Received: November 22, 2014
Accepted: January 8, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015025, DOI 10.4084/MJHID.2015.025
This article is available on PDF format at:

Maria Chiara Tisi1*, Giuseppe Ausoni1*, Maria Gabriella Vita2, Tommaso Tartaglione3, Mario Balducci4, Luca Laurenti1, Patrizia Chiusolo1, Stefan Hohaus1 and Simona Sica1
*the first two authors contributed equally
1 Institute of Hematology, Catholic University S. Cuore, Rome
2 Institute of Neurology, Catholic University S. Cuore, Rome
3 Institute of Radiology, Catholic University S. Cuore, Rome
4 Institute of Radiation Oncology, Catholic University S. Cuore, Rome

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Eleven cases of neurological defects
in T-ALL patients treated with nelarabine have been described in the
last 4 years, seven of these after stem cell transplantation (SCT) for
T Lymphoblastic Lymphoma (T-LBL). Most of these patients had an
unfavorable outcome or irreversible neurological damage. We now report
the case of a 41-year-old woman suffering from T-LBL who presented with
severe, but reversible myelopathy after receiving nelarabine-based
treatment and mediastinal radiotherapy, and we provide a review of the
literature on the topic. |
Introduction
Case Report
A 41-year-old woman with a medical history of thyroiditis presented
at our Institution with a mediastinal mass up to 9 centimeters in
diameter, without involvement of other organs or lymph nodes (LN). A
biopsy of the mass was performed, and a diagnosis of T Lymphoblastic
Lymphoma was established (Ki67 90%). The peripheral white blood cell
(WBC) count was normal, and a bone marrow biopsy was inconclusive. No
involvement of the central nervous system (CNS) was detected.
Chemotherapy according to the GMALL protocol[2] was
started, including intrathecal CNS prophylaxis with Methotrexate
alternated with Cytarabine. A complete remission (CR) by conventional
criteria[3] was achieved after two cycles of induction
chemotherapy, although a residual infiltrate of T lymphocytes (6%) was
documented in the bone marrow biopsy. The patient then underwent
mediastinal (2400 cGy), and cranial radiotherapy (2400 cGy) followed by
consolidation with HDAC/MITOX and HDMTX/ASP.[2] During
chemotherapy, major adverse effects were gastrointestinal symptoms
caused by a documented cytomegalovirus colitis. The planned treatment
was stopped ahead of schedule because the patient was not considered in
CR due to residual disease in the bone marrow. In order to enhance the
response in preparation for allogeneic stem cell transplantation, she
was then given nelarabine (two cycles of 1500 mg/square meter on days
1,3 and 5 of a 21-day cycle).
One month after the last dose of
nelarabine, she was submitted to an unrelated matched hematopoietic
stem cell transplant. During the conditioning regimen with busulfan and
cyclophosphamide, she developed progressive sensory loss in the lower
limbs, paraparesis, and ataxia, (grade 3 toxicity according to
NCI-CTCAE v4.03).[4] In addition, she complained of
urinary retention that required bladder catheterization. Treatment was
continued, and she received hematopoietic stem cells peripheral blood
G-CSF mobilized; cyclosporine, rabbit anti-thymocyte globulin and MTX
were administered as GVHD prophylaxis. Spinal Magnetic Resonance
Imaging (MRI) with gadolinium revealed a hyperintense T2w signal from
vertebral level D5 to D11, consistent with inflammatory myelitis (Figure 1a,c).
A lumbar puncture was performed that was negative for both leukemic
and/or infectious CNS involvement. The patient received steroid therapy
with dexamethasone 4 mg twice daily for 15 days. Later, when the
patient recovered from aplasia, intensive rehabilitation physical
therapy was started, with progressive improvement. The last MRI
performed 5 months later (Figure 1b,d)
showed the persistence of spinal cord alteration. At the moment of
writing this report, 22 months after the initial damage, the patient is
in complete remission and able to walk with a mobility aid (5/6
according to ADL-Activities of Daily Living score).
Discussion
A frequent major dose-limiting side effect of many chemotherapeutics
agents, including vinca alkaloids, taxanes, thalidomide and newer
agents such as bortezomib, is peripheral neuropathy. The incidence and
degree of neuropathy depends on the type of cytotoxic drug, the
duration of administration, the cumulative dose, and pre-existing
peripheral neuropathy. The damage is, in many cases, only partially
reversible, and sometimes even completely irreversible. In this study,
we report the case of 41-year-old woman suffering from severe
myelopathy after nelarabine treatment, mediastinal radiotherapy and
allogeneic stem cell transplantation for T-LBL.
Nelarabine is a
nucleoside pro-drug of 9-beta-D-arabinofuranosyl guanine (ara-G). It
was approved in October 2005 for the treatment of pediatric and adult
patients diagnosed with T-cell acute lymphoblastic leukemia (T-ALL) and
T-cell lymphoblastic lymphoma (T-LBL), refractory or relapsed after
treatment with at least two chemotherapeutic regimens.[5]
Clinical responses to nelarabine have been demonstrated in various
T-cell malignancies, but neuropathy is the most predominant adverse
effect associated with this drug. The incidence of neuropathy
correlates with the dose administered. The reported neurological
symptoms occurs around the 12th day
after the beginning of treatment; they are often preceded by transient
somnolence, malaise, and overt fatigue, occurring 6 to 8 days after the
initiation of nelarabine treatment.[5] The patient
described in our report developed a severe myelopathy with sensory
loss, paraparesis, ataxia and sphincteric dysfunction. Since leukemic
infiltration and ischemic, hemorrhagic or infectious etiology were
ruled out, the myelopathy was attributed to cumulative drug toxicity
from nelarabine and the damage caused on the spinal cord to the
mediastinal radiotherapy.
The neurological dose-limiting
toxicity of nelarabine was initially described in a phase I study by
Kurtzberg et al., where 72% of patients enrolled experienced a
neurological event.[6] Substantial neurological
toxicity was also observed in a phase II study by Berg et al., who
described a grade ≥3 neurological event in 18% of patients.[7]
DeAngelo et al. reported 39 refractory or relapsed T-ALL and T-LBL in
adults treated with nelarabine as single-agent: the drug showed a
substantial activity, with a complete remission rate of 31% and an
overall response rate of 41%. In this study there was only one grade 4
adverse event of the nervous system.[8]
To date,
eleven cases of irreversible neurological defects in T- ALL patients
treated with nelarabine have been described in the last 4 years,[9-14] seven of these after stem cell transplantation (SCT) for T-LBL.[10,11] Detailed clinical information on these previously reported cases are summarized in Table 1.
Patients received nelarabine either prior to SCT or after SCT for
lymphoma progression. The vast majority also received radiotherapy as
part of the planned treatment or in the conditioning regimen. In the
report from Kawakami et al, an excess of nelarabine neurotoxicity (up
to 50%) was detected after HLA-haploidentical SCT.[11]
In the recent paper from Ngo et al, concurrent administration of single
dose intrathecal cytosine arabinoside was felt to exert an additive
neurotoxic effect due to the close timing of administration to
nelarabine.[14 ] MRI findings, when reported, are
superimposable resulting in T2- weighted and FLAIR hyperintensity
predominantly at thoracic or cervical level. In conclusion, we
emphasize that the onset of not specific symptoms, like “symmetric
neurologic symptoms”, seldom reversible despite intensive
rehabilitation, should raise the suspicion for nelarabine toxicity in
patients who received a previous treatment with this active drug
usually after a short latency period, particularly if combined with
radiotherapy or intrathecal administration of cytotoxic drug.
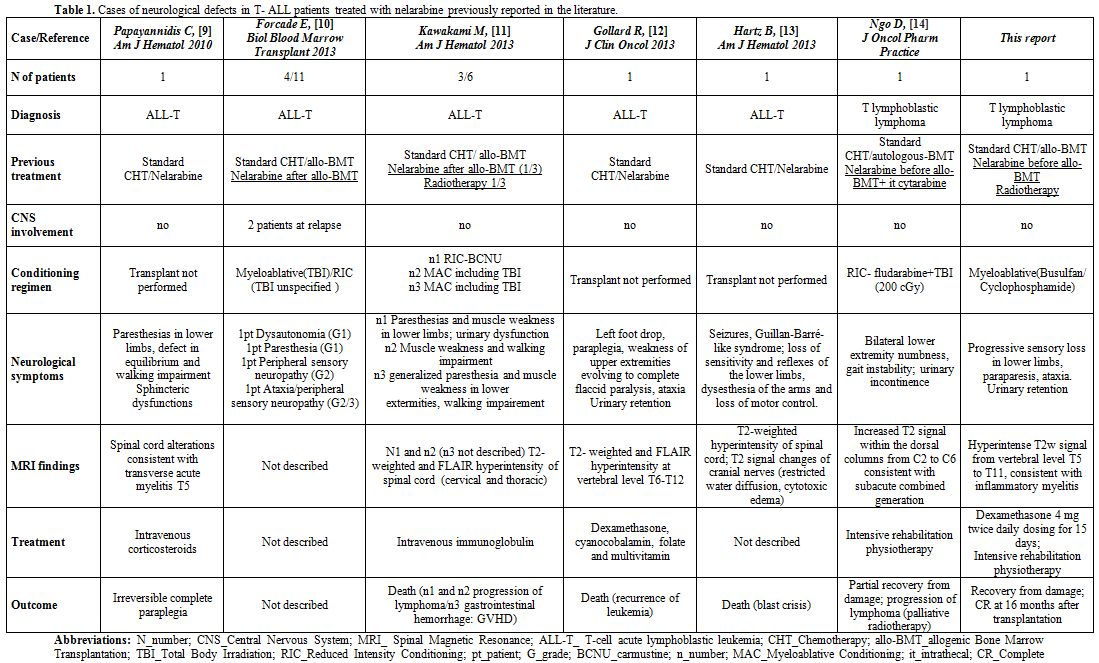 |
Table 1. Cases of neurological defects in T- ALL patients treated with nelarabine previously reported in the literature.. |
References













[TOP]