Received: January 28, 2015
Accepted: March 23, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015032, DOI 10.4084/MJHID.2015.032
This article is available on PDF format at:

Iyad M Ghonimat1, Lama H Nazer2, Flsteen Aqel1, Mohammad K Mohammad3, Feras I Hawari4 and Jennifer Le5
1Respiratory Therapy Services, King Hussein Cancer Center, Amman, Jordan
2Department of Pharmacy, King Hussein Cancer Center, Amman, Jordan
3ACDIMA Arab Company for Drug Industries & Medical Appliances, Amman, Jordan
4Pulmonary and critical care, King Hussein Cancer Center, Amman, Jordan
5University of California, San Diego Skaggs School of Pharmacy and Pharmaceutical Sciences, San Diego, CA, USA.

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Nebulized colistin (NC) is used for
the treatment of pneumonia due to multidrug-resistant Gram-negative
bacteria. In this one-year case-control study, our objective was to
evaluate the effect of NC on the ventilator circuit (VC) components.
The case group consisted of 25 mechanically-ventilated patients who
received NC for the treatment of nosocomial pneumonia while the control
group was 25 mechanically-ventilated patients who did not receive NC.
Respiratory therapists inspected the VC every 4 hrs and whenever a
ventilator alarm was reported. The VC component was changed if the
alarm did not subside after necessary measures were performed. Patients
from both groups were treated at the adult medical/surgical intensive
care unit at King Hussein Cancer Center. In the case group, 22 (88%)
patients required changing at least one of the circuit components (flow
sensor, exhalation membrane, or nebulizer kit). The median number of
changes (range) per patient of the flow sensor, exhalation membrane,
and nebulizer kit were: 2 (1-3), 2 (1-6), and 1 (1-2), respectively.
Large amounts of white crystals, which resembled the colistin powder,
were reported0 on the replaced VC components. The flow sensor was
changed in 2 control patients, but white crystals were absent. Crystals
obtained from one case subject were confirmed to be colistin by
chromatographic mass spectroscopy. Further studies are needed to
evaluate the effect of crystal formation on the efficacy of NC and
clinical outcomes. |
Introduction
Nebulized colistin (NC) has been widely used over the last decade in
critically ill patients for the treatment of multidrug resistant (MDR)
Gram-negative bacteria, mainly Pseudomonas aeruginosa and Acinetobacter
baumannii.[1] Several studies suggested that nebulized
colistin, as adjunct to systemic antibiotic treatment, improves
bacteriological and clinical response in patients with nosocomial
pneumonia.[2-6] In addition, the American Thoracic
Society-Infectious Disease Society of America Ventilator Associated
Pneumonia guidelines recommended adjunctive aerosolized
antibiotics in patients with MDR infections who are not responding well
to therapy.[7]
Despite its increased use, the
safety data of NC remains limited. In general, aerosolized
anti-infectives are attractive owing to their relative lack of systemic
toxicities.[8] However, serious adverse effects have
been reported with NC, including bronchospasm requiring mechanical
ventilation and leading to death.[8] Recently, we
raised the concern of crystal formation and frequent changes of the
ventilator circuit components in mechanically ventilated patients
treated with NC.[9] Up to our knowledge, there have
been no previous reports of these observations. To explore this
further, we conducted this prospective pilot case-control study to
evaluate the effect of NC on the components of the mechanical
ventilator in critically ill patients with nosocomial pneumonia.
Patients and methods
Study site and subjects.
The study was conducted in a 12-bed adult medical/surgical intensive
care unit (ICU) of a 170-bed comprehensive teaching cancer center, King
Hussein Cancer Center, in Amman, Jordan. The ICU manages
oncology-related and non-oncology related critical illnesses in cancer
patients. The ICU has a closed-unit model with high intensity staffing,
in accordance with the Leapfrog standards. This model was implemented
several years ago and has demonstrated improved clinical outcomes in
critically ill oncology patients.[10]
The study
included 25 consecutive mechanically- ventilated patients who received
NC for the treatment of nosocomial pneumonia due to multidrug
resistant gram negative bacteria (case group). The typically
administered dose of NC was 1 million units every 8 hrs. The control
group consisted of 25 consecutive mechanically ventilated patients who
did not receive any nebulized medications while they were on mechanical
ventilation. Patients on mechanical ventilation or NC for <24 hrs
were excluded.
Study design and methods.
This investigation was a one-year prospective pilot
case-control study (February, 2012 to April, 2013), approved by
the local institutional review board (IRB). The respiratory therapists
on a daily basis inspected the mechanical ventilator circuits for all
patients every 4 hrs and whenever a ventilator alarm was reported. The
ventilator used was the Galileo Gold Ventilator (Hamilton Medical AG,
Via Nova, Switzerland), along with the Intersurgical FlextubeTM
circle breathing system and the Hamilton single-use flow sensor. A
ventilator circuit component was changed if the alarm did not subside,
after all, necessary measures were performed and proved ineffective.
The
NC was prepared at the pharmacy using the product Colomycin® (Forest
Laboratories, UK), which is approved for inhalation and intravenous
uses. The preparation of NC was done under aseptic conditions,
according to the manufacturer’s recommendations: one million units of
colistimethate sodium was dissolved in 2 ml of normal saline, and then
refrigerated, with an expiration of 24 hours from the time of
preparation. The NC was administered by the respiratory therapist who
was assigned to the ICU patients. All respiratory therapists working in
the ICU are trained in managing critically ill patients and in the
administration of aerosolized medications. The NC was given after the
administration of any nebulized or inhaled
bronchodilator using the nebulizer kit (Plasti-Med®), and delivered
over 20 to 30 minutes. The nebulizer kit allowed the administration of
the aerosolized colistin, with a particle size of 2.7 micron MMAD (mass
median aerodynamic diameter), without interrupting the
ventilation cycle and according to the mechanical ventilator pressure.
During the administration of NC, the respiratory therapist remained in
proximity to the patient. Once the administration of NC was completed,
the nebulizer kit was disconnected by the respiratory therapist,
cleaned thoroughly, and stored by the patient’s bedside to use for
subsequent doses.
The patient demographics, length of stay, and
ICU mortality were recorded. In addition, the type of ventilator
component changed (flow sensor, exhalation membrane, or nebulizer kit),
the day(s) on which the ventilator component was changed and, the
number of changes was noted. When changing any of the ventilator
components, the respiratory therapist documented the reason for change
and described any unusual findings. A ventilator check list was used
when inspecting the ventilator circuit and to record the findings.
Subjects were monitored until they were extubated or transferred out of
the ICU, which ever occurred first.
To identify the content of the
white crystals, they were investigated in residue samples by the Arab
Company for Drug Industries & Medical Appliances (ACDIMA)
BioCenter, which is an international research organization specialized
in clinical studies and bioanalysis. An exhalation membrane that was
changed and had significant amount of white crystals was placed
in a tightly-closed container and then transported immediately
with ice to the analytical site of the ACDIMA BioCenter. The sample was
stored under -80oC until the time of
testing. We used the Colomycin® (Forest Laboratories, UK) product as a
control in the analysis. The identity of the residue sample was
investigated using Liquid chromatography coupled with an electrospray
ionization tandem mass spectrometry platform (Triple Quad Tandem Mass
Spectrometer, API 4000 LC-MS/MS). The collected crystals were dissolved
in sufficient quantity of methanol and injected into the instrument.
MS/MS spectra from samples, the accumulated residue, and the colistin
drug powder were scanned, and their fragmentation patterns were
elucidated under the conditions listed in Table 1.
Statistical analysis.
Descriptive statistics were used to report the results. Continuous data
were reported using mean with standard deviation (SD) and/or median
with range while categorical data were reported as counts and
percentages. The Chi-square or Fisher Exact test was used to compare
the categorical data while the t- test or Kruskal-Wallis test was used
to compare the continuous data. A significance criterion of p<0.05
was used in the analysis. All analysis was performed using SAS version
9.1 (SAS Institute Inc, Cary, NC).
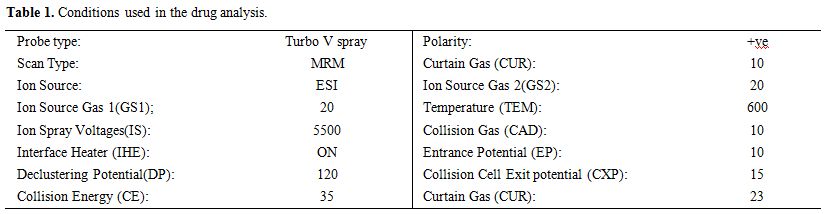 |
Table 1. Conditions used in the drug analysis. |
Results
The demographics and outcomes of the control and case groups are outlined in Table 2. Both groups were similar, except for a longer duration of ICU stay and a longer duration of mechanical ventilation in the case group, compared to the control group. During mechanical ventilation, both groups received adaptive support ventilation mode, with a minute ventilation (MV) ranging from 100-150%, PEEP between 5-14 cm H2O, and fraction of inspired oxygen (FiO2) between 40-100%.
| Table 2. Demographics and clinical characteristics. |
During mechanical ventilation, all subjects in both groups received albuterol, administered by a metered dose inhaler. Subjects did not receive any aerosolized antibiotics, except for the use of NC by the case group. All patients in the case group received intravenous and nebulized colistin for the treatment of nosocomial pneumonia, including ventilator-associated pneumonia, due to multidrug resistant Acinetobacter baumannii. Among those who received NC, 22 (88%) subjects required changing at least one of the circuit components (flow sensor, membrane, or nebulizer kit). At a median duration of NC use of 10 days, the median number of changes (range) per person in the flow sensor, exhalation membrane, and nebulizer kit were: 2 (1-3), 2 (1-6), and 1 (1-2), respectively (Table 3). Formation of large amounts of white crystals, which resembled the colistin powder, was observed in all ventilator components that were changed (Figure 1 and 2). The median duration to crystal formation was four days, (range 2-5), and crystals were found primarily in the exhalation membrane. All subjects with NC required at least one exhalation membrane change at a median of 7 days (range, 2-25) after initiating NC; this was followed by changing the flow sensor and nebulizer kit in 82% and 41% of the cases, respectively.
| Table 3. Subjects with crystal formation and obstruction of the ventilator circuit during nebulized colistin treatment (n=22) |
| Figure 1. Exhalation membrane; white crystals noted in the center. |
| Figure 2. Flow sensors; white crystals noted in the internal part for a patient who received nebulized colistin (left), while the other flow sensor for a patient who did not receive nebulized colistin (right). |
In the control group, the flow sensor was changed for two
patients (8%). There were no white crystals noted on the flow sensor,
and the replacement appeared to be due to a defect in the flow sensors.
The results of the MS/MS detection and identification of crystals
obtained from one subject using NC demonstrated the same fragmentation
pattern, as that obtained from the Colomycin® and both were consistent
with polymyxin behavior (Figures 3 and 4).
Polymyxins have a complex fragmentation behavior, and the spectra
reflects a similar pattern for both samples with base- peak ion mass at
597.56 (for the collected residue sample) and at 597.61 (for
Colomycin®).
| Figure 3. MS/MS spectra for the colistin product (Colomycin®) |
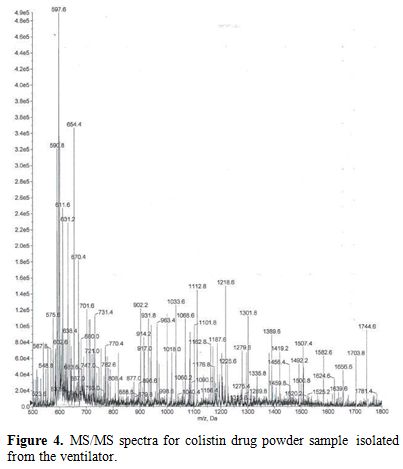 |
Figure 4. MS/MS spectra for colistin drug powder sample isolated from the ventilator. |
Discussion
This study demonstrated the high incidence of crystal formation on
various parts of the ventilator circuit in patients who received NC.
The crystals were found as early as two days after initiating NC. In
our previous case reports, crystal formation and the change in the
ventilator component were observed as early as one day after initiating
NC.[9] The aftermath of crystal formation was the
requirement for change in at least one of the ventilator components in
the majority of subjects receiving NC.
Based on the chromatography
analysis completed for one subject, it was confirmed that the crystals
were comprised of colistin. Although we did not analyze the crystals
for all subjects, we assumed that the content of the crystals were the
same since they all had similar general appearances. The origination of
the crystals is unknown. We hypothesize that colistimethate sodium
starts to crystallize shortly after its preparation, and once
administered, the high flow induced by the ventilator stimulates the
formation of more crystals that are apparent in the ventilator circuit.
The crystals formed in the prepared product were most likely non-
visible because the respiratory therapist typically inspects the
nebulizer solution prior to administration.
The observations
reported in this study raise two major issues: First is regarding the
amount of colistin reaching the site of infection in the lungs. The
concern of sub-therapeutic doses reaching the site of infection might
be considered, and future studies should focus on measuring the amount
of colistin that is lost in the ventilator circuit and through
exhalation to determine the actual amount of colistin reaching the
lungs. The second issue is whether the crystal formation has an effect
on the patients' ventilation and/or the ability of the ventilator
machine to operate accurately. The flow sensor in the ventilator
circuit provides the clinician with valuable data about the patients'
ventilation measures so that the ventilator setting can be adjusted
accordingly. However, if there are crystals formation in the flow
sensor, this might interfere with the accuracy of provided data.
Based
on the results of this study, we started changing the flow sensor every
three days for all mechanically ventilated patients receiving NC. This
is significantly more than what we typically do for our ventilated
patients who are not receiving NC and also more than the manufacturer's
recommendations. In fact, the manufacturer recommends changing
the flow sensor every two weeks, and we typically change it every 1-2
weeks. However, with our concern about the possible effect of the
crystals on the functionality of the flow sensor, and since the
ventilator alarm is a sign of the late stage of crystal formation, we
decided to change the flow sensor more frequently.
This study had
some limitations. First it was conducted in a single center therefore
affecting its reproducibility in another setting. Secondly, we did not
analyze the content of all crystals nor did we quantify the amount of
colistin that had crystallized. In addition, we did not match the
subjects in the case and control groups, but when comparing the two
groups, they were similar in demographics. Finally, we did not
correlate the findings with clinical outcomes.
Conclusion
This is the first study to describe a major complication associated with the administration of NC. The use of NC was associated with crystal formation and subsequent changes of the ventilator circuit components in about 90% of the subjects. Further investigations are imperative to confirm our findings and to evaluate the clinical implications of this complication.
References








 .
.[TOP]