Factors Influencing Adherence to Imatinib in Indian Chronic Myeloid Leukemia Patients: A Cross-Sectional Study
Jyotsna Kapoor1, Narendra Agrawal2, Rayaz Ahmed2, Sanjeev Kumar Sharma3, Anshul Gupta2 and Dinesh Bhurani2
1 Masters in
Clinical Research, Department of Hematology,
Rajiv Gandhi Cancer Institute and
Research Centre, Sector - 5, Rohini,
Delhi, India. PIN 110085
2 DM, Consultant
Hematology, Department of Hematology, Rajiv
Gandhi Cancer Institute and Research
Centre, Sector - 5, Rohini, Delhi,
India. PIN 110085
3 DM, Consultant Hematology,
Hemato-Oncology and BMT Unit, BLK Superspeciality Hospital, Rajendra
Place, New Delhi, India, PIN 110008
Corresponding author:Dinesh Bhurani, Department of
Hematology, Rajiv Gandhi Cancer Institute and Research Centre,
Sector-5, Rohini, Delhi, India. Mobile Number- 91-9971500861, Fax –
91-11-27051670. E-mail:
bhurani@gmail.com
Published: February 20, 2015
Received: November 9, 2014
Accepted: February 2, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015013, DOI
10.4084/MJHID.2015.013
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Adherence to imatinib (IM) is of
utmost importance in patients with chronic myeloid leukemia (CML) to
maximise treatment effectiveness. The main objective is to measure
adherence to IM by evaluating individual patient characteristics,
personal behaviour and, treatment related psychological factors
influencing adherence behaviour. Hundred patients receiving IM were
analysed for adherence behaviour using 9 item Morisky Medication
Adherence Scale (9-MMAS). Various factors were assessed for their
impact on adherence behaviour. These factors were age, gender, duration
of treatment, frequency and dosing of treatment, use of tobacco and
alcohol, educational qualification, employment status, monthly income,
side effects, financial assistance in treatment, social support,
knowledge about medicine and disease, concomitant drug burden,
polypharmacy, physician patient interaction, patient educational
sessions and prevalence of depression. Seventy five percent of patients
were found to be adherent. On univariate analysis, prevalence of
depression (p<0.000001), moderate severe depression (p<0.000001),
concomitant drug burden (p=0.036) and monthly income (p=0.015) were
found to be significantly influencing adherence. The final multivariate
model retained prevalence of depression with OR= 10.367 (95% CI, 3.112-
34.538) as independent predictor of adherence to therapy. This study
suggests that identification and treatment of depression among CML
patients may further enhance adherence to IM therapy. |
Introduction
More than a decade ago, revolution came in the treatment of CML with
the introduction of the Imatinib Mesylate (IM), a BCR- ABL tyrosine
kinase inhibitor. After 5 years of follow – up, continuous treatment of
chronic – phase CML with imatinib, as initial therapy, was found to
induce durable responses in a high proportion of patients.[1,2] With IM
being so effective, the allogenic stem cell transplantation no longer
remains the first line treatment, despite being a curative treatment.
Though IM is the first line treatment, few drawbacks are associated
with its use as it is still not considered to be a curative therapy; it
requires indefinite treatment on daily basis and ensuring optimal
adherence to treatment for long term. Adherence to medication has been
recently defined by an international panel of experts as ‘the process
by which patients take their medications as prescribed’ and this
process has three main components: initiation, implementation, and
discontinuation.[3]
Various studies and several case reports have
shown that non adherence to IM is common[4-10,13] and intertwined with
non-achievement of molecular responses[4,5,7,10] and event free
survival[8] emphasising strict adherence to the prescribed dose of IM
holds paramount importance to maximise treatment effectiveness in CML
patients. For example, a Belgian study found that one third of the
patients were non adherent and only 14% were adherent to all the
prescribed dose. On average, patients with suboptimal response had
significantly higher mean percentages of IM not taken (23.2%,standard
deviation [SD] 23.8) than did those with optimal response (7.3%, SD
19.3, P.005),[5] Marin et al found that 26% of the patients had
adherence rate <90% (considered to be nonadherent) and adherence is
a critical factor for achieving molecular responses in patients with
CML who achieve complete cytogenetic responses on IM.[4] Darkow et al
found 31% of nonadherence rate among US CML population using electronic
data of dispensation of IM and also found non- adherence led to
increased healthcare costs.[6] Adherence to IM have been also studied
in the past in Indian population using records of Glivec International
Patient Assistance Program (GIPAP) retrospectively in which one third
of the patients were found to be non-adherent to IM and concluded that
non- adherence to IM adversely affects event free survival (EFS) in
chronic phase CML (CP-CML) patients.[8]
There is scarce
availability of literature citing the potential reasons for
non-adherence to oral anticancer treatment[11] and few existing data on
reasons why CML patients might be non- adherent to IM. Treatment
related aspects (side effects, knowledge of disease or treatment,
financial cost of treatment etc.), individual patient characteristics
(gender, age) and personal factors (social support) have been found to
be influencing adherence in chronic illnesses.[11-13] We hypothesized
that these factors might affect adherence to IM in CML patients too.
Ganesan et al tried to explore reasons of non- adherence to IM in
Indian CML patients and assessed age, sex, economic status and Sokal
score.[8] No study has completely investigated the treatment related,
individual patient characteristics, personal and psychological factors
influencing adherence in Indian patients with CML so far. Therefore, we
conducted this personal interview based study to assess the adherence
of CML patients using 9 MMAS and to evaluate personal, treatment
related, and psychological factor associated with adherence at Rajiv
Gandhi Cancer Institute and Research Centre, India.
Methods
Study Design and Setting.
This study was carried out at Rajiv Gandhi Cancer Institute and
Research Centre, Delhi, India. All CML patients over 18 years of age
and below 80 years, with ongoing IM therapy for minimum duration of
three months, and who visited the outpatient department during a period
of February 2013 and May 2013 were considered for inclusion in the
study. Patients who were dumb and/or deaf or undergone allogenic
hematopoietic stem cell transplant were excluded from the study. The
questionnaires were available in Hindi and English, the patients who
did not understand these languages were excluded. The patients included
in the study were taking IM either 400mg/day or 600mg/day or 800mg/day.
The patients who were taking 600mg/day or 800mg/day were advised to
take half the dose after heavy meal in the morning and the other half
dose after heavy meal in the evening to manage the gastric side
effects. Optimal sample size was calculated and found to be 84 in
accordance with the previous adherence study conducted on Indian
population by Ganesan et al (30% of non-adherence rate was found), we
approximated the sample size to be 100.[8] The total number of patients
visiting the OPD within this period were 139 and 82.7% (115 patients)
of these fulfilled the inclusion criteria.
The questionnaire was
translated by official translators in Hindi allowing the majority of
patients to undergo personal interview in their native language. The
patients were given oral and written information regarding the study
when asked to participate. After giving oral and written consent for
participation, the study coordinator personally interviewed the
patients using questionnaires in their preferred language. This study
was approved by the Institutional Review Board of our centre. This
study was conducted in accordance with latest version of Declaration of
Helsinki.
Questionnaires. The questionnaire used consisted of 9-MMAS (to measure adherence behaviour), additional questionnaire
(to assess the factors influencing adherence except depression) and
PHQ-9 (to assess prevalence of depression). The questionnaire asked
about adherence behaviour, socio-demographic background, knowledge
about disease and medicine, social support, physician patient
relationship, role of patient educational sessions, side effects of
medicine, financial assistance in treatment, concomitant drug burden,
polypharmacy, details about therapy, and depression. Additional questionnaire
was partly devised from questionnaire, previously used by Jonsonn et
al9 and questions regarding role of patient educational sessions,
polypharmacy, financial assistance in treatment and concomitant drug
burden were added in view of our cohort. The internal consistency
reliability of the combined questionnaire to assess the factors
influencing adherence (additional questionnaire and PHQ-9), using
Crohnbach α was found to be 0.72.
Adherence Behaviour.
The 9-item Morisky Medication Adherence Scale (9-MMAS), a standardised
test, was used to measure adherence, with scores ranging from 1-13,
where 13 indicates perfects adherence. This test has been developed
from the well validated Morisky Green Test and the eight item
MMAS.[15,16] The internal consistency reliability of the English
version of 9- item MMAS, measured by the Crohnbach α, had a value of
0.89.[15] The 9- item MMAS is composed of 9 questions explores
adherence behaviour based on forgetfulness, negligence, interruption of
drug intake and restart of drug intake when symptoms worsen. Patients
scoring 11 or above in the summary score were classified as adherent.
This definition of adherence is based on how patients theoretically
would have completed the MMAS if they had taken at least 95% of
prescribed doses.
Factors Influencing Adherence.
Socio-demographic background composed of 8 questions asking about
gender, age, marital status, employment status, educational
qualification, monthly income, and use of tobacco or alcohol in any
form. For example, with regard to employment status, a question was
asked ‘Do you work?’ with an option of ‘Yes/No’. Knowledge about
Medicine and Disease composed of 5 questions along with subparts to
find out whether the respondents have basic knowledge about their
disease and treatment. For each correct answer ‘1’ was scored. Support
given by family, friends and colleagues was assessed using 10 questions
comprising of Yes/No option. A healthy and regular physician patient
interaction was assessed using a set of 7 questions followed by a Yes/
No option except one question. Questions included were ‘Do you visit
your physician at regular intervals?’, ‘Do you feel the physician is
very helpful to you?’ ‘Do you trust your physician?’etc. Patients were
interviewed whether they have attended the last patient educational
session on CML and if yes, did they found it helpful to find out the
role of patient educational sessions on adherence. Patients were
questioned about being financially assisted in treatment, if so, and
then what were the means of assistance. Concomitant drug burden was
defined as the assumption of additional drugs related to diseases other
than CML may affect the adherence to IM (Yes/No). Polypharmacy was
defined as taking at least one alternative medicine apart from IM for
CML (Yes/No) may affect the adherence to IM. Commonly used alternative
medicines were from ayurvedic, homeopathic and unani system of
medicine. Patients were also questioned about the side effects if they
ever had with the use of imatinib and if they had, the side effects
were recorded accordingly. The prevalence of depression among CML
patients was evaluated with a Patient Health Questionnaire-9 (PHQ -9),
a validated and standardized instrument with good specificity and
sensitivity. The PHQ-9 focuses on the nine signs and symptoms of
depression from DSM-IV. In addition, the sum score of PHQ-9 (0-27) is
used for screening purposes and for measuring depression severity. As a
severity measure the PHQ-9 score can range from 0-27, since each of the
9 items can be scored from 0 (Not at all) to 3 (Nearly every day).
Statistical Analysis
The
quantitative variables were presented with mean and SD, however the
categorical variables in frequencies along with respective percentages.
The reliability of all the domains of the questionnaire was tested by
Cronbach alpha. When comparing adherent with non-adherent patients, in
the univariate analysis, chi-squared test was used to analyze
categorical data (gender, use of tobacco/smoking, use of alcohol,
employment status, educational qualifications, patient educational
sessions, financial assistance in treatment, Side effects of Imatinib,
prevalence of depression, concomitant drug burden, polypharmacy, dose
of imatinib and frequency of dosing of imatinib), the independent
t-test was used to compare means (age, knowledge about medicine and
disease, social support, physician patient interaction and duration of
prescription of imatinib) and Mann-Whitney U test was used to compare
Monthly income.
Multiple
logistic regression analysis was used to identify factors associated
with adherence. For variable selection in the model, the backward
stepwise likelihood ratio method was used to perform regression
analysis with probability less than 0.3. Data were analyzed using SPSS
version 21.0 (2012, IBM Corp, Armonk, NY, USA) and p value <0.05 was
considered of statistical significance.
Results
In this study, 100 out of 115 eligible patients completed the
interview (response rate 86.9%) (Figure 1). 51% of the respondents were
interviewed in Hindi language.
|
|
Figure 1. Patient Recruitment Details |
Descriptive Statistics. Descriptive
statistical data of 100 patients analyzed are present in Table 1. The
majority of the respondents were male (63%) and the mean age was 41.08
years (range 18-70) and median duration of imatinib therapy was 30
months (range 3-101).
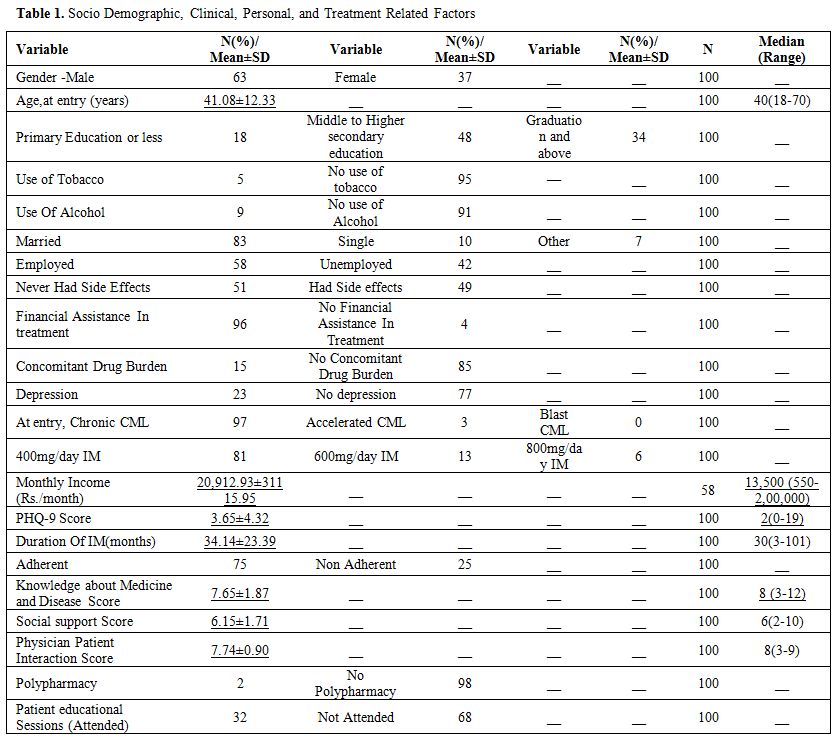 |
Table 1. Socio Demographic, Clinical, Personal, and Treatment Related Factors |
Adherence Behaviour. All patients included
in the study (n=100) completed the 9-MMAS. The median Morisky Score of
100 patients included was 12 (Range; 7-13). 75 (50 male and 25 female)
out of 100 patients had Morisky score ≥ 11, therefore classified as
adherent. Twenty two percent of the respondents scored 13, i.e. perfect
adherence. Forty six percent of the respondents had special routine or
reminder system which helps them taking medication. Ninety three
percent patients took their medicine prior to the day of interview.
None of the patients had summary score <5. Four out of twenty five
non adherent patients had summary score between 5 and 8.
Comparison
of variables with Adherence. The univariate analysis is presented in
Table 2 and 3. Among the quantitative variables, monthly income of the
patients was found to be significantly associated with adherence
(p-value 0.015). Among the categorical variables, prevalence of
depression (p value <0.000001), moderate severe depression
(p<0.000001) and concomitant drug burden (p value = 0.036) were
found to be significantly associated with adherence behaviour. Non
depressed people were more likely to be adherent (84.4% vs 43.5%).
Patients with no concomitant drug burden were more likely to be
adherent (78.8% vs 53.3%).
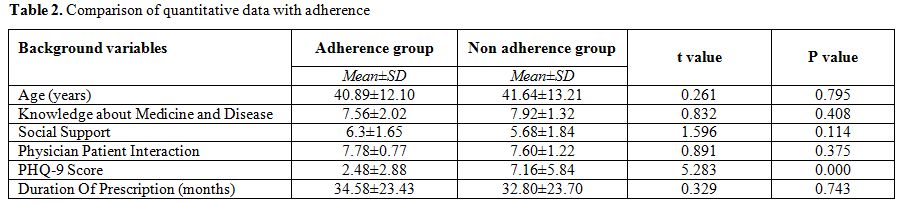 |
Table 2. Comparison of quantitative data with adherence |
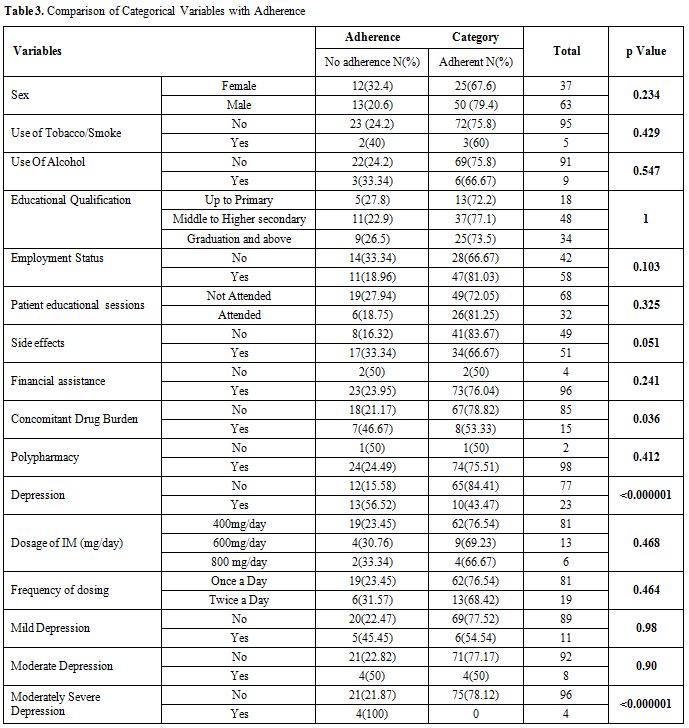 |
Table 3.Comparison of Categorical Variables with Adherence |
The results of the logistic regression
analysis of factors associated with adherence (9-MMAS summary score ≥
11), adjusted for covariates are presented in Table 4. The variables
included in the study were age, knowledge about medicine and treatment,
physician patient interaction, those who attended patient educational
sessions, male, depressed patients, smokers, alcoholics, educational
qualifications, employed patients, patients who had side effects, being
financially assisted in treatment, had concomitant drug burden, having
polypharmacy and dosage of imatinib. Full data were available for all
the 100 patients, who were included in the logistic regression
analysis. Prevalence of depression among CML patients remained
independently associated with adherence (OR= 10.367, 95% CI 3.112-
34.538).
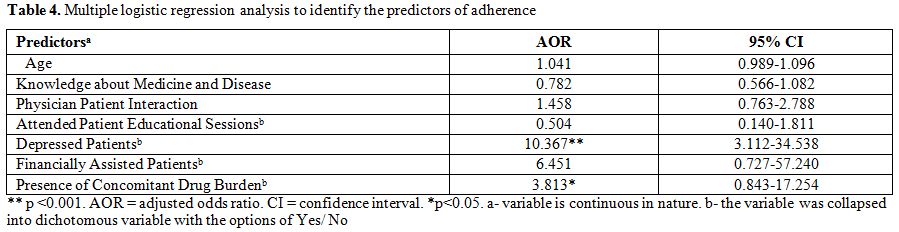 |
Table 4. Multiple logistic regression analysis to identify the predictors of adherence |
Discussion
The
objectives of the study were to assess the prevalence of adherence to
imatinib treatment in Indian CML patients, to evaluate the factors
associated with adherence. In this sample, 75% of the respondents were
classified as adherent. Factors associated with high adherence were no
concomitant drug burden, no prevalence of depression and monthly
income. As the questionnaire was also available in Hindi, participation
of patients who could not understand English was encouraged. The
response rate of patients was found to be fairly high (86.9%).Optimal adherence to imatinib therapy is crucial to maximize treatment effectiveness,[4,5,7,8] however the ability of the physician to recognize adherence is poor.[19]
Given the scanty data of CML literature, we selected the possible
factors to be associated with the adherence behaviour based on previous
studies in other chronic medical illnesses.[12,18,19,23]
The percentage of patients found to be non-adherent in our study (i.e.
25%), seems consistent with previous data indicating non adherence
rates of 25 to 50%.[19] Also, it is difficult to make
the comparisons regarding prevalence of non adherence in other studies
as this fluctuates as a function of methods used. However, our study
support previous findings that adherence to imatinib therapy is far
from optimal (i.e 75 % of patients have adherence rates ≥ 95%) in CML
patients.[5] As per our knowledge, only one study in a small cohort of 38 patients have found ‘good’ adherence to imatinib therapy.[9]Negative
significant association between the adherence and the prevalence of
depression among the Indian CML cohort was observed with a p value
<0.00001. 23% (n=23) of patients were found to be depressed, out of
which none of the patient was severely depressed. 47.82 %(n=11), 34.78%
(n=8) and 17.4% (n=4) patients were found to be mildly, moderately and
moderately severely depressed. We further analyzed the severity (mild,
moderate and moderately severe depression) of depression with adherence
and found moderate severely depressed patients to be significantly
associated with non-adherence (p<0.000001). Our study revealed that
non depressed patients are more likely to be adherent (84.4% vs 43.5%).
Prevalence of depression was found to be the only factor to be
associated with adherence through multivariate logistic regression
analysis with odds ratio of 10.367 with 95% confidence interval of odds
ratio to be between 3.112 and 34.538. Given the paucity of data in the
CML literature regarding the negative association between adherence and
depression, our findings are thus consistent with the meta-analysis
performed by Di Matteo et al which included 12 articles about
depression and noncompliance to medical treatment and 13 articles about
anxiety and noncompliance to medical treatment revealed a significant
and substantial relationship between depression and non-adherence to
medical treatment prescribed for chronic illnesses.[22]
A recent meta analysis on the depression and medication adherence of
patients with chronic diseases in U.S population by Grenard et al
estimated the odds of a depressed patient being non-adherent are 1.76
times the odds of a non-depressed patient across 31 studies and 18,245
participants.[24]In
our cohort, concomitant drug burden was found to be negatively
associated with adherence to imatinib therapy (p value – 0.036). Out of
15 patients on concomitant drugs, only 8 patients (53%) were found to
be adherent. Though our results contrasts with the results obtained
worldwide, which states that concomitant drug burden has a positive
association with adherence to imatinib therapy in CML patients.[5,13] Noens et al showed an association between more medication to be taken daily and better adherence to imatinib therapy.[5] A qualitative study by Eliasson et al [23]
reported that adherent patients referred to taking imatinib as being
part of their daily routine, possible to speculate that patients who
are already taking medications for other diseases might be facilitating
in fitting CML therapy into their regular overall medication taking
schedule. However, we might have observed such a contrasting result
because the concomitant drug burden in the previous studies was fairly
high (41.16% in Efficace F et al)[13] unlike our
study (15%) and only 46% of the patients in the 9-MMAS reported that
they had a special routine or a reminder system to facilitate their
medication taking behaviour.58%
patients were found to be working in our cohort of Indian CML patients
with a mean monthly income of Rs.20,912.93 (range Rs.550-2,00,000). Our
results showed monthly income to be associated with adherence to
imatinib therapy (p value- 0.015) through univariate analysis but this
was found to be insignificant when logistic regression analysis was
performed.There
is conflicting evidence in the literature whether age influences
adherence in CML patients. A study of 87 patients by Marin et al,[4]
showed that younger patients have lower adherence rate whereas older
patients with a median age of 53.8 years had an adherence rate of
greater than or equal to 90%. Unlike our study, did not show that
increasing age positively influences adherence (p value – 0.795). A study of Darkow et al[6]
on 267 patients showed adherence to be influenced by gender, non
adherence was significantly higher in women; in the present study this
difference was not observed (p = 0.234). Santoleri et al concluded that
frequency of dosing does not influence adherence to drug therapy.[20]
Though the imatinib is once a day dose, but patients prescribed
600mg/day or 800mg/day of imatinib were advised to take half the dose
in morning and other half in evening to manage the gastric side
effects. Similar results were obtained through this study (p value –
0.536). Imatinib therapy is prolonged and previous research has shown
that adherence for long – term drug therapies are lower, often no more
than 40-50%,[13] but our study reflected no
significant association between adherence and duration of prescription
(months) of imatinib( p= 0.743). The side effects of imatinib are
relatively mild, dyspepsia (21%) and edema (21%) was found to affect
the CML patients the most. As these side effects are mild, adherence
was found not to be influenced by side effects (p=0.051). Richardson et
al showed that patient educational programs including information on
disease and expected side effects were associated with better survival
in patients with hematologic malignancies.[26] Moon et al reported that a counselling programme was effective in improving compliance in CML patients receiving imatinib.[27]
But, our study did not reflected the similar results, as we found
patient educational sessions did not play a significant role in
influencing adherence (p value- 0.325)Backward
step wise multiple logistic regression analysis was used to find the
independent predictors of adherence. Initially, all the independent
variables were included in the model. Further, non-associated variables
were dropped one by one step wise and finally age, knowledge about
medicine and disease, physician patient interaction, patient
educational sessions, prevalence of depression, financial assistance
and concomitant drug burden were selected at 10th
step with probability less than 30%. The criterion of 30% was based on
the assumption to find the closely related variables with adherence.
Among all the selected variables, only depression was significantly (OR
10.367; 95% C.I, 3.112- 34.538) associated with the adherence. However,
other independent variables showed the closeness to the adherence.
Marin et al showed that younger patients have lower adherence.[4]
In HIV patients, the perceived very good contact with health care was
found to be associated with adherence to antiretroviral treatment.[14] Efficace et al found concomitant drug burden as an independent predictor of adherence in CML patients to IM.[13] Moon et al reported that a counselling programme was effective in improving compliance in CML patients receiving imatinib.[27]This
paper has number of strengths including, selection bias is likely to be
limited as the proportion of non-respondents was fairly small (15 of
115). A response rate of almost 87% is fairly good and the proportion
of eligible patients was also high (115 of 139). No internal attrition
was found. For appropriate results, the sample size approximation was
priorly done in accordance with the adherence study conducted on Indian
population.[8]This
paper, however, also has potential limitations. First, we might have
missed additional patient related and psychological factors that might
have found to be related to adherence in patients with other diseases.[25]
Second, we used non validated questionnaire to assess the factors
influencing adherence except depression and third, it is possible that
additional measures of adherence could have further contributed to a
more sensitive definition of adherence in our study. However, the
methods available for measuring adherence all have different strengths
and weaknesses; because of the complexity of the adherence behaviour
and problems with bias, none is optimal and self-report methods provide
a good estimation of medication adherence in an inexpensive manner over
a possible breadth of distribution and also have great advantages over
other methods.These
potential limitations notwithstanding, we are confident our results
extend findings of previous research in the field of adherence and
investigation of factors influencing adherence in CML on IM to suggest
key potential determinant of adherence behaviour. Physicians are
encouraged to pay attention to factors identified in this study could
help to promptly identify patients who might be at a heightened risk of
non adherence. Acknowledgments
We thank Dr. Jes Rafael for his valuable suggestions to improve the
design and conduct of the study. Dr. Tabassum, Dr. Shishir Seth played
a vital role in helping to recruit the patients in the study. We would
like to pay our gratitude to Dr. Ram Chandra Bajpai for performing the
statistical analysis and critically reviewing the manuscript. We are
indebted to Dr. Suman Pramanik for critical reading of the manuscript
and Mrs. Niharika Bhatia, Miss Priyanka Shrivastav for the
unconditional support in the conduct of the study. We thank all the
members and staff of Rajiv Gandhi Cancer Institute and Research Centre,
India.
References
- Druker BJ, Guilhot F, O' Brien SG et al. Five
year follow up of patients receiving imatinib for chronic myeloid
leukemia. N Eng J Med 2006; 355: 2408-2417.
http://dx.doi.org/10.1056/NEJMoa062867 PMid:17151364 
- Kantarjian
HM, Cortes J, La Rosee P, Hochhaus A. Optimising therapy for patients
with chronic myelogenous leukemia in chronic phase. Cancer 2010; 116:
1419-1430.
http://dx.doi.org/10.1002/cncr.24928 PMid:20120030 
- Vrijens
B, De Geest S, Hughes DA et al. A new taxonomy for describing and
defining adherence to medications. Br J Clin Pharmacol 2012; 73:
691–705. http://dx.doi.org/10.1111/j.1365-2125.2012.04167.x PMid:22486599 PMCid:PMC3403197

- Marin
D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Blea M, Apperley JF,
Syzdlo R et al. Adherence is the critical factor achieving molecular
responses in patients with chronic myeloid leukemia who have achieve
complete cytogenetic responses on imatinib. J Clin Oncol 2010; 28 :
2381 – 2388. http://dx.doi.org/10.1200/JCO.2009.26.3087 PMid:20385986

- Noens
L, van Lierde MA, De Beck R et al. Prevalence, determinants, and
outcome of non-adherence to imatinib therapy in patients with chronic
myeloid leukemia : The ADAGIO Study. Blood 2009 ;113 : 5401 -5411. http://dx.doi.org/10.1182/blood-2008-12-196543 PMid:19349618

- DarkowT,
Henk HJ, Thomas SK, et al. Treatment interruptions and non adherence
with imatinib and associated healthcare costs : A retrospective
analysis among managed care patients with chronic myelogenous leukemia.
Pharmacoeconomics2007; 25 :481 -496. http://dx.doi.org/10.2165/00019053-200725060-00004 PMid:17523753

- Ibrahim
AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Syzdlo R et al. Poor
adherence is the main reason for loss of CCyR and imatinib failure for
chronic myeloid leukemia patients on long term therapy. Blood 2011 ;
117 (14) : 3733 – 6. http://dx.doi.org/10.1182/blood-2010-10-309807 PMid:21346253

- Ganesan
P, Sagar TG, Dubashi B, Rajendranath R, Kannan K, Cyriac S et al. Non
adherence to imatinib adversely affects event free survival in chronic
phase chronic myeloid leukemia. Am J Hematol.2011 ; 86 (6) :471 -4. http://dx.doi.org/10.1002/ajh.22019 PMid:21538468

- JonssonS,
Olsson B, Soderberg J, Wadnenvik H. Good adherence to imatinib therapy
among patients with chronic myeloid leukemia – a single center
observational study. Ann Hematol 2012, 91 : 679- 685. http://dx.doi.org/10.1007/s00277-011-1359-0 PMid:22048790

- Campos
Dos Reis S, de Souze Quixada A, Nune S, Camelocid MD, de Souza J, de
Costa C et al. Adherence to imatinib in chronic myeloid leukemia : a
study of first decade of responses obtained at a Brazilian Hospital.
Rev Bras Hematol Hemoter. 2013 35 (3) :174-9
 .
.
- Ruddy
K, Mayer E, Patridge A. Patient adherence and persistence with oral
anticancer treatment. CA Cancer J Clin (2009), 59:56-66. http://dx.doi.org/10.3322/caac.20004 PMid:19147869

- Di Matteo MR. Social support and patient adherence to medical treatment : a meta- analysis. Health Psychol (2004), 23: 207-218. http://dx.doi.org/10.1037/0278-6133.23.2.207 PMid:15008666

- Efficace
F, Baccarani M, Rosti G et al. Investigating factors associated with
adherence behaviour in patients with chronic myeloid leukemia: an
observational patient-centered outcome study. Br J Cancer (2012),107
:904-909. http://dx.doi.org/10.1038/bjc.2012.348 PMid:22871884 PMCid:PMC3464760

- Sodergard
B, Halvarsson M, Tully MP, Mindouri S, Nordstom ML, Lindback S, et al.
Adherence to treatment in Swedish HIV – infected patients. J Clin Pharm
Ther 2006, 31 : 605 – 616. http://dx.doi.org/10.1111/j.1365-2710.2006.00782.x PMid:17176366

- Morisky
DE, Green LW, Levin DM. Concurrent and predictive validity of a self
reported measure of medication adherence. Med Care 1986, 24 : 67-74. http://dx.doi.org/10.1097/00005650-198601000-00007 PMid:3945130

- Morisky
DE, Ang A, Krousal – Wood M, Ward HJ. Predictive validity of a
medication adherence measure in an outpatient setting. J Clin
Hypertens2008, 10 : 348 – 354. http://dx.doi.org/10.1111/j.1751-7176.2008.07572.x

- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005, 353:487–497. http://dx.doi.org/10.1056/NEJMra050100 PMid:16079372

- Vermeire
E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to
treatment: three decades of research. A comprehensive review. J Clin
Pharm Ther 2001, 26: 331–342. http://dx.doi.org/10.1046/j.1365-2710.2001.00363.x PMid:11679023

- Di
Matteo MR. Variations in patients' adherence to medical
recommendations: a quantitative review of 50 years of research. Med
Care 2004, 42: 200–209 http://dx.doi.org/10.1097/01.mlr.0000114908.90348.f9 PMid:15076819

- Santoleri
F, Sorice P, Lasala R, Rizzo CR, Constantini A. Patient adherence and
persistence with imatinib, nilotinib, dasatinib in clinical practice.
Plos One 2013, 8; issue 2. PMid:23437249 PMCid:PMC3577678

- Krousal
Wood M, Hyre A, Muntner P, Morisky D. Methods to improve medication
adherence in patients with hypertension: Current status and future
directions. Curr Opi Cardiol 2005, 20 : 296 – 300.

- Dimatteo
MR, Leeper HS, Croghan TW. Depression is a risk factor for non
compliance with medical treatment :Meta-analysis of the effects of
anxiety and depression on patient adherence. Archives of Internal
Medicine 2000, 160 (14) : 210 -217. http://dx.doi.org/10.1001/archinte.160.14.2101

- Eliasson
L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia
patient's reasons for not adhering to the oral anticancer treatment
drug imatinib as prescribed. Leuk Res 2011;35:626-630. http://dx.doi.org/10.1016/j.leukres.2010.10.017 PMid:21095002

- Grenard
JL, Munjas BA, Adams JL, et al. Depression and medication adherence in
the treatment of chronic diseases in the United States: A
meta-analysis. Journal of General Internal Medicine. 2011;26:1175–1182.
http://dx.doi.org/10.1007/s11606-011-1704-y PMid:21533823 PMCid:PMC3181287

- Markkula
A, Hietala M, Henningson M, Ingvar C, Rose C, Jernstrom H (2012)
Clinical profiles predict early nonadherence to adjuvant endocrine
treatment in a prospective breast cancer cohort. Cancer Prev Res 5:
735–745 http://dx.doi.org/10.1158/1940-6207.CAPR-11-0442 PMid:22401981

- Richardson
JL, Shelton DR, Krailo M, Levine AM (1990) The effect of compliance
with treatment on survival among patients with hematologic
malignancies. J Clin Oncol 8: 356–364 PMid:2299375

- Moon
JH, Sohn SK, Kim SN, Park SY, Yoon SS, Kim IH, Kim HJ, Kim YK, Min YH,
Cheong JW, Kim JS, Jung CW, Kim DH (2011) Patient counseling program to
improve the compliance to imatinib in chronic myeloid leukemia
patients. Med Oncol 29: 1179–1185. http://dx.doi.org/10.1007/s12032-011-9926-8 PMid:21472487

[TOP]















 .
. 















