.
2014 15. [Epub ahead of print]

- Chouci-o
C, Goodman SA, Greer JP, Stein RS, Wolff SN, Dummer JS. Nocardial
infections in bone marrow transplant recipients. Clin Infect Dis. 1996;
23: 1012-9. http://dx.doi.org/10.1093/clinids/23.5.1012

- Pamukçuoglu
M, Emmez H, Tunçcan OG, Oner AY, Cirak MY, Senol E, Sucak GT. Brain
abscess caused by Nocardia cyriacigeorgica in two patients with
multiple myeloma: novel agents, new spectrum of infections. Hematology.
2014; 19: 158-62. http://dx.doi.org/10.1179/1607845413Y.0000000108 PMid:23906027

- Eisenblätter
M, Disko U, Stoltenburg-Didinger G, Scherübl H, Schaal KP, Roth A,
Ignatius R, Zeitz M, Hahn H, Wagner J. Isolation of Nocardia
paucivorans from the cerebrospinal fluid of a patient with relapse of
cerebral nocardiosis. J Clin Microbiol. 2002; 40: 3532-4. http://dx.doi.org/10.1128/JCM.40.9.3532-3534.2002 PMid:12202613 PMCid:PMC130694

- Khan
SH, Sanche SE, Robinson CA, Pirouzmand FN. paucivorans infection
presenting as a brain abscess. Can J Neurol Sci. 2006; 33: 426-7. http://dx.doi.org/10.1017/S0317167100005436 PMid:17168173

- König
C, Kleber M, Reinhardt H, Knop S, Wäsch R, Engelhardt M. Incidence,
risk factors, and implemented prophylaxis of varicella zoster virus
infection, including complicated varicella zoster virus and herpes
simplex virus infections, in lenalidomide-treated multiple myeloma
patients. Ann Hematol. 2014; 93: 479-84. http://dx.doi.org/10.1007/s00277-013-1951-6 PMid:24318541

- Borrello I. Can we change the disease biology of multiple myeloma? Leuk Res. 2012; 36: S3-12.

- Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J. 2005 22;7: E14-9. http://dx.doi.org/10.1208/aapsj070103 PMid:16146335 PMCid:PMC2751493

- Yang
B, Yu RL, Chi XH, Lu XC. Lenalidomide treatment for multiple myeloma:
systematic review and meta-analysis of randomized controlled trials.
PLoS One. 2013 14;8: e64354.

- Jung
SH, Bae SY, Ahn JS, Kang SJ, Yang DH, Kim YK, Kim HJ, Lee JJ.
Lymphocytopenia is associated with an increased risk of severe
infections in patients with multiple myeloma treated with
bortezomib-based regimens. Int J Hematol. 2013 ;97: 382-7. http://dx.doi.org/10.1007/s12185-013-1270-7 PMid:23355264

- Nucci
M, Anaissie E. Infections in patients with multiple myeloma in the era
of high-dose therapy and novel agents. Clin Infect Dis. 2009
15;49:1211-25. http://dx.doi.org/10.1086/605664 PMid:19769539

- Martínez
Tomás R, Menéndez Villanueva R, Reyes Calzada S, Santos Durantez M,
Vallés Tarazona JM, Modesto Alapont M, Gobernado Serrano M. Pulmonary
nocardiosis: risk factors and outcomes. Respirology. 2007;12: 394-400. http://dx.doi.org/10.1111/j.1440-1843.2007.01078.x PMid:17539844

- Raturi
R, Palacio C, Baluch A, Vargas J, Kenney P, Greene JN. Retrospective
analysis of opportunistic brain abscesses in patients with hematologic
malignancies. Infect Dis Clin Pract 2014;22: 96-103. http://dx.doi.org/10.1097/IPC.0b013e3182a1eca2
























