Received: September 29, 2014
Accepted: January 2, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015017, DOI 10.4084/MJHID.2015.017
This article is available on PDF format at:

N. Geetha1, K.P. Sreelesh1, M. J. Priya2, V.S. Lali1 and N. Rekha2
1
Department of Medical Oncology. Regional Cancer Centre. Trivandrum
695011, India
2 Department of Pathology. Regional Cancer
Centre. Trivandrum 695011, India

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Acute myeloid leukemia (AML) M6 is a rare form of AML accounting for < 5 % of all AML. Extramedullary involvement is very rarely seen in this entity. Skeletal lesion has not been described in AML M6 before. We discuss the case of a 17 year old boy with AML M6, who presented with osteolytic lesion of right humerus. He was treated with induction and consolidation chemotherapy. The present case is the first report in literature of AML M6 presenting with skeletal lesions. |
Introduction
Acute erythroid leukemia or Acute myeloid leukemia (AML) M6 is a rare form of AML. It accounts for < 5 % of all AML[1] AML M6 is otherwise known as Di Gugliemo syndrome, and it is a disease of adults. Extramedullary involvement is very rarely seen in this entity, and bone involvement is extremely rare. We present the case of a 17 year old boy with AML M6, who presented with predominant skeletal disease.
Case Report
A 17 year old boy presented with progressively increasing pain in right shoulder since 6 months, pain in right chest wall and gluteal region since 3 months. He gave history of intermittent fever and general weakness. A radiograph of right shoulder showed an irregular permeative type of lytic lesion involving proximal metadiaphyseal region of right humerus. Cortical breaks and interrupted periosteal reactions were present (Figure 1). He had undergone a biopsy from the humeral lesion prior to presenting to us.
Examination showed a sick boy with a performance status of 4, he had pallor, tenderness of right shoulder and hepatomegaly. His hemoglobin was 7.4gm%, total leucocyte count 3800/mm3, platelet was 1,67,000/mm3 and peripheral smear showed 6% abnormal cells. Serum chemistries were normal, and LDH was 532 IU/L (Normal 313-618U/L). Magnetic resonance imaging showed focal cortical lytic lesion in the head of right humerus and greater tuberocity, glenoid and corocoid process and right clavicle with moderate periosteal reaction (Figure 2). A computed tomogram showed permeative destruction of both shoulder joints and pelvic bones (Figure 3).
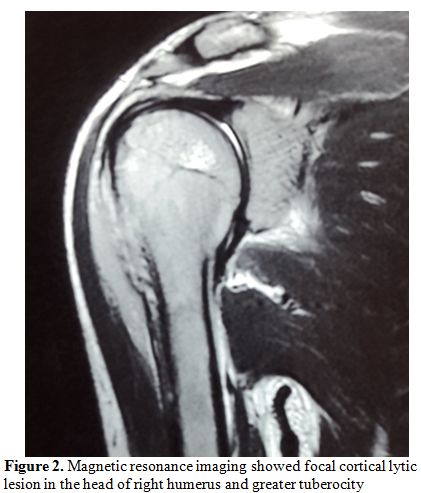 |
Figure 2. Magnetic resonance imaging showed focal cortical lytic lesion in the head of right humerus and greater tuberocity |
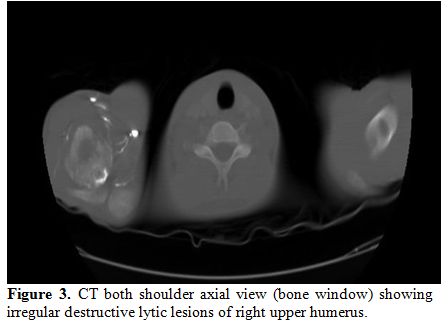 |
Figure 3. CT both shoulder axial view (bone window) showing irregular destructive lytic lesions of right upper humerus. |
A Tc99 bone scan showed hot spots over upper end of both humerii, trochanter of both femur, shaft of right femur (Figure 4).
A bone marrow study showed 64% myeloperoxidase-negative blasts with
scanty cytoplasm, blebbing, round nuclei and immature chromatin. The
remaining cells in marrow showed a Myeloid, erythroid ratio of 1:2.
Erythroid population showed dyserythropoiesis. Non erythroid population
showed 4% blasts. Megakaryocytes were absent. These blasts were
myeloperoixase negative and showed PAS block positivity. (Figure 5 and 6).
Flow cytometry from marrow showed the blasts to be negative for CD13,
CD33, CD64, CD117, cy MPO, cyCD61, CD10, CD19, CD2, CD3, CD4, CD5, CD5,
CD8, cyCD3, CD34, and HLA DR. The blasts were positive for glycophorin
A (Figure 7). Correlating the
morphology, differential count and immunophenotype of blasts, a
diagnosis of AML M6 (Pure erythroid leukaemia) was made. The biopsy
from the humerus shows spicules of bone with intervening neoplasm
showing tumor cells in sheets (Figure 8). Cells were negative for LCA, MIC2 (Figure 9 and 10).
The picture was compatible with AML M6 involving the bone. Bone marrow
cytogenetics was normal, and Bcr Abl was negative. He was treated with
induction chemotherapy with cytosine arabinoside and daunorubicin 7/3.
He achieved remission and symptom relief from bone pain. He received
further chemotherapy with FLAG for 3 cycles. His bone pain dissappeared
and there were healing changes in the humerus. However, he relapsed 4
months later and was put on supportive care. He died of progressive
disease at 10 months.
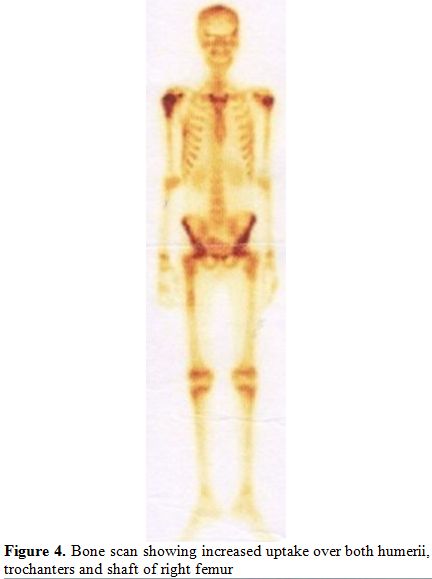 |
Figure 4. Bone scan showing increased uptake over both humerii, trochanters and shaft of right femur. |
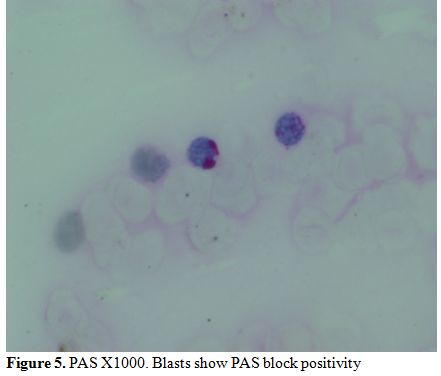 |
Figure 5. PAS X1000. Blasts show PAS block positivity. |
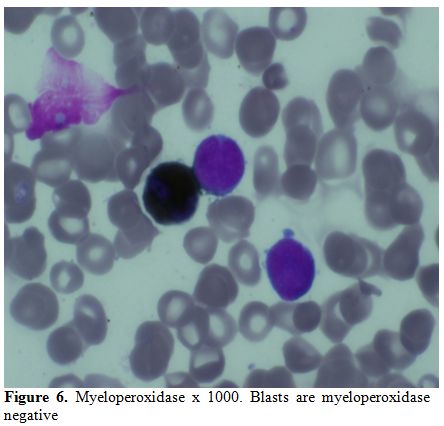 |
Figure 6. Myeloperoxidase x 1000. Blasts are myeloperoxidase negative. |
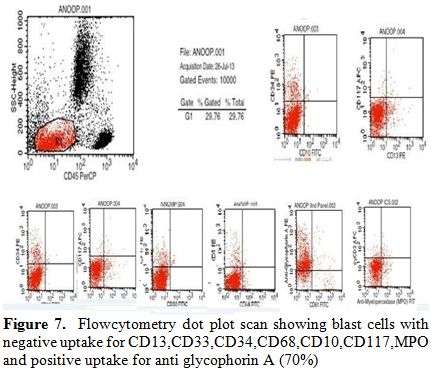 |
Figure 7. Flowcytometry dot plot scan
showing blast cells with negative uptake for
CD13,CD33,CD34,CD68,CD10,CD117,MPO and positive uptake for anti
glycophorin A (70%). |
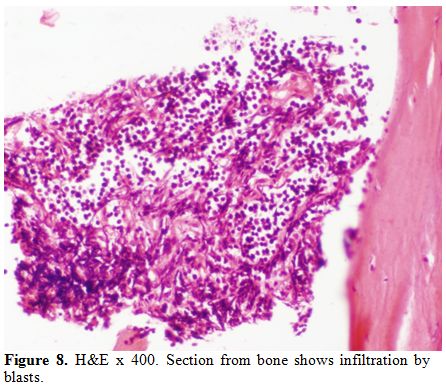 |
Figure 8. H&E x 400. Section from bone shows infiltration by blasts. |
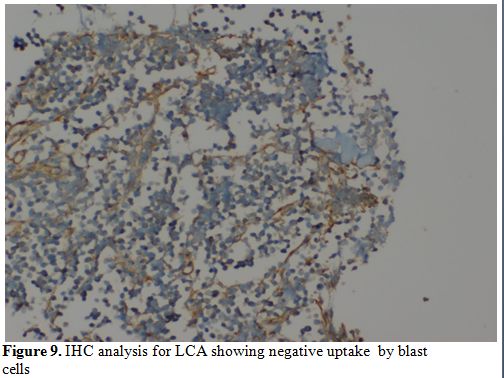 |
Figure 9. IHC analysis for LCA showing negative uptake by blast cells. |
 |
Figure 10.IHC analysis for mic 2 showing negative uptake by blast cells. |
Discussion
Although leukemia usually presents with pallor, bleeding tendencies, lymphadenopathy, and infections, rarely they present with skeletal manifestations. Such bone manifestations are more often found in lymphoid leukemias than myeloid. Osteolytic lesions of the skeleton associated with AML are uncommon. There are only few cases of AML associated with skeletal disease reported in literature (Table 1). Skeletal lesion has not been described in AML M6. The present case is the first report in literature of AML M6 presenting with skeletal lesions.
| Table 1. Patients with AML presenting with bone involvement reported in literature |
The radiological findings described in leukemias include
metaphyseal lucent bands, bone erosions, periosteal reactions, lytic
bone lesions, reduced bone density, permeative destruction and
vertebral collapse.[9] Bone lesions are more prevalent
in children than in adults since growing skeleton is an important site
for leukemic cell proliferation. Presence of bone lesions however do
not give a worse outcome compared to those without bone involvement.
Bone pain in acute leukemia is due to proliferation of bone marrow,
pressure effect, compression fractures and osteoporosis.[10]
The pathogenesis of bone destruction in leukemia remains poorly
defined. Abnormal production of parathyroid hormone by malignant cells
has been demonstrated.[11]
The hematologic
malignancies often presenting with osteolytic lesions are multiple
myeloma, non Hodgkin’s lymphoma such as adult Tcell lymphoma/leukemia,
anaplastic large cell lymphoma. Bone involvement can also rarely occur
in acute lymphoblastic leukemia and blast crisis of chronic myeloid
leukemia.[12] Other tumor presenting with predominant
bone destruction at this age is Ewing’s sarcoma. In the present case,
the bone was negative for LCA and MIC2, thus ruling out the possibility
of a lymphoid malignancy and Ewing’s sarcoma.
The expression of
glycophorin A on blast cells confirmed the diagnosis of erythroid
leukemia. The present case demonstrates the importance of evaluation of
skeleton in patients with AML presenting with bone pain.
References











[TOP]