Received: April 27, 2015
Accepted: May 20, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015041, DOI 10.4084/MJHID.2015.041
This article is available on PDF format at:

Matteo Parma1, Clara Viganò1, Monica Fumagalli1, Federica Colnaghi2, Arianna Colombo2, Federica Mottadelli2, Vincenzo Rossi2, Elena Elli1, Elisabetta Terruzzi1, Angelo Belotti1, Giovanni Cazzaniga2, Enrico Maria Pogliani3 and Pietro Pioltelli1
1 Haematology Division and BMT Unit, Ospedale San Gerardo, Monza, Italy.
2
Centro Ricerca Tettamanti, Clinica Pediatrica Università di Milano
Bicocca, Ospedale San Gerardo/Fondazione MBBM, Monza,
Italy.
3 Dipartimento di Scienze della Salute, Università di Milano Bicocca.

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background and Objectives:
Acute lymphoblastic leukaemia (ALL) carrying t(9;22) or t(4;11) genetic
abnormalities represents a very high risk subtype of disease (VHR-ALL).
Hematopoietic stem cell transplantation (HSCT) remains the best
curative option not only for t(4;11) ALL, but also for t(9;22) ALL in
the tyrosin-kinase inhibitors era. In the last years, low molecular
level of minimal residual disease (MRD) before HSCT was reported as one
of the best favourable indexes for survival in ALL. Here we observed
that even these patients can show a favourable outcome if submitted to
HSCT with very low MRD. Methods: We considered 18 consecutive VHR-ALL patients eligible to HSCT. 16 of them were transplanted in first remission, as soon as possible, employing myelo-ablative conditioning regimens. Molecular MRD has been evaluated before and after HSCT. Results: Immediately before HSCT, MRD revealed: complete molecular remission (MRDneg) for five patients, and a level <1x10-3 for seven patients. 100 days after HSCT we had: MRDneg for seven patients and a decrease for all the others after HSCT. After the tapering of immunosuppressive drugs, 13 patients reached the MRDneg in a median time of 8 months (range 3-16). In the intention to treat analysis, 14/18 patients are alive and disease free at the date of analysis. Overall survival and event free survival is of 78% and 66% respectively, with an average follow-up of 45 months (range 6-84) since HSCT. Conclusion: Early transplantation with low MRD level seems to be correlated with a favourable outcome also in VHR-ALL. |
Introduction
Acute lymphoblastic leukaemia (ALL) carrying the t(4;11)(q21;q23) or
t(9;22)(q34;q11) (Philadelphia chromosome) genetic abnormalities,
associated with MLL-AF4 and the BCR-ABL fusion transcripts
respectively, represents a very high risk subtype of the disease
(VHR-ALL).[1,2]
t(4;11) ALL has a major incidence in infant and adult population.[3] Typically it shows an early-B precursor immunophenotype (CD10 negative),[4,5]
and it is characterized by an extreme hyperleucocytosis upon onset.
Although the rate of complete remission (CR) after the induction
treatment is high (more than 90%), the occurrence of relapse and death
through the first two years is very elevated in the patients
treated with the sole chemotherapy: Consequently the long term
overall survival (OS) rate is low, around 20%-25%.[6]
By contrast, the efficacy of allogeneic haematopoietic stem cell
transplantation (HSCT) performed in first CR is clearly superior, with
a five years OS around 60%.[7,8] By the way, all the authors confirm this main indication in this disease.[9]
Differently from the t(4;11) ALL, the Philadelphia chromosome positive (Ph+)
ALL is more frequent in the adult population, with an incidence between
20-30%; whereas its incidence in the paediatric population is around
5-10%. In the tyrosin-kinase inhibitors (TKI) era, the combination of
chemotherapy and TKI has drastically increased the outcome. Nowadays,
in terms of complete haematologic and cytogenetic response, Ph+ ALL has a response rate of about 90% and OS at five years is around 40%, considering all the patients involved.[10]
Despite these improvements in non allografted patients, the OS at three
years is extremely low (less than 20%) while is in patients submitted
to HSCT around 50%. According to the recent analysis of UKALL/ECOG
trial, the favourable prognostic impact of Imatinib is due to the
generation of better conditions for HSCT; indeed patients, who did not
undergo HSCT, show a 5 years OS, which is very similar to those
patients treated with chemotherapy alone, in the pre-Imatinib era.[11] Also the GETH/GITMO trial has given a favourable outcome in a cohort of 45 patients affected by Ph+
ALL and submitted to Umbilical cord blood transplantation, showing a 5
years OS of 44%, extended to 60% in patients in molecular
remission before allograft.[12] This outcome given, very early HSCT seems to be the primary indication in Ph+ ALL patients suitable for this procedure.[9]
By contrast, there are recent reports, particularly from MD Anderson
Group, that underline how intensive chemotherapy associated with Imatib
or Dasantinib maintenance without HSCT can lead to a good outcome and
the overall survival is already similar to patients submitted to HSCT.[13]
Alternative strategies, such as monoclonal antibodies (Blinatumumab or
Inotuzumab-Ozagomicin) and Chimeric Antigen Receptor Modified T-Cells
(CAR T), seems to be a valid alternative in B-cells ALL, as reported in
some experimental trials.[14]
Furthermore, the
role of Minimal Residual Disease in ALL and its monitoring have become
crucial in the last few years. MRD in ALL is highly predictive of
relapse in those patients, who did not become negative after the first
courses of treatment or in those patients, who became positive after
having been previously negative.[15] For this reason
HSCT should be considered for all the patients who show MRD positivity
(MRD+) independently from the risk assessment group at the onset.
Moreover, quantitative molecular assay for MRD is very useful in
predicting the post-transplant outcome of these patients: in fact
patients showing MRD+ at lower levels have a better outcome compared to
those with higher levels. In particular, as reported in the experience
of Northern Italy Leukemia Group, 10-3
seems to be a real threshold in term of predicting the post-transplant
evolution, since patients who underwent HSCT with MRD value of 10-3 or more had an extremely unfavourable outcome.[16]
All the recent reports agree with the predictive role of MRD either in
patients submitted to HSCT or in patients non allografted. Also
the report of MDACC confirmed that a major molecular response obtained
in the first months of treatment has an excellent impact also in Ph+ALL patients not submitted to HSCT.[13]
In
this report, we analyzed the outcome of patients affected by t(4;11)
and Ph+ ALL and submitted to HSCT, by investigating the role of MRD,
both before and after the allograft.
Methods
Patients:
Hereinafter, we take into consideration a group of 18 consecutive
patients affected by VHR-ALL (5 with t(4:11) ALL and 13 with Ph+
ALL), treated in our division between January 2010 and December 2014
and eligible for HSCT upon onset of the disease. Our intention to treat
analysis excluded all the patients who, in the same period, were not
eligible to HSCT for clinical or other reasons. These two categories
are extremely different and not comparable, and this justifies the
exclusion of the last one from the analysis. Due to the fact that HSCT
has been considered the gold standard for VHR-ALL and the main option
proposed to these patients, we have not a control group of “not HSCT
patients” with similar clinical condition at baseline. Clinical
characteristics were: M/F ratio 5/13, mean age 46 years (21-65), none
of them showed CNS involvement. All of them have been treated with an
induction course according to an IVAP scheme: Idarubicin (12 mg/m2 day 1,2); Vincristine (1,4 mg/m2 day 1; 8; 15); Asparaginase (3000 UI/m2
for six administrations after day +8) and Prednisone (1 mg/Kg from day
1 to 21). Two patients died during the induction or consolidation
phase; the remaining 16 continued the treatment till HSCT. After the
induction, 14 patients underwent some consolidation courses while the
remaining 2, affected by Ph+ ALL,
continued with TKI only till HSCT. Although the consolidation programs
were comprehensive of different regimens, at least one course based on
high-dose Methotrexate-Cytarabine or high dose Cytarabine was given to
all patients. CNS prophylaxis has been carried out with an intrathecal
administration of a standard dose of Cytarabine (50 mg), Methotrexate
(12,5 mg) and 6-metil-Prednisolone (40 mg) during each chemotherapy
course. Imatinib has been administered in Ph+
patients for at least three weeks in each course regularly and has been
stopped for at least one week, to prevent possible resistant mutations.
400 mg x 2/day was the target dosage of Imatinib, adjustable in those
patients unable to tolerate this amount. One patient showed an early
relapse during the consolidation treatment, namely a T315I mutation in
the BCR-ABL gene, conferring resistance to all TKI, except for
Ponatinib.[17] Therefore, the patient has been
treated with Ponatinib (45 mg once a day), obtaining a second
haematological remission after two months.
After VHR-ALL was
diagnosed, donor research has been immediately activated, and HSCT has
been promptly performed as a donor was available. Patients, who could
not find a prompt donor, continued with the intensive consolidation
regimen till HSCT. 15 patients underwent HSCT upon first remission and
one upon second remission. Donors were: 8 siblings related, 7 matched
unrelated and one haploidentical. Six months was the average time for
the transplant (range 3-12), from the onset of disease. The number of
courses before HSCT goes from 1 to 7 and all the patients have been
treated according to the following standard mieloablative regimens:
TBI-Cyclofosfamide, Busulfan-Cyclofosfamide, Busufan-Fludarabine and
Busulfan-Thiotepa-Fludarabine. Complete data about the characteristics
at the onset, the treatment and the outcome are shown on Table 1.
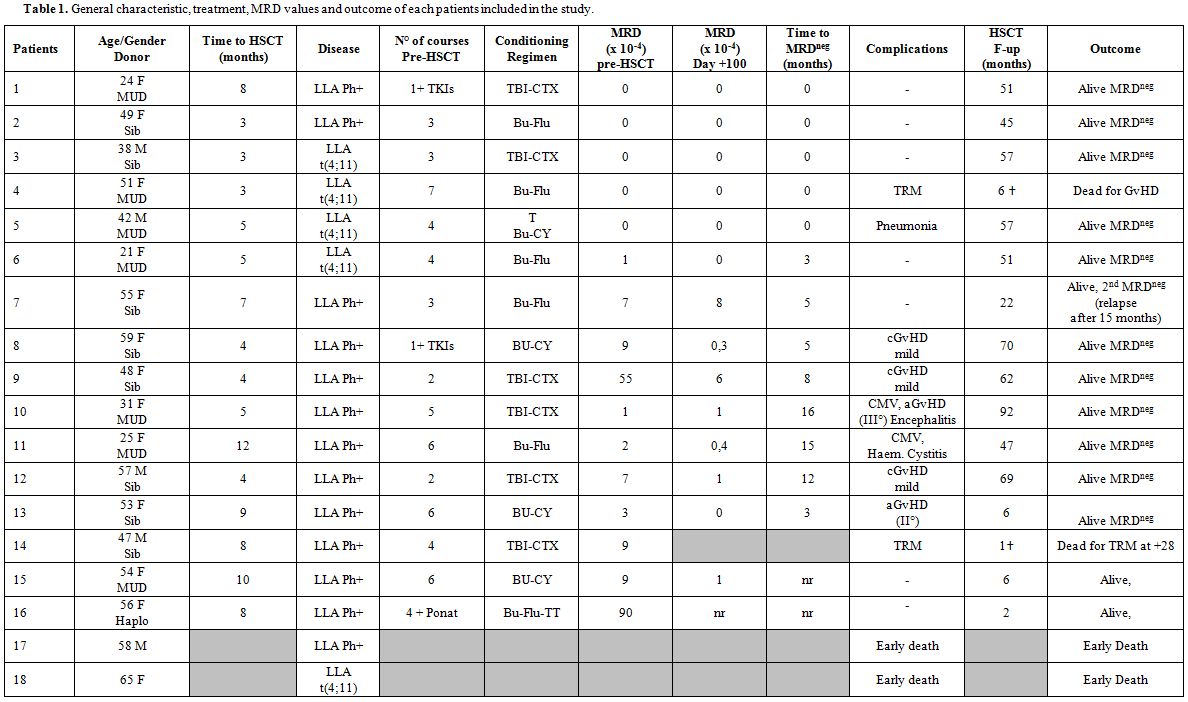 |
Table 1. General characteristic, treatment, MRD values and outcome of each patients included in the study. |
Laboratory Findings:
MRD analysis has been performed on bone marrow samples only. The
BCR-ABL fusion transcript was monitored by Real-time Quantitative PCR
(RQ-PCR) as previously described, by using an ABI Prism 7900HT Fast
Real-time Sequence Detection System (Life Technologies, Carlsbad, CA,
USA). RQ-PCR was conducted in triplicate, with a sensibility of 10-4.[18]
Copies of BCR-ABL fusion transcript molecules were calculated referring
to a plasmid standard curve (Ipsogen-Qiagen, Marseille, France), data
were normalized using ABL as housekeeping transcript, and results were
expressed as number of molecules of BCR-ABL any 104 ABL molecules.
A
qualitative Reverse-Transcriptase PCR (RT-PCR) analysis was employed
for MLL-AF4 fusion monitoring, by a two-steps (‘nested’) PCR approach.[19]
Briefly, the nested RT-PCR consisted of a second RT-PCR round by using
1 ul of the single RT-PCR amplification. PCR products have been
visualized on ethidium bromide stained agarose gels. The sensitivity of
single and nested RT-PCR rounds was 10-3 and 10-4, respectively. By this semi-quantitative approach, we assumed that a patient positive after single RT-PCR had an MRD level ≥10-3 and a patients negative after the single RT-PCR but positive after nested RT-PCR had an MRD level lower than 10-3 (but not negative). Only a patient negative after nested RT-PCR has been considered as truly MRD negative.[20]
Both for BCR-ABL and for MLL-AF4, complete molecular remission is defined as an undetectable MRD level (MRDneg).
Results
Soon before HSCT 14/16 patients showed an MRD level ≤1x10-3, namely: 5 patients had MRDneg, two patients had MRD level ≤1x10-4 and seven patients had an MRD level between 1x10-4 and 1x10-3. Only 2/16 patients showed an MRD level >1x10-3.
100 days after HSCT, MRD has been evaluated (MRD+100), in 14 patients:
one patient died before because of transplant related mortality (TRM),
another one has not reached 100 days yet at the time of analysis.
Significantly, all 5 patients who were MRDneg soon before HSCT remained
MRDneg; as for the other ones: 2 became MRDneg, 5 reduced their MRD to ≤1x10-4 and 2 showed MRD level between 1x10-4 and 1x10-3 (Figure 1).
t(4;11) ALL patients had an extremely low MRD level or were MRDneg
before HSCT and all of them remained MRDneg after transplantation
during the entire time observation.
Ph+ ALL patients showed a different trend 100 days after HSCT: 5 patients maintained an MRD+100 weakly positive (≤1X10-4):
we decided not to treat them with TKI but to try a rapid tapering of
immunosuppression. The same policy has been applied to a patient with a
higher MRD+100 (6x10-3) but in a reduction of 1 log compared to the MRD level before HSCT. In this way, we obtained a stable MRDneg
for five patients while one patient had a shorter follow-up at the time
of analysis. The other patient with higher MRD+100 (8x10-3)
was not treated because not immediately eligible for TKI.
Unfortunately, she showed a molecular relapse after 15 months after
obtaining an MRDneg, so she was
treated therefore with Imatinib 300 mg x 2/day (maximum tolerated
dosage), obtaining a second molecular remission in 3 months. The last 3
Ph+ ALL patients were MRDneg at 100 days after HSCT.
Overall MRDneg
has been reached in 13 patients during an average time of 10 months
(range 5-16); two patients had a very short follow-up at the date of
analysis and 1 died. After 47 months (range 2-67) since HSCT, the
outcome was as it follows. Two patients died from TRM (1 during HSCT
and one for GvHD occurred six months after HSCT), a patient showed
a molecular relapse 15 months after HSCT (and obtained a second
molecular remission with TKI treatment), all other patients maintained
the complete molecular remission. Considering that ten patients are in
stable remission for more than two years, they are highly likely to be
cured. Beside the two death from TRM, we also observed one patient who
developed acute GvHD (grade III) followed by a JC-virus correlated
encephalitis (with serious cerebral impairment): no other severe
transplant-related complication has been observed during the follow-up.
One bacterial pneumonia, two CMV reactivations, one haemorrhagic
cystitis, one acute GvHD (grade II) and three mild chronic GvHD had a
favourable outcome. After an average follow-up of 35 months (range
2-92) from HSCT, the estimated 5 years OS and event-free survival (EFS)
is 78% and 66% respectively, considering all the patients (Figure 2a), but it is 86% and 79% considering the allografted patients only (Figure 2b).
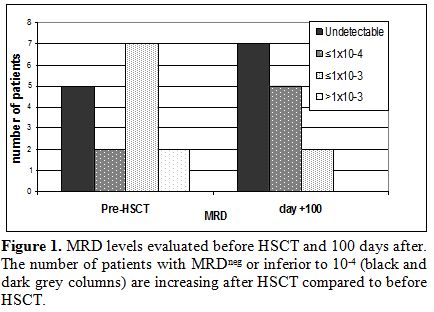 |
Figure 1. MRD levels evaluated before HSCT and 100 days after. The number of patients with MRDneg or inferior to 10-4 (black and dark grey columns) are increasing after HSCT compared to before HSCT. |
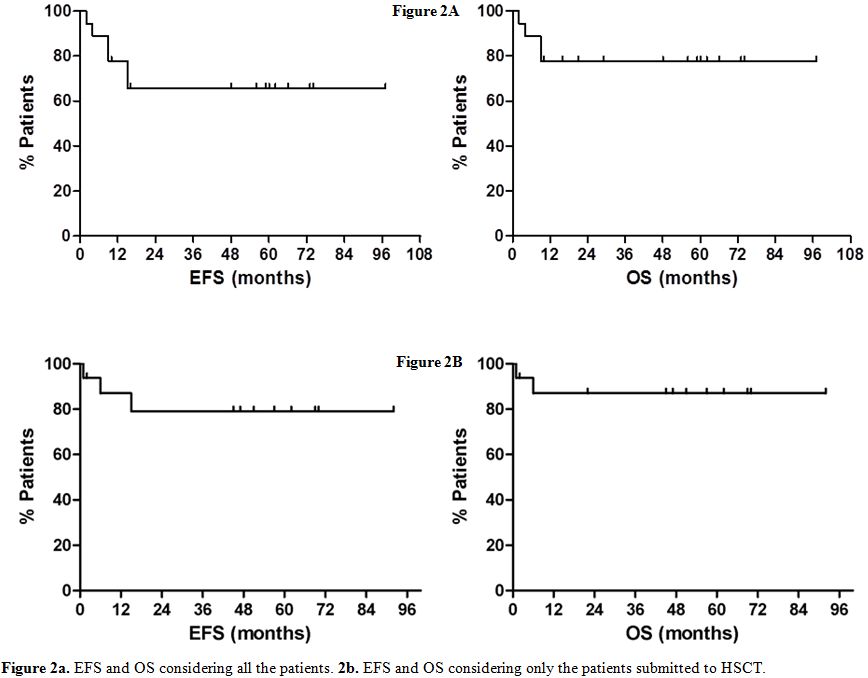 |
Figure 2a. EFS and OS considering all the patients. 2b. EFS and OS considering only the patients submitted to HSCT. |
Discussion
Our experience reflects many other literature reports, which confirm that t(4;11) and Ph+ ALL
may have a favourable outcome if patients are promptly submitted to
HSCT upon first remission. In this report we took only a cohort of
consecutive patients into consideration, who were suitable for HSCT,
since the onset, and we intentionally excluded all the others (not
eligible to HSCT), in order to evaluate the effect of a rapid
transplantation upon onset of the disease. With the limitation due to
the small number, we have observed that those patients, who underwent
HSCT, have a high cure rate. Furthermore, we considered the role of MRD
in influencing the outcome of the HSCT. Most of our patients have been
allografted with a deep molecular remission, with an MRD level <1x10-3
and all of them, except for one, showed a favourable outcome. The only
patient, who relapsed, was the only one that did not reduce again the
MRD three months after HSCT. It could be hypothesized that the graft
versus leukaemia effect has been less efficient in this case than in
the other ones. Many reports have claimed the positive impact of TKI in
Ph+ ALL, speculating on their role in giving better conditions for HSCT. In the TKI era, it seems therefore that Ph+
ALL can be allografted before, and under better state. Moreover TKI
generate a deeper remission status (in terms of MRD), which is a
prognostically favourable factor for the following HSCT. As previously
reported in the introduction session, an MRD level <10-3 is a favourable prognostic index for post HSCT outcome in not VHR-ALL who fails to obtain an MRDneg : we can suppose that also in Ph+ ALL
an MRD level below this cut-off has a favourable impact. A
similar partial conclusion may be applied for t(4;11) ALL: Indeed we
observed a good molecular response due to the intensive chemotherapy
regimens also in this case. HSCT performed promptly and in deep
molecular remission allowed a good outcome for these patients too.
In this context, a big issue is represented by all those patients who remained highly MRD+ (upper 10-3)
independently from the risk assessment at the onset: for all of them an
alternative strategy is necessary. TKI of second and third generation
for Ph+ ALL, nelarabine for T-ALL and
monoclonal antibodies (in example Blinatumomab) for Ph negative B-ALL
could be a good choice in these cases.[14]
At last, regarding Ph+ ALL who maintained a weakly MRD+100 positive (≤1x10-4)
after HSCT, we decided not to treat them with TKI but to try a rapid
tapering of immunosuppression. The goal of this strategy is to maximize
the graft versus leukaemia effect avoiding other drug administration.
Despite the limited positive experience here reported, in many cases
the only “Graft versus Leukemia” effect seems not to be sufficient to
control MRD positive in post-transplant setting. In this context, also
donor lymphocytes infusion (DLI) may be considered,[21]
also if this is a debated issue, considering the immunological escape
of lymphoblastic cells. Actually, in patients who remained or became
MRD positive after HSCT, many alternative strategies are object of
discussion and it should be taken into consideration the same options
contemplated for the patients who failed to obtain a deeper molecular
response before HSCT. For Ph positive ALL, the main option remains TKI,
particularly of second and third generation. The limitation of TKI in
this contest is the insurgence of mutations that confer resistance to
the drug during the time. Ponatinib is the powerful TKI, and the only
one active against T315I mutation, but also it has a limited duration
in time. Monoclonal antibodies such as Blinatumumab or
Inotuzumab-Ozagomicin seems to give a valid alternative particularly in
Ph negative ALL. Instead CAR-T have been so far applied only in few
experimental trials.
Conclusion
This experience seems to suggest that Ph+
ALL and t(4;11) ALL are likely to prove a favourable outcome, if
promptly submitted to HSCT, especially if the patients showing a deep
remission status, in terms of molecular MRD, soon before the allograft.
Moreover, a rapid tapering of immunosuppressive drugs seems to be
useful in MRD+100 minimally positive Ph+ ALL patients, allowing them to avoid the TKI treatment.
Extra in-depth studies are necessary to confirm these observations.
Acknowledgements
Fondazione Tettamanti and Comitato Maria Letizia Verga. Mrs Maria Cristina Facchinetti for English language consulting. .References
 .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
.  .
. [TOP]