Current Role of Autologous and Allogeneic Stem Cell Transplantation for Relapsed and Refractory Hodgkin Lymphoma
Luca Castagna1, Carmelo Carlo-Stella1,2, Rita Mazza1 and Armando Santoro1
1 Department of Hematology and Oncology, Humanitas Cancer Center, Humanitas Clinical and Research Center, Rozzano (Milano), Italy
2 Department of Medical Biotechnology and Translational Medicine, University of Milano, Milano, Italy
L.C. and C.C.-S. contributed equally to this manuscript.
Corresponding author: Armando Santoro, M.D.
Department of Oncology and Hematology, Humanitas Cancer Center,
Humanitas Clinical and Research Center. Via Manzoni, 56 - Rozzano
20089, Italy. Tel: +39 02 8224 4080, Fax: +39 02 8224 4590. E-mail:
armando.santoro@cancercenter.humanitas.it
Published: February 15, 2015
Received: November 11, 2014
Accepted: January 19, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015015, DOI
10.4084/MJHID.2015.015
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(http://creativecommons.org/licenses/by/2.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Classical Hodgkin lymphoma (cHL) is a
relatively rare disease, with approximately 9,200 estimated new cases
and 1,200 estimated deaths per year in the United States. First-line
chemo-radiotherapy leads to cure rates approaching 80% in patients with
advanced-stage disease. However, 25 to 30% of these patients are not
cured with chemotherapy alone (i.e., the ABVD regimen) and show either
primary refractoriness to chemotherapy, early disease relapse or late
disease relapse. Second-line salvage high-dose chemotherapy (HDC) and
autologous stem cell transplantation (SCT) have an established role in
the management of refractory/relapsed cHL, leading to durable responses
in approximately 50% of relapsed patients and a minority of refractory
patients. However, due to the poor responses to second-line salvage
chemotherapy and dismal long-term disease control of primary refractory
and early relapsed patients, their treatment represents an unmet
medical need. Allogeneic SCT represents, by far, the only strategy with
a curative potential for these patients; however, as discussed in this
review, it’s role in cHL remains controversial. Despite a general
consensus that early relapsed and primary refractory patients represent
a clinical challenge requiring effective treatments to achieve
long-term disease control, there has been no consensus on the optimal
therapy that should be offered to these patients. This review will
briefly discuss the clinical results and the main issues regarding
autologous SCT as well as the current role of allogeneic SCT. |
Introduction
Classical Hodgkin lymphoma (cHL) is a relatively rare disease, with
approximately 9,200 estimated new cases and 1,200 estimated deaths per
year in the United States.[1] First-line chemo-radiotherapy yields cure rates approaching 80% in patients with advanced-stage disease.[2,3] However, 25 to 30% of these patients are not cured with modern chemo-radiotherapy and show either primary refractoriness to chemotherapy, as defined by disease progression during or within 3 months of doxorubicin-based chemotherapy, early disease relapse (i.e., within 12 months after the end of first-line treatment) or late disease relapse.[4]
Second-line salvage high-dose chemotherapy (HDC) and autologous stem
cell transplantation (SCT) have become the standard of care for
refractory/relapsed cHL, leading to durable responses in approximately
50% of relapsed patients and a minority of refractory patients.[5-12]
However, due to the poor responses to second-line salvage chemotherapy
and dismal long-term disease control of primary refractory and
early-relapsed patients, their treatment represents an unmet medical
need. Despite a general consensus that these patients represent a
clinical challenge requiring effective treatments, there remains no
consensus on the optimal therapy to be offered to early relapsed and
primary refractory patients.[13,14] Disease
recurrence or progression after autologous SCT is associated with a
very poor prognosis and the median survival time from transplantation
failure ranges from 12 to 29 months in different series.[15-18] Various therapeutic options are currently available for relapsed/refractory cHL patients who fail autologous SCT.[19] Among these, brentuximab vedotin (BV), nivolumab and bendamustine have demonstrated extraordinary efficacy.[20-24]
However, both drugs are limited in terms of long-term disease control,
and by far, allogeneic SCT represents the only strategy with a curative
potential for multirelapsed and refractory patients.[25-27]
Nevertheless, among patients who receive allogeneic SCT, long-term
progression-free survival (PFS) does not exceed 25% to 35% in most
series, and disease relapse is associated with an exceedingly poor
outcome, with less than half of patients surviving for 3 years.[25,26,28-31]
This review will briefly discuss the clinical results and the main
issues regarding autologous SCT and the current role of allogeneic
SCT.
Autologous SCT
According to retrospective and prospective, as well as randomized
studies, HDC followed by autologous SCT can rescue 30 to 80% of
relapsed/refractory cHL patients. On average, 50% of patients who
receive autologous SCT relapse or progress within 12 months after
transplant. Randomized studies (Table 1)
have failed to report significantly improved overall survival (OS),
likely due to the “cross-over” to autologous SCT of patients failing
conventional therapy.[5,8,32]
The treatment-related mortality (TRM) in 3 randomized studies was
similar between HDC and conventional chemotherapy, likely due to the
relatively high toxicity of chemotherapy used in the conventional arm.[33] Although initial studies reported an average TRM of 10% (range, 3 - 17%), randomized studies (Table 1)
reported a lower TRM (3 - 4%), likely due to better supportive care,
the use of peripheral blood stem cells (PBSC) instead of bone marrow
(BM), and earlier referral of patients to autografting. Long-term
toxicity, including heart, lung and endocrine toxicities, as well as
infections, infertility, and secondary malignancies should also be
considered during counseling. A consensus study from several
cooperative groups suggested that as early as 6 months after the start
of HDC, patients should receive a specific follow-up for the early
detection of complications.[34] An analysis involving
more than 800 patients autografted for hematological malignancies who
survived more than 2 years after transplant showed that their risk of
late death was 13-fold higher than in the general population,
particularly in the first 2-5 years after HDC. For cHL patients, the
standardized mortality ratio (SMR) was 28, meaning that these patients
had a 28-fold increased risk of dying compared with the general
population. Furthermore, the most frequent specific causes of death
were secondary cancers and lung disease (SMR 30 and 29, respectively).[34]
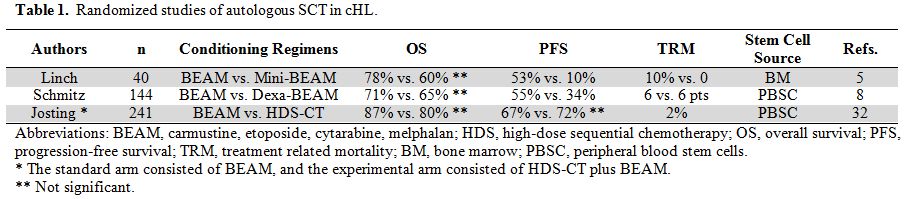 |
Table 1. Randomized studies of autologous SCT in cHL. |
Prognostic Factors and Risk-Adapted Strategies.
Factors shown to influence the outcome of relapsed/refractory patients
have led to the generation of prognostic scores for the risk
stratification of patients undergoing HDC and autologous SCT
(summarized in Table 2). The
most popular scoring system is the German Score (GS), which
incorporates 3 variables, including anemia, stage III-IV, and time to
relapse less than 12 months.[35,36] The GS was
validated by the randomized HDR2 study, which showed a 3-year PFS of
81%, 70%, 50%, and 14% in patients with adverse factors ranging from 1
to 4, respectively.[32] Majhail et al.[10]
analyzed 141 patients and identified the 3 following variables as being
predictive of outcome: chemoresistance, B symptoms at relapse, and
persistence of disease at transplant. According to this score, the
figures for 5-year PFS were 67%, 37% and 9% for patients with 0-1, 2,
and 3 factors, respectively. Similarly, the 5-year OS was significantly
different among the 3 groups, with respective values of 71%, 49%, and
13%.[10]
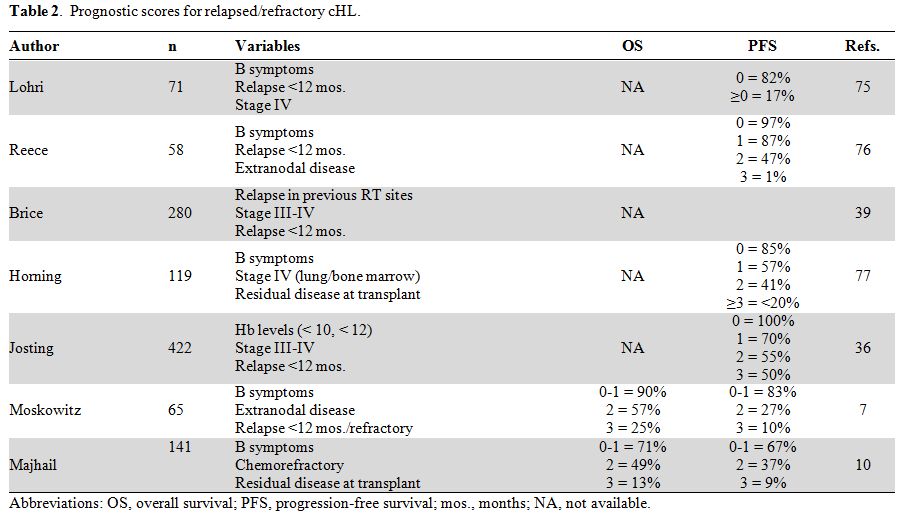 |
Table 2. Prognostic scores for relapsed/refractory cHL. |
Prognostic scores have also been used prospectively to
evaluate the clinical impact of risk-adapted therapeutic programs.
Moskowitz et al.[37] used standard-dose ICE for
low-risk patients, intensified-ICE for intermediate-risk patients, and
ICE plus autologous SCT for high-risk patients and showed that
risk-adapted augmentation of salvage treatment improved event-free
survival in higher risk patients.
Morschhauser et al.[38] subsequently tested the prognostic score proposed by Brice et al.[39]
This score included advanced stage disease, duration of first response
shorter than 12 months, disease relapse in irradiated fields, and
refractoriness to first-line chemotherapy. Intermediate-risk patients
received conventional salvage chemotherapy followed by BEAM, whereas
high-risk patients (chemorefractory or bearing more than 2 risk
factors) were treated with intensified salvage chemotherapy and double
autologous SCT (CBV-Mx or BEAM and TAM or BAM).[38]
The 5-year freedom from second failure (FF2F) and OS rates were 46% and
57% in the high-risk group and 73% and 85% in the intermediate-risk
group. The overall efficacy of salvage chemotherapy was not optimal, as
the objective response rate (ORR) was 63%, and this value was even
lower among high-risk patients (ORR 54%, CR/Cru 23%).[38]
Although the results obtained with tandem autologous SCT in the poor
prognosis group were better than those reported in other trials (Table 3),
they are still unsatisfactory, further supporting the requirement for
new therapeutic strategies. A study from the Royal Marsden involving
patients with relapsed or refractory disease and a 10-year follow-up
reported PFS and OS figures of 49% and 37%, respectively.
Chemosensitive disease and a Hasenclever index <3 at SCT were the
two prognostic factors for OS and PFS.[40]
Primary Refractory cHL.
Chemorefractoriness to first-line therapy represents the strongest
factor predicting a poor outcome after autologous SCT. These patients
were not included in randomized trials, and autografting resulted in
30% to 40% durable PFS, once again supporting the general concept of
poorer outcome in chemorefractory patients compared with chemosensitive
patients (Table 3). In a study
from the German group, 206 primary progressive patients were analyzed
and 153 received salvage chemotherapy, of which only 70 (34%) were
autografted, whereas 47 received salvage radiotherapy.[36]
The 5-year FF2F and OS for all patients were 17% and 26%, respectively;
the same figures for patients treated with HDC were 31% and 43%,
respectively. The identification of three prognostic factors, including
an age >50 years, failure to obtain temporary remission after
first-line chemotherapy, and poor performance status, enabled the
design of a prognostic score. Combining these factors, the 5-year OS
ranged from 56% (absence of adverse factors) to 0% (presence of all 3
factors).[36] Uncontrolled disease prior to
autologous SCT, either stable or progressive, was included for a small
group of very high-risk patients and generated an OS ranging from 11%
to 37% (Table 4). Furthermore,
in most of the studies dealing with mixed cohorts of patients with
relapsed or refractory disease, the absence of chemosensitivity before
autografting negatively influenced the outcome. Therefore, biomarkers
enabling the early identification of chemorefractory patients (such as
CD68 expression on macrophages,[41] PD-1/PD-L1 expression on Hodgkin Reed-Sternberg cells or microenvironment cells,[42]
etc.), novel agents specifically targeting tumor cells along with the
tumor microenvironment at the genetic or epigenetic level, as well as
innovative therapeutic strategies are urgently needed for
chemorefractory patients.
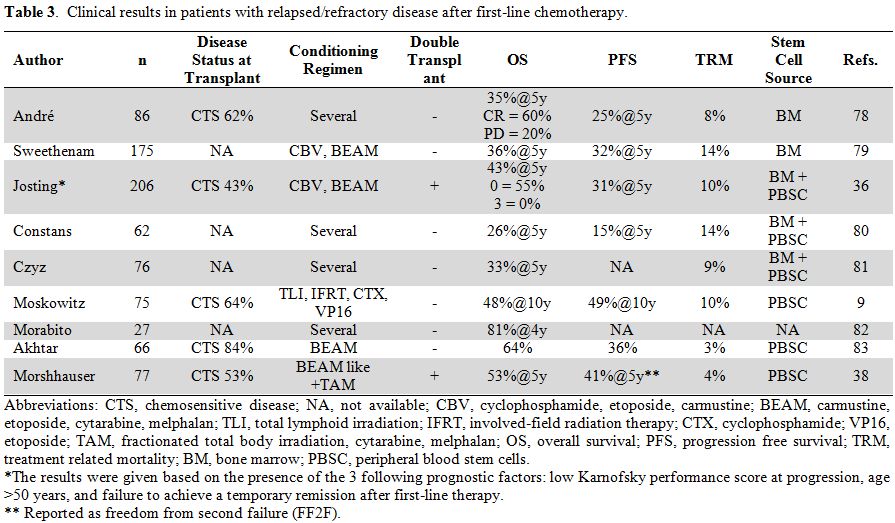 |
Table 3. Clinical results in patients with relapsed/refractory disease after first-line chemotherapy. |
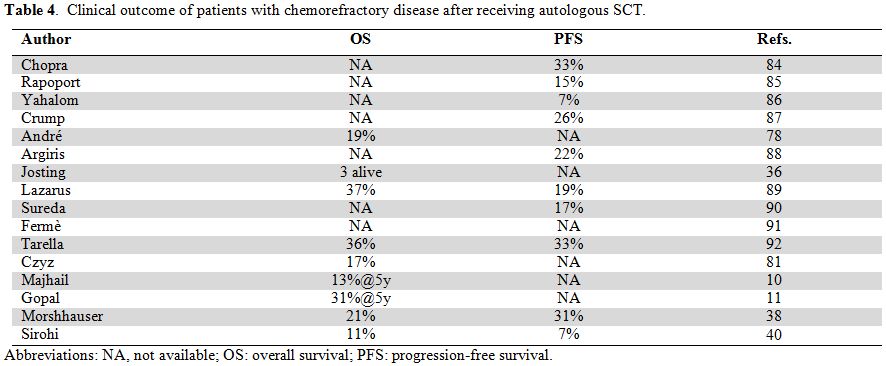 |
Table 4. Clinical outcome of patients with chemorefractory disease after receiving autologous SCT. |
Conditioning Regimens.
The potential benefit of a conditioning regimen has not been adequately
explored in the autologous setting. Two randomized studies applied the
BEAM conditioning regimen,[5,8] which
was introduced several years ago but not previously tested in
randomized trials. Nevertheless, this regimen is considered the gold
standard for autologous transplantation. When salvage chemotherapy
followed by BEAM was compared with a more intensive high-dose
sequential therapy (HDS-CT), the outcomes were not different, although
the toxicities were higher in the HDS-CT arm.[32]
Evidence emerging from several recent studies also supports the concept
that alternative conditioning regimens are not more effective and/or
less toxic than BEAM. In the event that a randomized study comparing
BEAM with newer regimens is not performed, the BEAM regimen may be
considered the gold standard. However, due to drug constraints on
carmustine, this drug is often replaced by a variety of agents,
including fotemustine,[43] bendamustine,[44] and thiotepa.[45]
Role of PET Imaging.
The extensive use of 18F-fluorodeoxyglucose positron emission
tomography (FDG-PET) over the past 10 years has resulted in significant
changes in the outcomes of relapsed/refractory patients, as some
patients classified as PR or SD, or rarely PD after salvage
chemotherapy, may in fact be in metabolic CR. The bottom line is that
FDG-PET segregates patients into 2 groups: positive and negative. The
available data show that a positive FDG-PET before autografting
identifies patients with poorer outcome than those with negative
FDG-PET.[37] However, the outcome of the FDG-PET
positive group (OS 40-58%, PFS 23-40%) is often unsatisfactory, and
newer approaches should be tested for their ability to obtain FDG-PET
negativity. However, the early application of allogeneic SCT in FDG-PET
positive patients was reported by the English group, with encouraging
results (3-year PFS 68% and OS 88%).[46]
Interestingly, the use of FDG-PET overcame the impact of prognostic
factors (B symptoms, early relapse/refractoriness), with the exception
of extra-nodal localization.[47] Castagna et al. also showed that in the context of salvage therapy, interim FDG-PET could predict PFS.[48]
Prospective studies are currently ongoing, in which the treatment
strategy is changed based on the FDG-PET results, after first-line or
second-line chemotherapy. Devillier et al.[49]
recently published a retrospective study on 111 patients, confirming
the predictive value of the response by FDG-PET at autografting (5-year
PFS and OS, 79% vs. 23% and 90% vs. 55% in FDG-PET negative and
positive patients, respectively). Furthermore, in FDG-PET positive
patients, the outcome was better if they received a double transplant.[49]
Therefore, defining the therapeutic response with FDG-PET represents
the most relevant improvement in the treatment of advanced cHL,
challenging most of the data generated in recent years.[47]
The
prognosis of patients who fail autologous SCT is poor.15 A joint EBMT
and GITMO retrospective analysis on 462 patients who relapsed or
progressed after autologous SCT showed a median time from SCT to
relapse of 7 months (range, 1 - 78) and a 5-year OS for the entire
cohort of 32%.[16] In multivariate analysis, early
relapse, stage IV, bulky disease, poor performance status, and age ≥50
years were significantly associated with survival, and 3 groups (0, 1,
≥2 factors) showed different OS rates (62%, 37%, and 12%,
respectively).[16] Thus, patients with refractory
disease and patients failing autologous SCT represent an unmet medical
need requiring innovative treatment.[50]
Allogeneic SCT
Clinical results from retrospective trials of allogeneic SCT
reported in the early nineties were disappointing, likely due to the
inclusion of heavily pretreated patients, who had received extended
radiotherapy and were allografted in the presence of active disease
after myeloablative conditioning with bone marrow stem cells (reviewed in Sureda et al.[51]).
Allogeneic SCT has been associated with a high TRM due to the high
incidence of graft versus host disease (GVHD) and fatal infections
post-transplantation. The poor outcome of cHL patients after allogeneic
SCT may reflect, in part, the advanced status of the disease at
transplantation and the poor performance status of the patient
population that was allografted. Furthermore, the high TRM present in
the conventional allogeneic SCT setting has never allowed proper
evaluation of a possible graft-versus-Hodgkin's effect. In the late
nineties, this scenario changed substantially with the introduction of
reduced intensity conditioning (RIC) and non-myeloablative conditioning
(NMAC) regimens (Table 5). As a
matter of fact, a clinically significant reduction of TRM below 30% was
reported by several investigators and resulted in a renewed interest in
allogeneic SCT. On average, PFS ranged from 20% to 42% and OS from 25%
to 57%. Such a wide variability is mainly due to the heterogeneity of
patients included in these retrospective trials. Despite representing
an increasingly used procedure, allogeneic SCT remains a matter of
discussion, and several controversial issues are currently under
investigation.
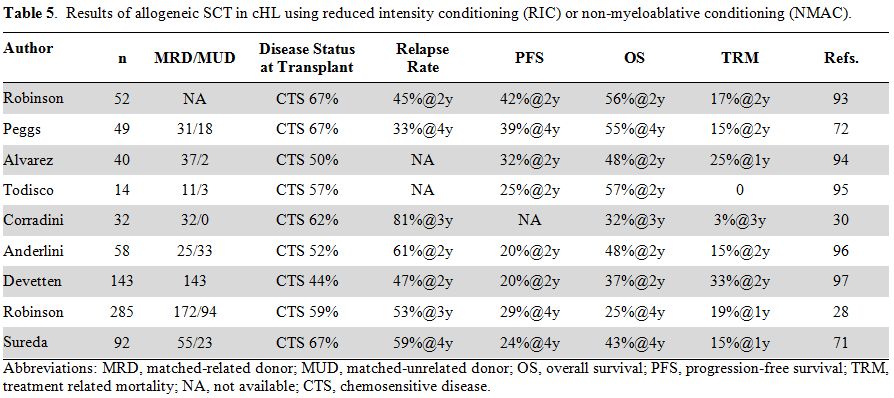 |
Table 5. Results of allogeneic SCT in cHL using reduced intensity conditioning (RIC) or non-myeloablative conditioning (NMAC). |
One general question that needs to be addressed is how
allogeneic SCT compares with other therapies. In the absence of
randomized trials, figures extrapolated from retrospective studies have
to be considered with caution. An EBMT/GITMO study retrospectively
analyzed the risk factors predicting the outcome of cHL patients
relapsing after autologous SCT.[16] A total of 462
patients were treated with either conventional chemotherapy eventually
supplemented by radiotherapy (64%), a second autologous SCT (9%) or
allogeneic SCT (29%). At a median follow-up of 49 months, 2-year and
5-year OS rates were 55% and 32%. In multivariate analysis, allogeneic
SCT was associated with a trend towards improved survival (P = 0.08).[16]
In fact, the OS at 5 years was 48% for patients receiving allogeneic
SCT (RIC) and 32% for those treated with conventional
chemotherapy/radiotherapy, with a median survival time of 45 and 19
months, respectively. Independent risk factors predicting a poor OS
were early relapse within the first 6 months after HDC, stage IV
disease, bulky disease, presence of B symptoms, a Karnofsky performance
status under 80% and age of 50 years or older. Patients presenting with
none of these risk factors had a 5-year OS rate of 62%, whereas among
patients presenting with one risk factor, the 5-year OS rate was 37%.
In contrast, patients with two or more risk factors had a poor clinical
outcome, with a 5-year OS rate of only 12%.
Novel Agents and Allogeneic SCT.
Several retrospective studies have suggested that allogeneic SCT should
be considered a therapeutic option in patients relapsing or progressing
after autografting.[25,46,52] The current availability of active, although non-curative drugs, such as BV,[20,21] nivolumab,[22] bendamustine,[23,24,53] histone deacetylase inhibitors,[54,55] mTOR inhibitors,[56] kinase inhibitors,[57,58] and immunomodulatory drugs,[59]
has allowed substantially high rates of objective responses in patients
who previously failed autologous SCT, thus resulting in significant
improvements of the quality and quantity of clinical responses achieved
by patients who became eligible for allogeneic SCT after having failed
autografting. Recently, Chen et al.[60] compared a
small cohort of patients (n = 21) receiving BV before allogeneic SCT
with historical controls (n= 23). The BV cohort showed better 2-year
PFS (59% vs. 26%) and OS (71% vs. 56%), with a lower relapse rate (24%
vs. 57%) and 1-year NRM of 9.5% vs. 17%. Interestingly, these
treatments shared a good toxicity profile, thus allowing patients to
achieve a good performance status at the time of allografting.
Allogeneic
SCT could also be a viable option for patients who are refractory to
salvage chemotherapy, especially because better results are obtained
when this treatment is applied earlier.[61] Indeed, the survival of these patients is poor, and most of them die from disease progression.[62]
The availability of novel agents resulting in objective responses may
eventually result in increased eligibility for allogeneic SCT (Figures 1, 2).
|
|
Figure 1. Treatment algorithm for relapsed/refractory cHL |
|
|
Figure 1. Treatment algorithm for cHL relapsing or progressing following Auto-SCT |
Recently, the UK group reported interesting results in
patients who were FDG-PET positive after salvage chemotherapy and
treated with allogeneic SCT. For most of these patients, the
conditioning regimen consisted of BEAM plus Campath, and the results
were encouraging because the 3-year NRM, PFS, and OS rates were 24%,
68%, and 80%, respectively.[46] In general, for
patients refractory to salvage CT, allogeneic SCT should be considered,
provided that good disease control is achieved prior to
transplantation.[63]
Conditioning Regimens.
The type of conditioning regimen to be used prior to allogeneic SCT
represents another matter of discussion. There is a consensus that RIC
should be preferred to MAC regimens. Indeed, in a retrospective
registry-based study, Sureda et al. reported that patients receiving
MAC had lower OS rates than those treated with RIC.[26] However, it
should be noted that after MAC, even though NRM was higher, the relapse
rate was lower, meaning that new and less toxic myeloablative regimens
should be prospectively evaluated.
Prognostic Factors.
Several prognostic factors associated with different outcomes after
allogeneic SCT have been reported. In a large retrospective study from
EBMT, Robinson et al.[28] reported that prognostic factors may help to define different patient populations with significantly different outcomes (Table 6);
the most important and recurrent factor was the disease status before
allogeneic SCT, as patients not achieving CR at the time of
transplantation experienced shorter survival, increased toxicity and
relapse. Furthermore, in patients allografted after autologous SCT, the
interval between relapse and autografting (cut-off 6 months) was a
protective prognostic factor. In contrast with other studies, which
demonstrated a reduction of relapse in patients experiencing chronic
GVHD (cGVHD),[26,63] the EBMT study failed to show a link between the development of cGVHD and survival.[28]
 |
Table 6. Prognostic factors at allogeneic SCT (adapted from Robinson et al.[28]). |
Donor Source.
The vast majority of allografting in cHL stemmed from studies using
either an HLA-identical sibling or a matched unrelated donor (MUD).
With a median NRM of 10% (range, 3-25%), the use of HLA-identical
siblings is considered a standard option due to its good toxicity
profile. Because only 25-30% of patients have an HLA-identical sibling,
searching for a MUD is mandatory, despite the consistent increase in
median NRM to 28% (range 16-34). In recent years, great interest has
been focused on haploidentical family donors (HLA-haplo). Encouraging
results have been obtained using the Baltimore approach, combining NMAC
regimens, T cell-replete BM and post-transplant cyclophosphamide (Cy).[64] This scheme is well tolerated and has shown a remarkably low NRM, with good OS in a variety of hematological malignancies.[65,66]
Two retrospective studies have reported the activity of transplantation
from haploidentical family donors. Burroughs et al. compared the
results obtained in patients receiving transplantation from a matched
related donor (MRD), MUD, or haploidentical family donor.[67]
The PFS, NRM, and relapse rates were significantly lower after
haploidentical transplantation than transplantation using other stem
cell sources. Furthermore, the incidence of acute and chronic GVHD was
equally lower in the haploidentical group.[67] More recently, Raiola et al. reported [26]
cHL patients grafted from haploidentical family donors with rates of
PFS, OS, relapse, and NRM of 63%, 77%, 31%, and 4%, respectively.[65] Additionally, this study confirmed the low incidence of both acute GVHD (grade 2-4, 24%) and cGVHD (9%).[65]
Altough preliminary and based on a limited number of patients, the
extraordinary efficacy of this strategy of haploidentical transplant
suggests a peculiar role of the conditioning regimen in eliciting an
HL-specific immune activity.
Management of Disease Relapse after Allogeneic SCT.
Notwithstanding the reduction of NRM and GVHD, disease relapse
following allogeneic SCT ranges from 31% to 81% in different series and
still represents a major issue that needs to be addressed. In
particular, the survival of relapsing patients is dismal. Ram et al.
analyzed the outcome of 26 cHL patients and reported that the 3-year OS
was 47%, with a median time from allografting to relapse of 6 months
(range, 0.5-29 months). Different therapies were administered,
including withdrawal of immunosuppressive therapy, standard
chemotherapy eventually combined with radiotherapy, donor lymphocyte
infusion (DLI), or a second allogeneic transplantation. This translated
to an ORR of 78%, which was, however, associated with a high risk of
further progression.[31] A second retrospective study
in 28 cHL patients reported a survival rate of 49% and identified late
relapse (cut off 100 days), achievement of CR/PR, and localized nodal
or extra-nodal relapse as significant predictive factors.[68]
We reported a series of 97 HL patients receiving allogeneic SCT at
either Humanitas Cancer Center (Rozzano, Italy) or Institut Paoli
Calmettes (Marseille, France). Thirty-three (34%) patients relapsed
after a median time from allografting of 4.5 months (range, 0.3-17
months). In this series, the median follow-up time was 46 months
(range, 1-160 months), and the 2-y PFS and OS were 17% and 33%. We also
confirmed that patients with late relapse showed a better prognosis
(Castagna L. et al., manuscript in preparation).
Survival
data from the EBMT/GITMO study, as well as other series, strongly
suggest that allogeneic SCT is feasible and appears to be active in at
least one third of multi-relapsed patients. However, this treatment
modality cannot be considered a standard procedure and should be
offered to carefully selected chemosensitive patients included in
clinical studies. However, the availability of new active drugs to be
used alone or in combination, and eventually associated with DLI, could
substantially change this scenario.
The implementation of novel
agents, such as BV, nivolumab, and bendamustine, for the treatment of
multi-relapsed cHL patients has improved the outcome of these patients
and will significantly impact the history of multi-relapsed cHL in the
near future when the results of combination studies become available.
Two studies have reported similar efficacy data of BV used as single
agent in patients with recurrent disease after allogeneic SCT.[69,70]
The largest study of BV after allografting failure involved 24 patients
who received a median of 8 cycles (range, 1-16) of BV at a median of 42
months (range, 6-116) after allografting. After a median follow-up time
of 34 weeks, these patients showed ORR and CR rates of 50% and 38%,
respectively, with a median PFS of 7.8 months, whereas the median OS
was not reached.[69] The toxicity profile was good, without any impact on GVHD or CMV reactivation.[69] The largest cohort study of bendamustine in cHL patients with recurrent disease after allogeneic SCT was recently reported.[23]
In a multicenter retrospective study, 45 and 22 patients received
bendamustine for disease recurrence after autologous and allogeneic
SCT, respectively; most of these patients received 90 mg/m2
x 2 days (73%). The CR+PR rates for patients treated with bendamustine
due to recurrence after autologous or allogeneic SCT were 56% and 59%,
respectively, whereas the same figures for patients achieving SD+PD
were 44% and 41%, respectively. After a median follow-up time of 13
months, the PFS was 49%, and OS was 70% at 1 year. The median PFS was
10 months, whereas the median OS was not established. Toxicities were
manageable, with grade 3-4 hematological toxicity being evident in less
than 20% of patients. The most common extra-hematological toxicities
were fever and febrile neutropenia.[23]
DLI has
been used frequently, resulting in an average ORR ranging from 40% to
80%. However, in most cases, the duration of the response was short and
almost all patients relapsed.[71] Of special interest
are the data from the English group, showing that disease relapse was
extremely rare in patients receiving DLI when in CR after allogeneic
SCT and with mixed chimerism. Overall, the 4-year OS was 59%. This
result may confirm the immunological effect of donor lymphocytes in the
situation of minimal residual disease.[72] DLI has
also been combined with other drugs. In a proof-of-principle study,
Teurich et al. treated 4 patients with the combination of BV plus DLI
and demonstrated an immunological effect on HL cell lines mediated by
heterogeneous CD161-positive lymphocytes.[73] In
addition, all patients showed a metabolic response. In a multicenter
retrospective study, Sala et al. assessed 18 patients receiving
bendamustine, 9 of them in association with DLI, and the 1-year OS and
PFS rates were 59% and 30%, respectively.[74]
Conclusions
Autologous SCT have become the standard of care for
refractory/relapsed cHL, leading to durable responses in approximately
50% of relapsed patients and a minority of refractory patients (Figure 1).
Furthermore, the current availability of active, yet non-curative,
drugs has significantly improved the management of autografting
failures, allowing for substantially increased rates of objective
responses. In particular, these treatments have resulted in significant
quantitative and qualitative improvements in the clinical responses of
patients who have subsequently become eligible for allogeneic SCT after
having failed autografting (Figure 2).
Patients achieving PET-negativity after a second salvage regimen may do
well with autologous SCT even though they were PET-positive after the
first salvage regimen.[47] However, retrospective
data in the setting of haploidentical SCT report a low TRM and suggest
the existence of clinically relevant, graft-induced immune effects,
thus suggesting that allogeneic SCT can be offered to chemorefractory
cHL patients, as well as to those patients who fail autologous SCT and
achieve CR or PR using novel agents.[61] Despite the
reduction of NRM and GVHD, disease relapse still represents the major
issue in the setting of allogeneic SCT failure. Novel biomarkers for
the early identification of relapsing and refractory patients, as well
as novel agents specifically targeting genetic or epigenetic changes in
both tumor cells and the tumor microenvironment, are needed for
refractory patients. Together, the integration of novel prognostic
biomarkers, novel agents and allogeneic SCT will significantly impact
the history of multi-relapsed and refractory patients, overcoming the
issues of chemorefractoriness as well as disease relapse. Finally, the
long-term toxicities of such treatments should be carefully evaluated,
and specific follow-up, which ideally would be given in specialized
clinics, should become part of global care.
Acknowledgments
This work was supported in part by funding from the Ministry of
Health (RF #2010-2313979 to C.C.-S.) and the Italian Association for
Cancer Research (AIRC, grant #15835 to C.C.-S.).
References
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29.http://dx.doi.org/10.3322/caac.21208 PMid:24399786

- Santoro
A, Bonadonna G, Valagussa P, Zucali R, Viviani S, Villani F, Pagnoni
AM, Bonfante V, Musumeci R, Crippa F, et al. Long-term results of
combined chemotherapy-radiotherapy approach in Hodgkin's disease:
superiority of ABVD plus radiotherapy versus MOPP plus radiotherapy. J
Clin Oncol. 1987;5:27-37. PMid:2433409

- Engert
A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, Berger B,
Greil R, Willborn KC, Wilhelm M, Debus J, Eble MJ, Sokler M, Ho A, Rank
A, Ganser A, Trumper L, Bokemeyer C, Kirchner H, Schubert J, Kral
Z, Fuchs M, Muller-Hermelink HK, Muller RP, Diehl V. Reduced treatment
intensity in patients with early-stage Hodgkin's lymphoma. N Engl J
Med. 2010;363:640-652. http://dx.doi.org/10.1056/NEJMoa1000067 PMid:20818855

- Canellos
GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. Treatment of
Hodgkin lymphoma: a 50-year perspective. J Clin Oncol. 2014;32:163-168.
http://dx.doi.org/10.1200/JCO.2013.53.1194 PMid:24441526

- Linch
DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, Chopra R,
Milligan D, Hudson GV. Dose intensification with autologous bone-marrow
transplantation in relapsed and resistant Hodgkin's disease: results of
a BNLI randomised trial. Lancet. 1993;341:1051-1054. http://dx.doi.org/10.1016/0140-6736(93)92411-L

- Brice
P, Divine M, Simon D, Coiffier B, Leblond V, Simon M, Voilat L, Devidas
A, Morschhauser F, Rohrlich P, Andre M, Lepage E, Ferme C. Feasibility
of tandem autologous stem-cell transplantation (ASCT) in induction
failure or very unfavorable (UF) relapse from Hodgkin's disease (HD).
SFGM/GELA Study Group. Ann Oncol. 1999;10:1485-1488. http://dx.doi.org/10.1023/A:1008343823292 PMid:10643540

- Moskowitz
CH, Nimer SD, Zelenetz AD, Trippett T, Hedrick EE, Filippa DA, Louie D,
Gonzales M, Walits J, Coady-Lyons N, Qin J, Frank R, Bertino JR, Goy A,
Noy A, O'Brien JP, Straus D, Portlock CS, Yahalom J. A 2-step
comprehensive high-dose chemoradiotherapy second-line program for
relapsed and refractory Hodgkin disease: analysis by intent to treat
and development of a prognostic model. Blood. 2001;97:616-623. http://dx.doi.org/10.1182/blood.V97.3.616 PMid:11157476

- Schmitz
N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, Boissevain F,
Zschaber R, Muller P, Kirchner H, Lohri A, Decker S, Koch B,
Hasenclever D, Goldstone AH, Diehl V. Aggressive conventional
chemotherapy compared with high-dose chemotherapy with autologous
haemopoietic stem-cell transplantation for relapsed chemosensitive
Hodgkin's disease: a randomised trial. Lancet. 2002;359:2065-2071. http://dx.doi.org/10.1016/S0140-6736(02)08938-9

- Moskowitz
CH, Kewalramani T, Nimer SD, Gonzalez M, Zelenetz AD, Yahalom J.
Effectiveness of high dose chemoradiotherapy and autologous stem cell
transplantation for patients with biopsy-proven primary refractory
Hodgkin's disease. Br J Haematol. 2004;124:645-652. http://dx.doi.org/10.1111/j.1365-2141.2003.04828 PMid:14871252

- Majhail
NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, Arora M,
Ramsay NKC, Orchard PJ, MacMillan ML, Burns LJ. Long-term results of
autologous stem cell transplantation for primary refractory or relapsed
Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006;12:1065-1072. http://dx.doi.org/10.1016/j.bbmt.2006.06.006 PMid:17084370

- Gopal
AK, Metcalfe TL, Gooley TA, Pagel JM, Petersdorf SH, Bensinger WI,
Holmberg L, Maloney DG, Press OW. High-dose therapy and autologous stem
cell transplantation for chemoresistant Hodgkin lymphoma: the Seattle
experience. Cancer. 2008;113:1344-1350. http://dx.doi.org/10.1002/cncr.23715 PMid:18623377 PMCid:PMC2700660

- Viviani
S, Di Nicola M, Bonfante V, Di Stasi A, Carlo-Stella C, Matteucci P,
Magni M, Devizzi L, Valagussa P, Gianni AM. Long-term results of
high-dose chemotherapy with autologous bone marrow or peripheral stem
cell transplant as first salvage treatment for relapsed or refractory
Hodgkin lymphoma: a single institution experience. Leukemia &
Lymphoma. 2010;51:1251-1259. http://dx.doi.org/10.3109/10428194.2010.486090 PMid:20528244

- Younes
A. Novel treatment strategies for patients with relapsed classical
Hodgkin lymphoma. Hematology Am Soc Hematol Educ Program. 2009:507-519.
http://dx.doi.org/10.1182/asheducation-2009.1.507 PMid:20008236

- Canellos
GP. Brentuximab vedotin and panobinostat: new drugs for Hodgkin's
lymphoma--can they make one of medical oncology's chemotherapy success
stories more successful? J Clin Oncol. 2012;30:2171-2172. http://dx.doi.org/10.1200/JCO.2011.39.6416 PMid:22547611

- Moskowitz
AJ, Perales M-A, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z,
Vanak J, Zelenetz AD, Moskowitz CH. Outcomes for patients who fail high
dose chemoradiotherapy and autologous stem cell rescue for relapsed and
primary refractory Hodgkin lymphoma. Br J Haematol.
2009;146:158-163. http://dx.doi.org/10.1111/j.1365-2141.2009.07727.x PMid:19438504 PMCid:PMC3278667

- Martinez
C, Canals C, Sarina B, Alessandrino EP, Karakasis D, Pulsoni A, Sica S,
Trneny M, Snowden JA, Kanfer E, Milpied N, Bosi A, Guidi S, de Souza
CA, Willemze R, Arranz R, Jebavy L, Hellmann A, Sibon D, Oneto R, Luan
JJ, Dreger P, Castagna L, Sureda A, for the Lymphoma Working Party of
the European Group for B, Marrow T, the Gruppo Italiano Trapianto di
Midollo O. Identification of prognostic factors predicting outcome in
Hodgkin's lymphoma patients relapsing after autologous stem cell
transplantation. Ann Oncol. 2013. http://dx.doi.org/10.1093/annonc/mdt206

- Arai
S, Fanale M, DeVos S, Engert A, Illidge T, Borchmann P, Younes A,
Morschhauser F, McMillan A, Horning SJ. Defining a Hodgkin lymphoma
population for novel therapeutics after relapse from autologous
hematopoietic cell transplant. Leukemia & Lymphoma.
2013;54:2531-2533. http://dx.doi.org/10.3109/10428194.2013.798868 PMid:23617324

- Crump
M. Management of Hodgkin lymphoma in relapse after autologous stem cell
transplant. Hematology Am Soc Hematol Educ Program. 2008:326-333.
http://dx.doi.org/10.1182/asheducation-2008.1.326 PMid:19074105 
- Younes
A. Beyond chemotherapy: new agents for targeted treatment of lymphoma.
Nat Rev Clin Oncol. 2011;8:85-96.
http://dx.doi.org/10.1038/nrclinonc.2011.20 PMid:21151205
PMCid:PMC3192435
- Younes A, Bartlett NL,
Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A.
Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N
Engl J Med. 2010;363:1812-1821. http://dx.doi.org/10.1056/NEJMoa1002965 PMid:21047225

- Younes
A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren
R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH,
Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R.
Results of a pivotal phase II study of brentuximab vedotin for patients
with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol.
2012;30:2183-2189. http://dx.doi.org/10.1200/JCO.2011.38.1350 PMid:22454421 PMCid:PMC3646316

- Ansell
SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster
SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH,
Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1
Blockade with Nivolumab in Relapsed or Refractory Hodgkin's Lymphoma. N
Engl J Med. 2014;in press.

- Anastasia
A, Carlo-Stella C, Corradini P, Salvi F, Rusconi C, Pulsoni A, Hohaus
S, Pregno P, Viviani S, Brusamolino E, Luminari S, Giordano L, Santoro
A. Bendamustine for Hodgkin lymphoma patients failing autologous or
autologous and allogeneic stem cell transplantation: a retrospective
study of the Fondazione Italiana Linfomi. Br J Haematol.
2014;166:140-142. http://dx.doi.org/10.1111/bjh.12821 PMid:24606548

- Moskowitz
AJ, Hamlin PA, Jr., Perales MA, Gerecitano J, Horwitz SM, Matasar MJ,
Noy A, Palomba ML, Portlock CS, Straus DJ, Graustein T, Zelenetz AD,
Moskowitz CH. Phase II study of bendamustine in relapsed and refractory
Hodgkin lymphoma. J Clin Oncol. 2013;31:456-460. http://dx.doi.org/10.1200/JCO.2012.45.3308 PMid:23248254 PMCid:PMC3862960

- Sarina
B, Castagna L, Farina L, Patriarca F, Benedetti F, Carella AM, Falda M,
Guidi S, Ciceri F, Bonini A, Ferrari S, Malagola M, Morello E, Milone
G, Bruno B, Mordini N, Viviani S, Levis A, Giordano L, Santoro A,
Corradini P. Allogeneic transplantation improves the overall and
progression-free survival of Hodgkin lymphoma patients relapsing after
autologous transplantation: a retrospective study based on the time of
HLA typing and donor availability. Blood. 2010;115:3671-3677. http://dx.doi.org/10.1182/blood-2009-12-253856 PMid:20220116

- Sureda
A, Robinson S, Canals C, Carella AM, Boogaerts MA, Caballero D, Hunter
AE, Kanz L, Slavin S, Cornelissen JJ, Gramatzki M, Niederwieser D,
Russell NH, Schmitz N. Reduced-intensity conditioning compared with
conventional allogeneic stem-cell transplantation in relapsed or
refractory Hodgkin's lymphoma: an analysis from the Lymphoma Working
Party of the European Group for Blood and Marrow Transplantation. J
Clin Oncol. 2008;26:455-462. http://dx.doi.org/10.1200/JCO.2007.13.2415 PMid:18086796

- Corradini P, Sarina B, Farina L. Allogeneic transplantation for Hodgkin's lymphoma. Br J Haematol. 2011;152:261-272. http://dx.doi.org/10.1111/j.1365-2141.2010.08492.x PMid:21155760

- Robinson
SP, Sureda A, Canals C, Russell N, Caballero D, Bacigalupo A, Iriondo
A, Cook G, Pettitt A, Socie G, Bonifazi F, Bosi A, Michallet M,
Liakopoulou E, Maertens J, Passweg J, Clarke F, Martino R, Schmitz N,
EBMT LWPot. Reduced intensity conditioning allogeneic stem cell
transplantation for Hodgkin's lymphoma: identification of prognostic
factors predicting outcome. Haematologica. 2009;94:230-238.
http://dx.doi.org/10.3324/haematol.13441 PMid:19066328 PMCid:PMC2635413 
- Armand
P, Kim HT, Ho VT, Cutler CS, Koreth J, Antin JH, LaCasce AS, Jacobsen
ED, Fisher DC, Brown JR, Canellos GP, Freedman AS, Soiffer RJ, Alyea
EP. Allogeneic transplantation with reduced-intensity conditioning for
Hodgkin and non-Hodgkin lymphoma: importance of histology for outcome.
Biol Blood Marrow Transplant. 2008;14:418-425. http://dx.doi.org/10.1016/j.bbmt.2008.01.008 PMid:18342784 PMCid:PMC2364453

- Corradini
P, Dodero A, Farina L, Fanin R, Patriarca F, Miceli R, Matteucci P,
Bregni M, Scime R, Narni F, Pogliani E, Locasciulli A, Milani R,
Carniti C, Bacigalupo A, Rambaldi A, Bonifazi F, Olivieri A, Gianni AM,
Tarella C, Gruppo Italiano Trapianto di Midollo O. Allogeneic stem cell
transplantation following reduced-intensity conditioning can induce
durable clinical and molecular remissions in relapsed lymphomas:
pre-transplant disease status and histotype heavily influence outcome.
Leukemia. 2007;21:2316-2323. http://dx.doi.org/10.1038/sj.leu.2404822 PMid:17597807

- Ram
R, Gooley TA, Maloney DG, Press OW, Pagel JM, Petersdorf SH, Shustov
AR, Flowers ME, O'Donnell P, Sandmaier BM, Storb RF, Gopal AK.
Histology and time to progression predict survival for lymphoma
recurring after reduced-intensity conditioning and allogeneic
hematopoietic cell transplantation. Biol Blood Marrow Transplant.
2011;17:1537-1545. http://dx.doi.org/10.1016/j.bbmt.2011.03.010 PMid:21536145 PMCid:PMC3176968

- Josting
A, Muller H, Borchmann P, Baars JW, Metzner B, Dohner H, Aurer I,
Smardova L, Fischer T, Niederwieser D, Schafer-Eckart K, Schmitz N,
Sureda A, Glossmann J, Diehl V, DeJong D, Hansmann ML, Raemaekers J,
Engert A. Dose intensity of chemotherapy in patients with relapsed
Hodgkin's lymphoma. J Clin Oncol. 2010;28:5074-5080. http://dx.doi.org/10.1200/JCO.2010.30.5771 PMid:20975066

- Rancea
M, Monsef I, von Tresckow B, Engert A, Skoetz N. High-dose chemotherapy
followed by autologous stem cell transplantation for patients with
relapsed/refractory Hodgkin lymphoma. Cochrane Database Syst Rev.
2013;6:CD009411. PMid:23784872

- Bhatia
S, Robison LL, Francisco L, Carter A, Liu Y, Grant M, Baker KS, Fung H,
Gurney JG, McGlave PB, Nademanee A, Ramsay NK, Stein A, Weisdorf DJ,
Forman SJ. Late mortality in survivors of autologous hematopoietic-cell
transplantation: report from the Bone Marrow Transplant Survivor Study.
Blood. 2005;105:4215-4222. http://dx.doi.org/10.1182/blood-2005-01-0035 PMid:15701723 PMCid:PMC1895040

- Josting
A, Franklin J, May M, Koch P, Beykirch MK, Heinz J, Rudolph C, Diehl V,
Engert A. New prognostic score based on treatment outcome of patients
with relapsed Hodgkin's lymphoma registered in the database of the
German Hodgkin's lymphoma study group. J Clin Oncol. 2002;20:221-230. http://dx.doi.org/10.1200/JCO.20.1.221 PMid:11773173

- Josting
A, Rueffer U, Franklin J, Sieber M, Diehl V, Engert A. Prognostic
factors and treatment outcome in primary progressive Hodgkin lymphoma:
a report from the German Hodgkin Lymphoma Study Group. Blood.
2000;96:1280-1286. PMid:10942369

- Moskowitz
CH, Yahalom J, Zelenetz AD, Zhang Z, Filippa D, Teruya-Feldstein J,
Kewalramani T, Moskowitz AJ, Rice RD, Maragulia J, Vanak J, Trippett T,
Hamlin P, Horowitz S, Noy A, O'Connor OA, Portlock C, Straus D, Nimer
SD. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin
lymphoma and the significance of pre-transplant functional imaging. Br
J Haematol. 2010;148:890-897. http://dx.doi.org/10.1111/j.1365-2141.2009.08037.x PMid:20085577 PMCid:PMC3920913

- Morschhauser
F, Brice P, Ferme C, Divine M, Salles G, Bouabdallah R, Sebban C,
Voillat L, Casasnovas O, Stamatoullas A, Bouabdallah K, Andre M, Jais
JP, Cazals-Hatem D, Gisselbrecht C, Group GSS. Risk-adapted salvage
treatment with single or tandem autologous stem-cell transplantation
for first relapse/refractory Hodgkin's lymphoma: results of the
prospective multicenter H96 trial by the GELA/SFGM study group. J Clin
Oncol. 2008;26:5980-5987. http://dx.doi.org/10.1200/JCO.2007.15.5887 PMid:19018090

- Brice
P, Bouabdallah R, Moreau P, Divine M, Andre M, Aoudjane M, Fleury J,
Anglaret B, Baruchel A, Sensebe L, Colombat P. Prognostic factors for
survival after high-dose therapy and autologous stem cell
transplantation for patients with relapsing Hodgkin's disease: analysis
of 280 patients from the French registry. Societe Francaise de Greffe
de Moelle. Bone Marrow Transplant. 1997;20:21-26.
http://dx.doi.org/10.1038/sj.bmt.1700838 PMid:9232251 
- Sirohi
B, Cunningham D, Powles R, Murphy F, Arkenau T, Norman A, Oates J,
Wotherspoon A, Horwich A. Long-term outcome of autologous stem-cell
transplantation in relapsed or refractory Hodgkin's lymphoma. Ann
Oncol. 2008;19:1312-1319. http://dx.doi.org/10.1093/annonc/mdn052 PMid:18356139

- Steidl
C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ,
Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK,
Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES,
Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated
macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med.
2010;362:875-885. http://dx.doi.org/10.1056/NEJMoa0905680 PMid:20220182 PMCid:PMC2897174

- Shi
L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune
suppression in patients with hematological malignancies. J Hematol
Oncol. 2013;6:74. http://dx.doi.org/10.1186/1756-8722-6-74 PMid:24283718 PMCid:PMC3851976

- Musso
M, Scalone R, Marcacci G, Lanza F, Di Renzo N, Cascavilla N, Di
Bartolomeo P, Crescimanno A, Perrone T, Pinto A. Fotemustine plus
etoposide, cytarabine and melphalan (FEAM) as a new conditioning
regimen for lymphoma patients undergoing auto-SCT: a multicenter
feasibility study. Bone Marrow Transplant. 2010;45:1147-1153. http://dx.doi.org/10.1038/bmt.2009.318 PMid:19898504

- Visani
G, Malerba L, Stefani PM, Capria S, Galieni P, Gaudio F, Specchia G,
Meloni G, Gherlinzoni F, Giardini C, Falcioni S, Cuberli F, Gobbi M,
Sarina B, Santoro A, Ferrara F, Rocchi M, Ocio EM, Caballero MD,
Isidori A. BeEAM (bendamustine, etoposide, cytarabine, melphalan)
before autologous stem cell transplantation is safe and effective for
resistant/relapsed lymphoma patients. Blood. 2011;118:3419-3425. http://dx.doi.org/10.1182/blood-2011-04-351924 PMid:21816830

- Tombleson
RL, Green MR, Fancher KM. Putting caution in TEAM: high-dose
chemotherapy with autologous HSCT for primary central nervous system
lymphoma. Bone Marrow Transplant. 2012;47:1383-1384.
http://dx.doi.org/10.1038/bmt.2012.48 PMid:22426753 
- Thomson
KJ, Kayani I, Ardeshna K, Morris EC, Hough R, Virchis A, Goldstone AH,
Linch DC, Peggs KS. A response-adjusted PET-based transplantation
strategy in primary resistant and relapsed Hodgkin Lymphoma. Leukemia.
2013;27:1419-1422. http://dx.doi.org/10.1038/leu.2012.318 PMid:23135356

- Moskowitz
CH, Matasar MJ, Zelenetz AD, Nimer SD, Gerecitano J, Hamlin P, Horwitz
S, Moskowitz AJ, Noy A, Palomba L, Perales MA, Portlock C, Straus D,
Maragulia JC, Schoder H, Yahalom J. Normalization of pre-ASCT, FDG-PET
imaging with second-line, non-cross-resistant, chemotherapy programs
improves event-free survival in patients with Hodgkin lymphoma. Blood.
2012;119:1665-1670. http://dx.doi.org/10.1182/blood-2011-10-388058 PMid:22184409 PMCid:PMC3790950

- Castagna
L, Bramanti S, Balzarotti M, Sarina B, Todisco E, Anastasia A,
Magagnoli M, Mazza R, Nozza A, Giordano L, Rodari M, Rinifilo E, Chiti
A, Santoro A. Predictive value of early 18F-fluorodeoxyglucose positron
emission tomography (FDG-PET) during salvage chemotherapy in
relapsing/refractory Hodgkin lymphoma (HL) treated with high-dose
chemotherapy. Br J Haematol. 2009;145:369-372. http://dx.doi.org/10.1111/j.1365-2141.2009.07645.x PMid:19344403

- Devillier
R, Coso D, Castagna L, Brenot Rossi I, Anastasia A, Chiti A, Ivanov V,
Schiano JM, Santoro A, Chabannon C, Balzarotti M, Blaise D, Bouabdallah
R. Positron emission tomography response at the time of autologous stem
cell transplantation predicts outcome of patients with relapsed and/or
refractory Hodgkin's lymphoma responding to prior salvage therapy.
Haematologica. 2012;97:1073-1079.
http://dx.doi.org/10.3324/haematol.2011.056051 PMid:22271893 PMCid:PMC3396680 
- Moskowitz
CH, Nadamanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI,
Stiff PJ, Gianni AM, Carella AM, Osmanov D, Bachanova V, Sweetenham J,
Sureda A, Huebner D, Larsen EK, Hunder NNH, Walewski J. The Aethera
Trial: Results of a Randomized, Double-Blind, Placebo-Controlled Phase
3 Study of Brentuximab Vedotin in the Treatment of Patients at Risk of
Progression Following Autologous Stem Cell Transplant for Hodgkin
Lymphoma. Blood. 2014;124:673.

- Sureda
A, Schmitz N. Role of allogeneic stem cell transplantation in relapsed
or refractory Hodgkin's disease. Ann Oncol. 2002;13 Suppl 1:128-132. http://dx.doi.org/10.1093/annonc/13.S1.128 PMid:12078894

- Castagna
L, Sarina B, Todisco E, Magagnoli M, Balzarotti M, Bramanti S, Mazza R,
Anastasia A, Bacigalupo A, Aversa F, Soligo D, Giordano L, Santoro A.
Allogeneic stem cell transplantation compared with chemotherapy for
poor-risk Hodgkin lymphoma. Biol Blood Marrow Transplant.
2009;15:432-438. http://dx.doi.org/10.1016/j.bbmt.2008.12.506 PMid:19285630

- Corazzelli
G, Angrilli F, D'Arco A, Ferrara F, Musto P, Guarini A, Cox MC,
Stelitano C, Storti S, Iannitto E, Falorio S, Califano C, Amore A,
Arcamone M, De Filippi R, Pinto A. Efficacy and safety of bendamustine
for the treatment of patients with recurring Hodgkin lymphoma. Br J
Haematol. 2013;160:207-215. http://dx.doi.org/10.1111/bjh.12120 PMid:23167437

- Younes
A, Oki Y, Bociek RG, Kuruvilla J, Fanale M, Neelapu S, Copeland A,
Buglio D, Galal A, Besterman J, Li Z, Drouin M, Patterson T, Ward MR,
Paulus JK, Ji Y, Medeiros LJ, Martell RE. Mocetinostat for relapsed
classical Hodgkin's lymphoma: an open-label, single-arm, phase 2 trial.
Lancet Oncol. 2011;12:1222-1228. http://dx.doi.org/10.1016/S1470-2045(11)70265-0

- Younes
A, Sureda A, Ben-Yehuda D, Zinzani PL, Ong TC, Prince HM, Harrison SJ,
Kirschbaum M, Johnston P, Gallagher J, Le Corre C, Shen A, Engert A.
Panobinostat in patients with relapsed/refractory Hodgkin's lymphoma
after autologous stem-cell transplantation: results of a phase II
study. J Clin Oncol. 2012;30:2197-2203. http://dx.doi.org/10.1200/JCO.2011.38.1350 PMid:22547596

- Johnston
PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM, Micallef
IN, Porrata LF, Ansell SM, Reeder CB, Roy V, Witzig TE. A Phase II
trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin
lymphoma. Am J Hematol. 2010;85:320-324. PMid:20229590

- Guidetti
A, Carlo-Stella C, Locatelli SL, Malorni W, Mortarini R, Viviani S,
Russo D, Marchiano A, Sorasio R, Dodero A, Farina L, Giordano L, Di
Nicola M, Anichini A, Corradini P, Gianni AM. Phase II study of
perifosine and sorafenib dual-targeted therapy in patients with
relapsed or refractory lymphoproliferative diseases. Clin Cancer Res.
2014;20:5641-5651. http://dx.doi.org/10.1158/1078-0432.CCR-14-0770 PMid:25239609

- Guidetti
A, Carlo-Stella C, Locatelli SL, Malorni W, Pierdominici M, Barbati C,
Mortarini R, Devizzi L, Matteucci P, Marchiano A, Lanocita R, Farina L,
Dodero A, Tarella C, Di Nicola M, Corradini P, Anichini A, Gianni AM.
Phase II study of sorafenib in patients with relapsed or refractory
lymphoma. British Journal of Haematology. 2012;158:108-119. http://dx.doi.org/10.1111/j.1365-2141.2012.09139.x PMid:22571717

- Fehniger
TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, Fenske TS,
Hurd DD, Goy A, Schneider SE, Keppel CR, Wagner-Johnston ND, Carson KR,
Bartlett NL. A phase 2 multicenter study of lenalidomide in relapsed or
refractory classical Hodgkin lymphoma. Blood. 2011;118:5119-5125. http://dx.doi.org/10.1182/blood-2011-07-362475 PMid:21937701 PMCid:PMC3217400

- Chen
R, Palmer JM, Tsai NC, Thomas SH, Siddiqi T, Popplewell L, Farol L,
Nademanee A, Forman SJ. Brentuximab vedotin is associated with improved
progression-free survival after allogeneic transplantation for hodgkin
lymphoma. Biol Blood Marrow Transplant. 2014;20:1864-1868. http://dx.doi.org/10.1016/j.bbmt.2014.06.037 PMid:25008328

- Castagna
L, Crocchiolo R, Giordano L, Bramanti S, Carlo-Stella C, Sarina B,
Chiti A, Mauro E, Gandolfi S, Todisco E, Balzarotti M, Anastasia A,
Magagnoli M, Brusamolino E, Santoro A. High dose melphalan with
autologous stem cell support in FDG PET-refractory Hodgkin lymphoma
patients as bridge to second transplant. Bone Marrow Transplant. 2014;
in press.

- Ardeshna
KM, Kakouros N, Qian W, Powell MG, Saini N, D'Sa S, Mackinnon S, Hoskin
PJ, Goldstone AH, Linch DC. Conventional second-line salvage
chemotherapy regimens are not warranted in patients with malignant
lymphomas who have progressive disease after first-line salvage therapy
regimens. Br J Haematol. 2005;130:363-372. http://dx.doi.org/10.1111/j.1365-2141.2005.05603.x PMid:16042685

- Peggs KS, Anderlini P, Sureda A. Allogeneic transplantation for Hodgkin lymphoma. Br J Haematol. 2008;143:468-480. PMid:18710379

- Luznik
L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA,
Piantadosi S, Kaup M, Ambinder RF, Huff CA, Matsui W, Bolanos-Meade J,
Borrello I, Powell JD, Harrington E, Warnock S, Flowers M, Brodsky RA,
Sandmaier BM, Storb RF, Jones RJ, Fuchs EJ. HLA-haploidentical bone
marrow transplantation for hematologic malignancies using
nonmyeloablative conditioning and high-dose, posttransplantation
cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641-650. http://dx.doi.org/10.1016/j.bbmt.2008.03.005 PMid:18489989 PMCid:PMC2633246

- Raiola
A, Dominietto A, Varaldo R, Ghiso A, Galaverna F, Bramanti S, Todisco
E, Sarina B, Giordano L, Ibatici A, Santoro A, Clavio M, Bacigalupo A,
Castagna L. Unmanipulated haploidentical BMT following
non-myeloablative conditioning and post-transplantation CY for advanced
Hodgkin's lymphoma. Bone Marrow Transplant. 2014;49:190-194. http://dx.doi.org/10.1038/bmt.2013.166 PMid:24185585

- Castagna
L, Bramanti S, Furst S, Giordano L, Crocchiolo R, Sarina B, Mauro E,
Morabito L, Bouabdallah R, Coso D, Balzarotti M, Broussais F, Cheick
JE, Stella CC, Brusamolino E, Blaise D, Santoro A. Nonmyeloablative
conditioning, unmanipulated haploidentical SCT and post-infusion CY for
advanced lymphomas. Bone Marrow Transplant. 2014. http://dx.doi.org/10.1038/bmt.2014.197

- Burroughs
LM, O'Donnell PV, Sandmaier BM, Storer BE, Luznik L, Symons HJ, Jones
RJ, Ambinder RF, Maris MB, Blume KG, Niederwieser DW, Bruno B, Maziarz
RT, Pulsipher MA, Petersen FB, Storb R, Fuchs EJ, Maloney DG.
Comparison of outcomes of HLA-matched related, unrelated, or
HLA-haploidentical related hematopoietic cell transplantation following
nonmyeloablative conditioning for relapsed or refractory Hodgkin
lymphoma. Biol Blood Marrow Transplant. 2008;14:1279-1287. http://dx.doi.org/10.1016/j.bbmt.2008.08.014 PMid:18940683 PMCid:PMC2647369

- Wudhikarn
K, Brunstein CG, Bachanova V, Burns LJ, Cao Q, Weisdorf DJ. Relapse of
lymphoma after allogeneic hematopoietic cell transplantation:
management strategies and outcome. Biol Blood Marrow Transplant.
2011;17:1497-1504. http://dx.doi.org/10.1016/j.bbmt.2011.02.009 PMid:21338707 PMCid:PMC3132225

- Gopal
AK, Ramchandren R, O'Connor OA, Berryman RB, Advani RH, Chen R, Smith
SE, Cooper M, Rothe A, Matous JV, Grove LE, Zain J. Safety and efficacy
of brentuximab vedotin for Hodgkin lymphoma recurring after allogeneic
stem cell transplantation. Blood. 2012;120:560-568. http://dx.doi.org/10.1182/blood-2011-12-397893 PMid:22510871 PMCid:PMC3731651

- Carlo-Stella
C, Ricci F, Dalto S, Mazza R, Malagola M, Patriarca F, Viviani S, Russo
D, Giordano L, Castagna L, Corradini P, Santoro A. Brentuximab Vedotin
in patients with Hodgkin lymphoma and a failed allogeneic stem cell
transplantation: results from a named patient programme at four Italian
centers. The Oncologist. 2014; in press.

- Sureda
A, Canals C, Arranz R, Caballero D, Ribera JM, Brune M, Passweg J,
Martino R, Valcarcel D, Besalduch J, Duarte R, Leon A, Pascual MJ,
Garcia-Noblejas A, Lopez Corral L, Xicoy B, Sierra J, Schmitz N.
Allogeneic stem cell transplantation after reduced intensity
conditioning in patients with relapsed or refractory Hodgkin's
lymphoma. Results of the HDR-ALLO study - a prospective clinical trial
by the Grupo Espanol de Linfomas/Trasplante de Medula Osea (GEL/TAMO)
and the Lymphoma Working Party of the European Group for Blood and
Marrow Transplantation. Haematologica. 2012;97:310-317. http://dx.doi.org/10.3324/haematol.2011.045757 PMid:21993674 PMCid:PMC3269494

- Peggs
KS, Hunter A, Chopra R, Parker A, Mahendra P, Milligan D, Craddock C,
Pettengell R, Dogan A, Thomson KJ, Morris EC, Hale G, Waldmann H,
Goldstone AH, Linch DC, Mackinnon S. Clinical evidence of a
graft-versus-Hodgkin's-lymphoma effect after reduced-intensity
allogeneic transplantation. Lancet. 2005;365:1934-1941. http://dx.doi.org/10.1016/S0140-6736(05)66659-7

- Theurich
S, Malcher J, Wennhold K, Shimabukuro-Vornhagen A, Chemnitz J, Holtick
U, Krause A, Kobe C, Kahraman D, Engert A, Scheid C, Chakupurakal G,
Hallek M, von Bergwelt-Baildon M. Brentuximab vedotin combined with
donor lymphocyte infusions for early relapse of Hodgkin lymphoma after
allogeneic stem-cell transplantation induces tumor-specific immunity
and sustained clinical remission. J Clin Oncol. 2013;31:e59-63. http://dx.doi.org/10.1200/JCO.2012.43.6832 PMid:23269992

- Sala
E, Crocchiolo R, Gandolfi S, Bruno-Ventre M, Bramanti S, Peccatori J,
Sarina B, Corti C, Ciceri F, Santoro A, Marktel S, Castagna L.
Bendamustine Combined with Donor Lymphocytes Infusion in Hodgkin's
Lymphoma Relapsing after Allogeneic Hematopoietic Stem Cell
Transplantation. Biol Blood Marrow Transplant. 2014. http://dx.doi.org/10.1016/j.bbmt.2014.05.024

- Lohri
A, Barnett M, Fairey RN, O'Reilly SE, Phillips GL, Reece D, Voss N,
Connors JM. Outcome of treatment of first relapse of Hodgkin's disease
after primary chemotherapy: identification of risk factors from the
British Columbia experience 1970 to 1988. Blood. 1991;77:2292-2298.
PMid:1709382

- Reece
DE, Connors JM, Spinelli JJ, Barnett MJ, Fairey RN, Klingemann HG,
Nantel SH, O'Reilly S, Shepherd JD, Sutherland HJ, et al. Intensive
therapy with cyclophosphamide, carmustine, etoposide +/- cisplatin, and
autologous bone marrow transplantation for Hodgkin's disease in first
relapse after combination chemotherapy. Blood. 1994;83:1193-1199.
PMid:8118023

- Horning
SJ, Chao NJ, Negrin RS, Hoppe RT, Long GD, Hu WW, Wong RM, Brown BW,
Blume KG. High-dose therapy and autologous hematopoietic progenitor
cell transplantation for recurrent or refractory Hodgkin's disease:
analysis of the Stanford University results and prognostic indices.
Blood. 1997;89:801-813. PMid:9028311

- Andre
M, Henry-Amar M, Pico JL, Brice P, Blaise D, Kuentz M, Coiffier B,
Colombat P, Cahn JY, Attal M, Fleury J, Milpied N, Nedellec G, Biron P,
Tilly H, Jouet JP, Gisselbrecht C. Comparison of high-dose therapy and
autologous stem-cell transplantation with conventional therapy for
Hodgkin's disease induction failure: a case-control study. Societe
Francaise de Greffe de Moelle. J Clin Oncol. 1999;17:222-229.
PMid:10458237

- Sweetenham
JW, Carella AM, Taghipour G, Cunningham D, Marcus R, Della Volpe A,
Linch DC, Schmitz N, Goldstone AH. High-dose therapy and autologous
stem-cell transplantation for adult patients with Hodgkin's disease who
do not enter remission after induction chemotherapy: results in 175
patients reported to the European Group for Blood and Marrow
Transplantation. Lymphoma Working Party. J Clin Oncol.
1999;17:3101-3109. PMid:10506605

- Constans
M, Sureda A, Terol MJ, Arranz R, Caballero MD, Iriondo A, Jarque I,
Carreras E, Moraleda JM, Carrera D, Leon A, Lopez A, Albo C,
Diaz-Mediavilla J, Fernandez-Abellan P, Garcia-Ruiz JC,
Hernandez-Navarro F, Mataix R, Petit J, Pascual MJ, Rifon J,
Garcia-Conde J, Fernandez-Ranada JM, Mateos MV, Sierra J, Conde E,
Group GTC. Autologous stem cell transplantation for primary refractory
Hodgkin's disease: results and clinical variables affecting outcome.
Ann Oncol. 2003;14:745-751. http://dx.doi.org/10.1093/annonc/mdg206 PMid:12702529

- Czyz
J, Szydlo R, Knopinska-Posluszny W, Hellmann A, Gozdzik J, Hansz J,
Smolewski P, Robak T, Osowiecki M, Walewski J, Avigdor A, Nagler A,
Zemelka T, Pawlicki M, Sawicki Z, Wojtukiewicz M, Kachel L, Holowiecki
J, Charlinski G, Jedrzejczak WW. Treatment for primary refractory
Hodgkin's disease: a comparison of high-dose chemotherapy followed by
ASCT with conventional therapy. Bone Marrow Transplant.
2004;33:1225-1229. http://dx.doi.org/10.1038/sj.bmt.1704508 PMid:15094747

- Morabito
F, Stelitano C, Luminari S, Mammi C, Marcheselli L, Callea V, Gentile
M, Polimeno G, Merli F, Molica S, Gobbi P, Angrilli F, Brugiatelli M,
Federico M. The role of high-dose therapy and autologous stem cell
transplantation in patients with primary refractory Hodgkin's lymphoma:
a report from the Gruppo Italiano per lo Studio dei Linfomi (GISL).
Bone Marrow Transplant. 2006;37:283-288.
http://dx.doi.org/10.1038/sj.bmt.1705235 PMid:16327815 
- Akhtar
S, El Weshi A, Abdelsalam M, Hussaini H, Janabi I, Rahal M, Maghfoor I.
Primary refractory Hodgkin's lymphoma: outcome after high-dose
chemotherapy and autologous SCT and impact of various prognostic
factors on overall and event-free survival. A single institution result
of 66 patients. Bone Marrow Transplant. 2007;40:651-658. http://dx.doi.org/10.1038/sj.bmt.1705792 PMid:17660837

- Chopra
R, McMillan AK, Linch DC, Yuklea S, Taghipour G, Pearce R, Patterson
KG, Goldstone AH. The place of high-dose BEAM therapy and autologous
bone marrow transplantation in poor-risk Hodgkin's disease. A
single-center eight-year study of 155 patients. Blood.
1993;81:1137-1145. PMid:8443375

- Rapoport
AP, Rowe JM, Kouides PA, Duerst RA, Abboud CN, Liesveld JL, Packman CH,
Eberly S, Sherman M, Tanner MA, et al. One hundred autotransplants for
relapsed or refractory Hodgkin's disease and lymphoma: value of
pretransplant disease status for predicting outcome. J Clin Oncol.
1993;11:2351-2361. PMid:8246024

- Yahalom
J, Gulati SC, Toia M, Maslak P, McCarron EG, O'Brien JP, Portlock CS,
Straus DJ, Phillips J, Fuks Z. Accelerated hyperfractionated
total-lymphoid irradiation, high-dose chemotherapy, and autologous bone
marrow transplantation for refractory and relapsing patients with
Hodgkin's disease. J Clin Oncol. 1993;11:1062-1070. PMid:8501492

- Crump
M, Smith AM, Brandwein J, Couture F, Sherret H, Sutton DM, Scott JG,
McCrae J, Murray C, Pantalony D, et al. High-dose etoposide and
melphalan, and autologous bone marrow transplantation for patients with
advanced Hodgkin's disease: importance of disease status at transplant.
J Clin Oncol. 1993;11:704-711. PMid:8478664

- Argiris
A, Seropian S, Cooper DL. High-dose BEAM chemotherapy with autologous
peripheral blood progenitor-cell transplantation for unselected
patients with primary refractory or relapsed Hodgkin's disease. Ann
Oncol. 2000;11:665-672. http://dx.doi.org/10.1023/A:1008396525292 PMid:10942053

- Lazarus
HM, Loberiza FR, Jr., Zhang MJ, Armitage JO, Ballen KK, Bashey A,
Bolwell BJ, Burns LJ, Freytes CO, Gale RP, Gibson J, Herzig RH,
LeMaistre CF, Marks D, Mason J, Miller AM, Milone GA, Pavlovsky S,
Reece DE, Rizzo JD, van Besien K, Vose JM, Horowitz MM. Autotransplants
for Hodgkin's disease in first relapse or second remission: a report
from the autologous blood and marrow transplant registry (ABMTR). Bone
Marrow Transplant. 2001;27:387-396. http://dx.doi.org/10.1038/sj.bmt.1702796 PMid:11313668

- Sureda
A, Arranz R, Iriondo A, Carreras E, Lahuerta JJ, Garcia-Conde J, Jarque
I, Caballero MD, Ferra C, Lopez A, Garcia-Larana J, Cabrera R, Carrera
D, Ruiz-Romero MD, Leon A, Rifon J, Diaz-Mediavilla J, Mataix R, Morey
M, Moraleda JM, Altes A, Lopez-Guillermo A, de la Serna J,
Fernandez-Ranada JM, Sierra J, Conde E, Grupo Espanol de
Linformas/Transplante Autologo de Medula Osea Spanish Cooperative G.
Autologous stem-cell transplantation for Hodgkin's disease: results and
prognostic factors in 494 patients from the Grupo Espanol de
Linfomas/Transplante Autologo de Medula Osea Spanish Cooperative Group.
J Clin Oncol. 2001;19:1395-1404. PMid:11230484

- Ferme
C, Mounier N, Divine M, Brice P, Stamatoullas A, Reman O, Voillat L,
Jaubert J, Lederlin P, Colin P, Berger F, Salles G. Intensive salvage
therapy with high-dose chemotherapy for patients with advanced
Hodgkin's disease in relapse or failure after initial chemotherapy:
results of the Groupe d'Etudes des Lymphomes de l'Adulte H89 Trial. J
Clin Oncol. 2002;20:467-475. http://dx.doi.org/10.1200/JCO.20.2.467 PMid:11786576

- Tarella
C, Cuttica A, Vitolo U, Liberati M, Di Nicola M, Cortelazzo S, Rosato
R, Rosanelli C, Di Renzo N, Musso M, Pavone E, Santini G, Pescarollo A,
De Crescenzo A, Federico M, Gallamini A, Pregno P, Romano R, Coser P,
Gallo E, Boccadoro M, Barbui T, Pileri A, Gianni AM, Levis A. High-dose
sequential chemotherapy and peripheral blood progenitor cell
autografting in patients with refractory and/or recurrent Hodgkin
lymphoma: a multicenter study of the intergruppo Italiano Linfomi
showing prolonged disease free survival in patients treated at first
recurrence. Cancer. 2003;97:2748-2759.
http://dx.doi.org/10.1002/cncr.11414 PMid:12767087 
- Robinson
SP, Goldstone AH, Mackinnon S, Carella A, Russell N, de Elvira CR,
Taghipour G, Schmitz N, Lymphoma Working Party of the European Group
for B, Bone Marrow T. Chemoresistant or aggressive lymphoma predicts
for a poor outcome following reduced-intensity allogeneic progenitor
cell transplantation: an analysis from the Lymphoma Working Party of
the European Group for Blood and Bone Marrow Transplantation. Blood.
2002;100:4310-4316. http://dx.doi.org/10.1182/blood-2001-11-0107 PMid:12393626

- Alvarez
I, Sureda A, Caballero MD, Urbano-Ispizua A, Ribera JM, Canales M,
García-Conde J, Sanz G, Arranz R, Bernal MT, de la Serna J, Díez JL,
Moraleda JM, Rubió-Félix D, Xicoy B, Martínez C, Mateos MV, Sierra J.
Nonmyeloablative stem cell transplantation is an effective therapy for
refractory or relapsed hodgkin lymphoma: results of a spanish
prospective cooperative protocol. Biol Blood Marrow Transplant.
2006;12:172-183. http://dx.doi.org/10.1016/j.bbmt.2005.09.009 PMid:16443515

- Todisco
E, Castagna L, Sarina B, Mazza R, Anastasia A, Balzarotti M, Banna G,
Tirelli U, Soligo D, Santoro A. Reduced-intensity allogeneic
transplantation in patients with refractory or progressive Hodgkin's
disease after high-dose chemotherapy and autologous stem cell infusion.
Eur J Haematol. 2007;78:322-329. http://dx.doi.org/10.1111/j.1600-0609.2007.00814.x PMid:17253967

- Anderlini
P, Saliba R, Acholonu S, Giralt SA, Andersson B, Ueno NT, Hosing C,
Khouri IF, Couriel D, de Lima M, Qazilbash MH, Pro B, Romaguera J,
Fayad L, Hagemeister F, Younes A, Munsell MF, Champlin RE.
Fludarabine-melphalan as a preparative regimen for reduced-intensity
conditioning allogeneic stem cell transplantation in relapsed and
refractory Hodgkin's lymphoma: the updated M.D. Anderson Cancer Center
experience. Haematologica. 2008;93:257-264. http://dx.doi.org/10.3324/haematol.11828 PMid:18223284 PMCid:PMC4238917

- Devetten
MP, Hari PN, Carreras J, Logan BR, van Besien K, Bredeson CN, Freytes
CO, Gale RP, Gibson J, Giralt SA, Goldstein SC, Gupta V, Marks DI,
Maziarz RT, Vose JM, Lazarus HM, Anderlini P. Unrelated donor
reduced-intensity allogeneic hematopoietic stem cell transplantation
for relapsed and refractory Hodgkin lymphoma. Biol Blood Marrow
Transplant. 2009;15:109-117. http://dx.doi.org/10.1016/j.bbmt.2008.11.011 PMid:19135949 PMCid:PMC2929570

[TOP]







































































































