Received: January 8, 2015
Accepted: September 9, 2015
Mediterr J Hematol Infect Dis 2015, 7(1): e2015058, DOI 10.4084/MJHID.2015.058
This article is available on PDF format at:

Ola Khorshid1, Alfred Elias Namour1, Mosaad M El-Gammal1, Tarek Yakout Mahmoud1, Catherine Fortpied1, Raafat Abdel-Malek2 and Safaa Ramadan1
1 National Cancer Institute, Cairo University, Cairo, Egypt
2 Kasr Al-Ainy school of Medicine, Cairo University

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Cladribine induces durable
complete remission (CR) in approximately 85% of hairy cell leukemia
(HCL) patients. In Egypt, cladribine is mainly used as IV continuous
infusion at a dose of 0.1 mg/kg/day for 7 days and as SC bolus
injection at a dose of 0.14 mg/kg/day for 5 days. We aimed to compare
the outcome and toxicity between these two regimens. We retrospectively
collected data from HCL patients treated at the National Cancer
Institute and its affiliated center, Nasser Institute, Cairo, Egypt.
Forty-nine patients were identified, 18 treated with the IV regimen (IV
group) and 31 with the SC regimen (SC group). Forty-one patients were
newly diagnosed. Patient characteristics were balanced across the two
groups. The CR rates in the IV and the SC group were 94% and 97%,
respectively. The main complications in the IV group and the SC were
neutropenia G3-4 (67% vs. 87%), mucositis mainly G1-2 (67% vs 32%) and
infections (mainly viral, 78% vs 34%). In the IV group, five patients
died, three of progression and infection, one of unknown cause and one
of late heart failure. In the SC group, one patient died of disease
progression and one of second cancer. After 33.5 months, median
follow-up, the 3-year event free survival was 60% and 96%, respectively
(p=0.104). The 3-year overall survival was 81% and 100%, respectively
(p=0.277). In conclusion, SC cladribine is an excellent alternative to
the IV regimen for the treatment of HCL. |
Introduction
Hairy cell leukemia (HCL) is a rare indolent B-cell leukemia. It
accounts for approximately 2% of all leukemias and is characterized by
male preponderance (M:F ratio is 4:1). The majority of patients are
usually diagnosed over the age of 40 years. Main symptoms are related
to cytopenia and/or splenomegaly.[1,2] The diagnosis
is confirmed by bone marrow evaluation with flow cytometry. A panel of
pan-B cell markers and antigens commonly expressed on hairy cells
(CD19, CD20, CD22, CD103, CD11c, CD123 and CD25) is used to diagnose
HCL cells.[1-4] Annexin A1, which is not expressed in any other small B-cell lymphoproliferation, is currently the most specific HCL marker.[1,5] Tiacci et al have identified BRAF-V600E mutation as the disease-defining genetic event among patients with classical HCL.[6,7]
Indications
for treatment are the presence of constitutional symptoms, symptoms due
to splenomegaly, significant cytopenia (absolute neutrophil count (ANC)
<1x109/L, platelet count < 100 × 109/L,
or hemoglobin level <10g/dL), recurrent infections and autoimmune
complications. Main treatment objectives are to control symptoms,
normalize blood counts, and achieve lengthy remission. Over the years
treatment options have evolved from moderate success with low-dose
chlorambucil, splenic irradiation or even anthracyclines, through
splenectomy and IFN-α, and eventually to the current treatment of
choice: the purine nucleoside analogues pentostatin and Cladribine.[1,2]
Excellent outcomes are obtained with purine analogues and true primary resistance is seldom observed.[1,2,8,9] CR can be achieved in 80–85% of patients and more than 90% of patients are alive at 10 years.[10,11] Relapse rate is about 40% in the first 5–10 years and is rare after 10 years.[1,2,10-15] Second CRs can be obtained in more than 75% of patients using the same agents.[12,15]
Cladribine
can be administered via intravenous (IV) or subcutaneous (SC) route.
The 7-day IV continuous infusion of cladribine at a dose of 0.1
mg/kg/day is considered the standard regimen in the treatment of HCL
patients.[1,2,16-20] Alternative treatment schedules such as short daily or weekly infusion.[8] The SC injection produces equal (100%) cladribine bioavailability as IV.[21,22]
Cladribine is the first treatment choice due to its convenient
administration schedules that seems to be more advantageous to both
patients and physicians.[1,2]
At the NCI-Cairo,
Egypt, the two commonly used regimens of cladrabine are IV continuous
infusion at a dose of 0.1 mg/kg/day for 7 days[10] and SC bolus injection at a dose of 0.14 mg/kg/day for 5 days.[23]
In this study, we compared outcome and toxicity among HCL patients
treated with cladribine by SC bolus injection to patients treated with
clardribine by continuous IV infusion.
Patients and Methods
Results
Clinical characteristics of patients. The relevant characteristics of patients in each treatment group are compared in Table 1. A total of 49 patients were identified: 18 patients in the IV group and 31 in the SC group. Forty-one patients were newly diagnosed and 8 were previously treated. Previous treatment was chemotherapy (n=4), interferon alpha (n=2) and splenectomy (n=2). The median age was 47, range 26-74 years and 78% were males. The clinical characteristics were overall well balanced for patients across the IV and SC groups.
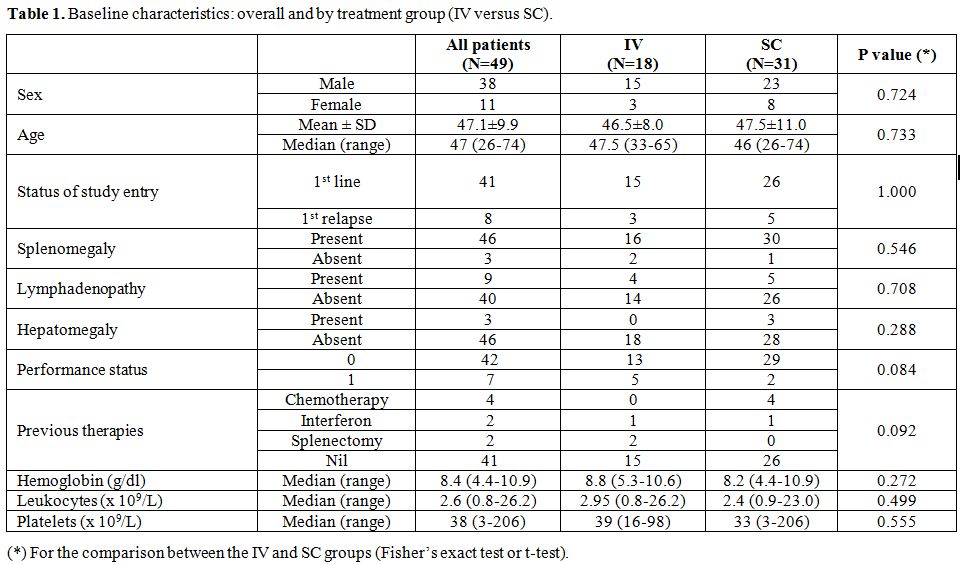 |
Table 1. Baseline characteristics: overall and by treatment group (IV versus SC). |
Responses to cladribine.
After treatment with cladribine, CR was achieved in 94% of patients
(17/18) in IV group and in 97% of patients (30/31) in the SC group (Table 2A).
At first evaluation post-treatment (months 3-4), 12 patients were still
in PR: two in the IV and 10 in the SC group. At the subsequent
evaluation, in the IV group one patient achieved CR and the other died
of disease progression and infection neutropenia. Whereas, in the SC
group, 9 patients could reach CR, 5 with no further therapy and 4
following a 2nd cycle of clardribine. The tenth patient progressed after the 2nd
cycle of clardribine and was lost to follow-up. This patient was
previously treated with combination chemotherapy and presented with
splenomegaly and lymphadenopathy.
We also did the same analysis
restricted to the subset of patients who received cladribine at onset
of disease and with single cladribine course in the IV and SC group. CR
rates were not different between the two groups, but again time to CR
was longer in the SC group. CR rates at week 12 and at subsequent
evaluation were 64% and 100% in the SC group, while it was 93% at the
two time points in the IV group (Table 2B).
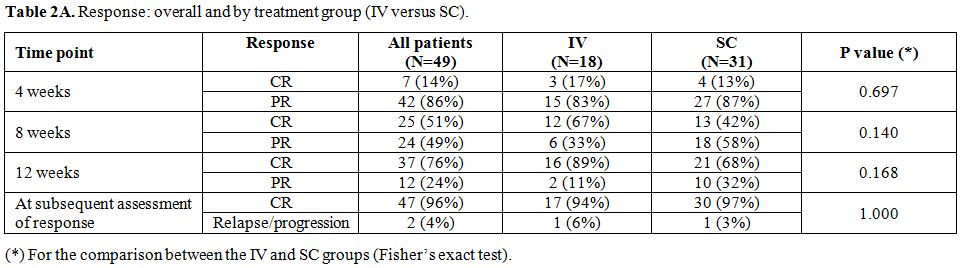 |
Table 2A. Response: overall and by treatment group (IV versus SC). |
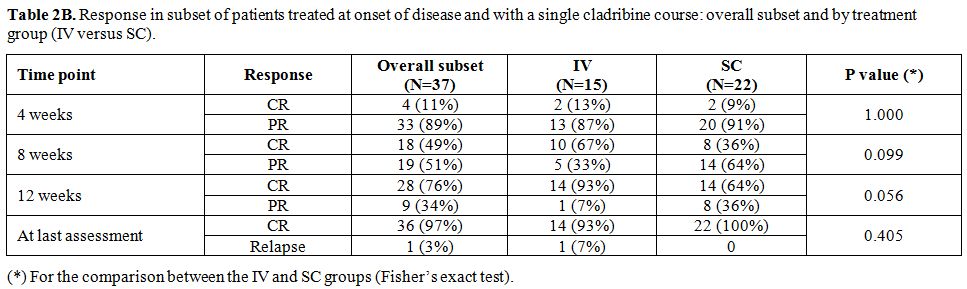 |
Table 2B. Response in subset of patients treated at onset of disease and with a single cladribine course: overall subset and by treatment group (IV versus SC). |
Toxicities. Blood values at diagnosis and at day 10 (nadir) and non-hematologic complications are presented in Table 3A and B.
At day 10, there was no difference in the changes in the median blood
values between the two groups. Neutropenia G3-4 in the IV group and the
SC occurred in 67% (n=12) and 87% (n=27) of patients, respectively.
Mucositis was significantly more in the IV group compared to the SC
group: 67% (n=12) vs 32% (n=10) P <0.001, respectively. Three
patients in the IV group had G3 mucositis and all other patients had
GI-II mucositis.
As regard to fever and infectious
complications, the rates were 78% (n=14) and 34% (n=11), respectively.
Infections occurred mainly within 10 weeks after treatment. In most
patients, the infectious agent couldn’t be identified. However, herpes
simplex infection was documented in 6 patients (5 in the IV group and 1
and the SC group). In each group, one patient suffered from fungal
infection. Fever without any identified source of infection was
observed in 4 patients in the IV and in 7 in the SC group.
In the SC group and following the 2nd cycle of treatment one patient had mild bilateral foot drop and one had mild shoulder tendinitis.
Other
non-hematologic toxicities were mild (G1-2) and in the form of nausea,
vomiting, elevated liver enzymes, cough, headache and bony pains.
Late
toxicities included heart failure in one patient in the IV group and
second cancer in one patient in the SC group. Causes of death in the IV
and SC groups are summarized in Table 3C and are detailed below.
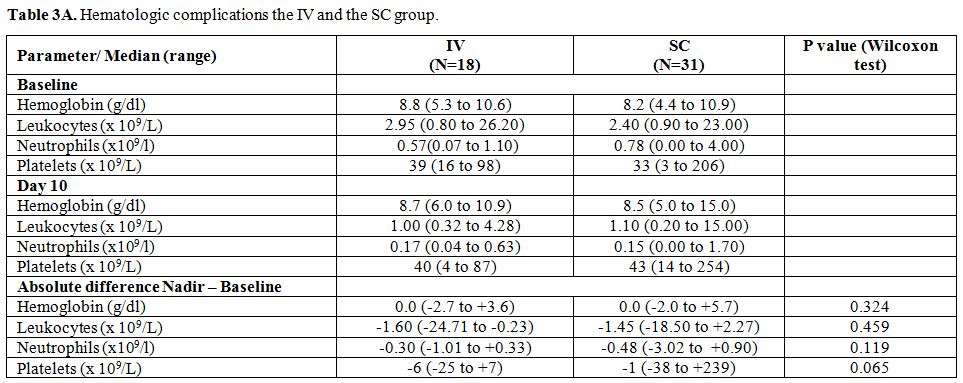 |
Table 3A. Cladribine toxicity in the IV and the SC. Hematologic complications the IV and the SC group. |
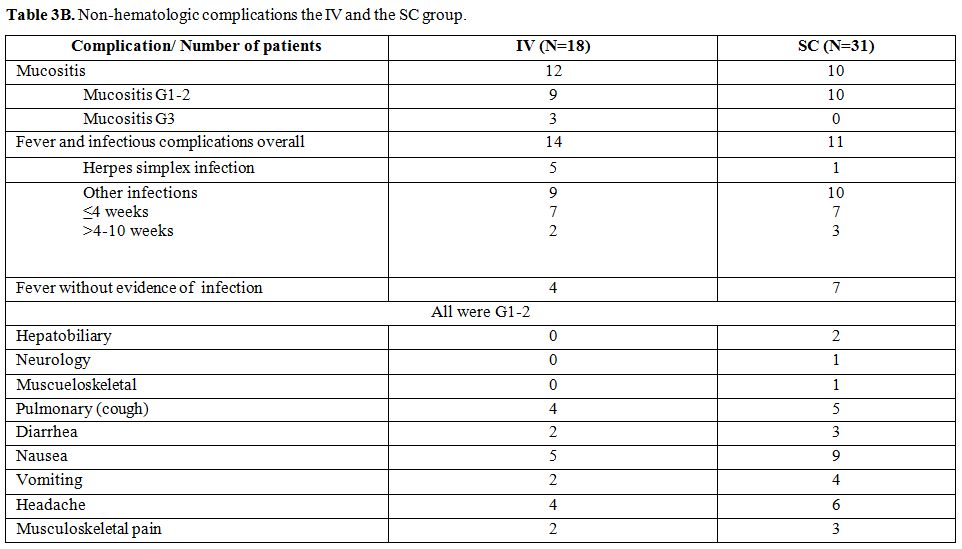 |
Table 3B. Cladribine toxicity in the IV and the SC. Non-hematologic complications the IV and the SC group. |
 |
Table 3C. Cladribine toxicity in the IV and the SC. Causes of death in the IV and the SC group. |
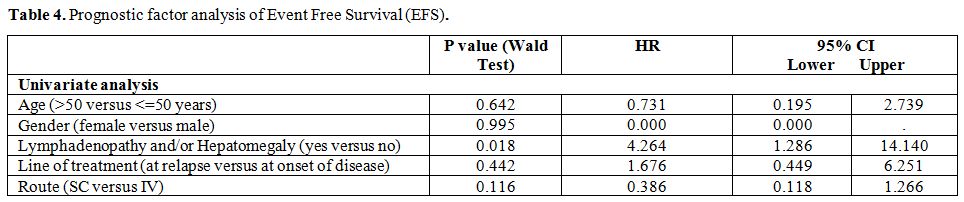 |
Table 4. Prognostic factor analysis of Event Free Survival (EFS). |
| Figure 2. EFS of the IV and SC cladribine group. |
| Figure 3. OS of the IV and the SC group. |
Discussion
To our knowledge, this study that compared HCL patients treated with
SC cladribine vs IV cladribine is the first from a developing country.
We reported the clinical features and the outcome of 49 Egyptian HCL
patients treated with cladribine in the period between 2004 and 2010.
The clinical presentation of patients in this series is similar to the
literature.[1] The results show that SC cladribine is
safe and produces a remission rate similar to the IV route.
Importantly, the two regimens resulted in high EFS. The SC route was
associated with non-significant but important longer DFS (median 63 vs
53 months and 3-year EFS 96% vs 60%). EFS than the IV regimen.
The
overall CR rate of this series was 96% which matches well to CR rates
reported in previous studies which ranged between 87%-100%.[1,2] Interestingly, the time to achieve CR was longer in the SC group (table 2A and B),
and 5 patients required a second course of cladribine to reach CR.
While 89% of patients were in CR at first evaluation in the IV group,
only 68% of the SC group reached CR. At the subsequent assessment the
rates improved to 94% and 97%, respectively. Studies investigating
the efficacy of the 7 day and 5 day regimens of SC cladribine reported
CR rates of 81% and 76%, respectively.[23,26] Therefore, in this study, the estimated CR rate is very encouraging for SC cladribine.
Cladribine is a generally well-tolerated[1,2,23,26-29] drug, and cytopenia and culture negative fever are the most commonly expected side effects.[1,2,27] Infections are also a common toxicity and occur mainly during the first weeks of treatment along with cytopenia.[1,2,27]
In a review by Maevis et al, the frequency of fever among HCL patients
treated with cladribine ranged between 40–69%, and not all fevers were
associated with signs of infection.[2] The authors suggested that fever may be a sign of cytokine release in HC rather than infection in some patients.[2]
In accordance, the most frequent toxicity in this study was a
myelosuppression (approximately 80% of patients). Fever (with or
without signs of infection) was seen in 35% of all patients, and
infections were diagnosed mainly in the first 10 weeks post-therapy.
Nevertheless, infectious episodes (mainly herpes simplex) and mucositis
were more frequent in the IV group.
A number of investigators have indicated that the risk of developing second malignancy is higher in HCL patients.[18,30]
Whether this is related to common genetic susceptibility or due to
treatment exposure is not known. In a study of 358 patients with HCL
treated with cladribine, 8% (27/358) developed second tumors.[18]
According to the Surveillance, Epidemiology and End Results (SEER)
data, of 3104 patients with HCL, the incidence of second cancer was 32%
compared to 23% in the general population (SIR=1.2; 95% CI 1.1-1.4 for
all, 6.6 for Hodgkin lymphoma, 5.0 for non-Hodgkin lymphoma and 3.6 for
thyroid cancer.[30] In our study, one patient (2%)
died of diffuse large B cell lymphoma 70 months after CDA therapy.
Without longer follow up, analysis of late effects such as second
cancers will not be valuable.
In a recent literature review, the 5-year PFS of patients treated with cladribine was found to be in the range of 72–84%.[2]
In correspondence to this range, the overall 3-year EFS of patients in
this report was 77%. However, patients in the IV group experienced more
failures than patients in the SC group (3-year EFS was 60% vs 96%) and
median EFS was shorter in patients in the IV than in the SC group (53
vs 63 months, respectively). This trend did not reach statistical
significance in the univariate analysis. A number of studied evaluated
SC cladribine as alternative to the IV route. In one study no relapse
was seen after 20 months follow up of 73 HCL patients treated with the
7 day SC schedule.[26] In the second, 62 patients received the 5 day SC regimen and the 18 months-EFS was 68%.[23]
A comparison between the different results is not possible due to
variability of end point definitions and methods on analysis.
Different prognostic factors have been previously described, such as cytopenia[1,12,13] and lymphadenopathy at presentation[14,31] and response to purine analogues.[12,14,25,29] Two main studies indicated response to purine analogs as the most significant factor for DFS.[12,25]
Therefore we compared EFS between patients in CR and patients in PR and
no statistically significant difference between the two subgroups was
found.
Albeit non-significant, the 3-year OS for patients
treated with the SC regimen was 100% compared to 81% in patients
treated with the IV regimen. This is very supportive to the
effectiveness of the SC regimen. Main causes of death in the IV group
were complications related to either disease progression or treatment
re-challenge.
Being a rare condition, conducting prospective
studies in HCL is a real challenge. Limitations to retrospective
studies include heterogeneity in data recording and data collection as
well as variability in treatment decisions among centers. However, data
collected from single center are usually more homogenous as is the case
of the current series. Therefore, this study would provide valuable
information on such rare disease.
In conclusion, this is the
first report of a large series of Egyptian HCL patients comparing the
SC to the IV cladribine regimen. Our data confirms that SC cladribine
is an excellent alternative to the IV regimen. SC cladribine is well
tolerated and is associated with favorable EFS. Together with the
convenience of the SC route, this regimen should be recommended as of
choice to treat HCL patients in institutes with limited resources such
as those in developing countries.
References























 .
. 






[TOP]