Received: August 14, 2015
Accepted: November 26, 2015
Mediterr J Hematol Infect Dis 2016, 8(1): e2016009, DOI 10.4084/MJHID.2016.009
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Objectives: Low-dose
cytarabine (LD-AraC) is still regarded as the standard of care in
elderly patients with acute myeloid leukemia (AML) ‘unfit’ for
intensive chemotherapy. In this study, we reported our experience with
LD-AraC in patients ≥ 70 years old and compared the results to those of
intensive chemotherapy, best supportive care (BSC), or hypomethylating
agents in the same age population. Methods: Between 2000 and 2014, 60 patients received LD-AraC at 20 mg once or twice daily by subcutaneous injection for 10 consecutive days every 4-6 weeks. Results: Complete remission rate with LD-AraC was 7% versus 56% with intensive chemotherapy and 21% with hypomethylating agents. Median overall survival (OS) of patients treated with LD-AraC was 9.6 months with 3-year OS of 12%. Survival with LD-AraC was better than with BSC only (P=0.001). Although not statistically significant, intensive chemotherapy and hypomethylating agents tended to be better than LD-AraC in terms of OS (median: 12.4 months and 16.1 months, respectively). There was no clear evidence that a beneficial effect of LD-AraC was restricted to any particular subtype of patients, except for cytogenetics. There was a trend for a better OS in LD-AraC treated patients in the setting of clinical trials as compared with those treated outside of a clinical trial. Conclusions: Despite a trend in favor of intensive chemotherapy and hypomethylating agents over LD-AraC, no real significant advantage could be demonstrated, while LD-AraC showed a significant advantage comparatively to BSC. All this tends to confirm that LD-AraC can still represent a baseline against which new promising agents may be compared either alone or in combination. |
Introduction
Despite multiple advances in AML therapy, the treatment outcome for
older patients with acute myeloid leukemia (AML) is unsatisfactory,
especially for patients in their latter years. As people are living
longer, the incidence of AML is increasing. The treatment outcome for
patients aged 70 years or older has not improved significantly over the
last two decades in spite of improved supportive care. Most of these
patients do not receive intensive chemotherapy either because they
decline or because they are not considered fit enough for such therapy.
The basis on which patients are not considered fit enough for intensive
chemotherapy remains not clear and varies considerably from one
investigator to another. Clearly performance status remains an
important factor in therapy planning. However, evaluation of ‘fitness’
remains unclear. Recent reports have shown that geriatric assessment
methods, with a focus on cognitive and physical function, improve risk
stratification and may inform interventions to improve outcomes for
older AML patients.[1] Others showed that candidacy for intensive therapy should be based on biological features of disease rather than on age.[2]
Although
low-dose cytarabine (LD-AraC) has not been adopted universally, it
still represents a treatment reference (at least in Europe) for
patients considered ‘unfit’ for intensive chemotherapy. LD-AraC was
investigated extensively more than 20 years ago. LD-AraC has been used
in various schedules showing responses that included complete remission
(CR).[3-9] Its mechanism of action is still not
completely clear, acting potentially through cytotoxic action and/or
through induction of apoptosis by differentiation induction.[10,11]
LD-AraC is relatively well tolerated and can be given in an outpatient
care setting. However, it can induce excess cytopenia although this may
be a prerequisite for efficacy. In the literature, 10% to 20% of
patients have been reported to achieve CR.[3-9,12]
Randomized studies between intensive and non-intensive treatments
showed better responses in intensively treated patients, but no
significant differences in terms of survival.[13,14]
LD-AraC has been demonstrated to be more beneficial than best
supportive care and hydroxyurea among patients not fit for intensive
therapy, although fitness was not defined for patients’ age >70
years.[12] LD-AraC therapy still represents a
baseline against which novel drugs may be compared either alone or in
addition to LD-AraC. Currently, the role of lower-intensity regimens is
under active investigations.[12,15-19]
Recent randomized trials comparing DNA hypomethylating agents,
azacitidine or decitabine, with LD-AraC found improved CR rates and
better survival with hypomethylating agents.[15,19]
These
studies urged us on analyzing our series of elderly patients treated
with LD-AraC with the aim to evaluate whether this treatment could
still represent a standard therapy in this patient population to which
new treatments should be compared. We, therefore, evaluated the
efficacy of LD-AraC, in a single institution experience, in patients
aged 70 years or older, and compared it to that of other treatments
received by patients of the same age (intensive chemotherapy, best
supportive care (BSC), and lower intensity therapy based on
hypomethylating agents).
Patients and Methods
Patients:
In total, 234 patients (aged 70 years or older) with newly diagnosed
AML have been seen in the Department of Hematology at Lyon-University
Hospital from 2000 to 2014. From 2000 to 2006, patients with PS ≤2
were considered ‘fit’ by the local physician and received an intensive
treatment approach systematically. No specific criteria for defining
‘fitness’ were used. After 2006, a more ‘personalized’ treatment using
either intensive chemotherapy or lower-intensity therapies (including
LD-AraC, decitabine or azacitidine) based on the clinical judgment of
the treating physician and the availability of clinical trials were
proposed.[20] Most patients older than 70 years
received, therefore, a non-intensive option. Any type of AML (de novo
or secondary) was considered. Acute promyelocytic leukemia and blast
transformation of chronic myeloid leukemia were excluded. Among the 234
patients (aged 70 years or older) with newly diagnosed AML, 60 patients
(16%) received LD-AraC. They were compared to 85 patients treated with
intensive therapy (anthracycline- and cytarabine-based chemotherapy),
34 patients treated with hypomethylating agents (12 by decitabine and
22 by azacitidine), and 43 patients receiving only BSC. The 12
remaining patients received other treatments in the setting of
investigational trials and were not considered for the study.
Treatment:
On entry, patients received LD-AraC 20 mg once or twice daily
(according to physician’s choice) by subcutaneous (sc) injection for 10
consecutive days. Subsequent courses of LD-AraC were administered at
intervals of 4 to 6 weeks. Regarding the control groups, intensive
chemotherapy consisted of a combination of intermediate-dose cytarabine
with an anthracycline. Azacitidine was given at the dose of 75 mg/m2/day for 7 consecutive days by sc injection, and decitabine was administered by intravenous route once daily at 20 mg/m2
for 5 consecutive days. Subsequent courses of these low-intensity
treatments were administered at intervals of 4 to 6 weeks until disease
progression. All clinical trials received approval from the
institutional review board and were conducted in accordance with the
Declaration of Helsinki. All participants gave their written informed
consent. Policies with regard to blood product support, antibiotic and
anti-fungal prophylaxis, and treatment of febrile neutropenia were
determined by established local practice. BSC consisted only in the
application of these policies plus eventually the administration of
hydroxyurea in order to control white blood cell (WBC) count in case of
the proliferative disease. Patients receiving intensive chemotherapy
were systematically hospitalized for induction chemotherapy (median
hospitalization duration: 36 days) and consolidation chemotherapy
courses. Blood product transfusions were systematically administered
when hemoglobin was ≤80 g/l and platelets ≤20x109/l.
Requirements for transfusions were the same for patients treated with
lower intensity therapies (LD-AraC or hypomethylating agents) while
platelets were only transfused to patients with bleedings in the case
of treatment by BSC alone. Hospitalization was reserved for patients
with infectious complications or other severe complications for
patients belonging to two last groups of treatment.
Endpoints:
CR was defined by bone marrow aspiration, which was required to consist
of more than 50% normal cellularity with evidence of trilineage
maturation and less than 5% bone marrow blasts, no evidence of
extramedullary disease, and regeneration of the peripheral neutrophil
count to 1.0x109/l and the platelet count to 100x109/l.
The persistence of myelodysplastic features did not exclude the
diagnosis of CR. Response to therapy was evaluated after one or two
courses for patients treated with intensive chemotherapy, and after 4
to 6 courses for those treated by lower-intensity treatments. Overall
survival (OS) was the primary endpoint. It defines the time from
starting treatment to death from any cause. For remitters, disease-free
survival (DFS) is the time from CR to first event (recurrence or death
in CR).
Statistical analyses:
Surviving patients were censored at the end of September 2014 when
follow-up was up to date for 95% of patients. Descriptive statistics
was used to characterize patients and their disease. Categorical
variables were compared between treatment options by Fischer exact
tests. Continuous variables were analyzed by parametric tests (t tests)
or nonparametric tests (Wilcoxon) as appropriate. Estimated
probabilities of survival were calculated using the Kaplan-Meier
method, and the log-rank test evaluated differences between survival
distributions. All variables tested by univariate analyzes were
included in the multivariate analysis. Multivariate analyzes used the
Cox proportional hazard method for survival. Hazard ratios (HRs) with
95% confidence intervals (CIs) were calculated for the main endpoint.
An HR <1 indicated a benefit for one factor over another. All P
values are two-tailed, with a P value ≤0.05 considered statistically
significant. Computations were performed using BMDP PC-90 statistical
program (BMDP Statistical Software, Los Angeles, CA, USA).
Results
Between 2000 and 2014, 60 patients (aged 70 years or older) with newly diagnosed AML, including 35 de novo AML and 25 secondary AML, were treated in our Institution by LD-AraC. Characteristics and outcome of these patients were compared to those of patients treated during the same period of time by intensive chemotherapy (85 patients), hypomethylating agents (34 patients), or only BSC (43 patients). The characteristics of patients, split by the treatment type at onset, are provided in Table 1, which shows, as expected, differences according to the distinct therapeutic approaches.
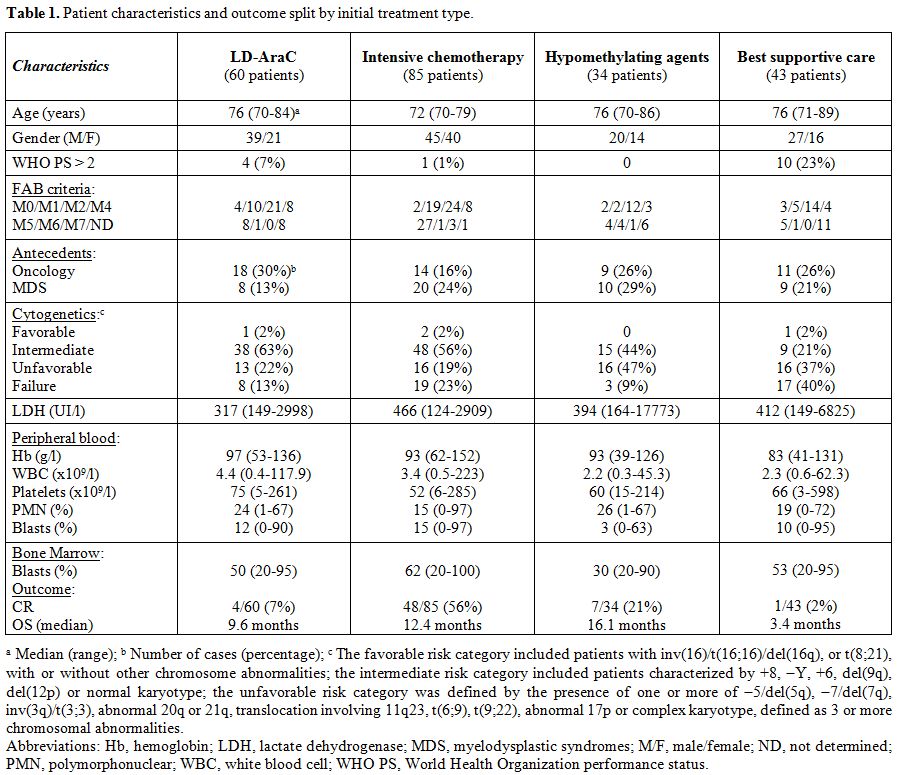 |
Table 1. Patient characteristics and outcome split by initial treatment type. |
The median number of treatment courses given was 5 for
LD-AraC (range: 1 – 20+) with a median length of treatment of 5.1
months (range: 0.9 – 28+ months). The overall CR rate of patients
treated with LD-AraC was 7% (4 of the 60 patients). The median number
of courses to achieve CR was 4 (range, 3-9 courses). The CR rate was
significantly better in patients treated by intensive chemotherapy
(48/85 patients; 56%) (P<0.0001) and in patients treated by
hypomethylating agents (7/34 patients; 21%) (P=0.09). Median OS of
patients treated with LD-AraC was 9.6 months (95% CI, 5.8-13.5 months)
with 3-year OS of 12%.
Survival with LD-AraC was better than that with BSC only (median OS: 9.6 months vs. 3.4 months; P=0.001) (Figure 1).
Although not statistically significant, intensive chemotherapy tended
to be better than LD-AraC in terms of OS (median OS: 12.4 months vs. 9.6 months; 3-year OS: 27% vs. 12%; P=0.07) (Figure 2).
However, differences in favor of intensive chemotherapy were only
confirmed for patients aged less than 75 years (median: 12.7 months vs. 9.2 months; 3-year OS: 28% vs. 10%). In patients aged ≥ 75 years, median OS was better with LD-AraC (9.6 months vs.
2.8 months). Although there was a trend for better results with
hypomethylating agents, no significant differences were observed when
compared with LD-AraC (median OS: 16.1 months with hypomethylating
agents vs. 9.6 months with LD-AraC; 3-year OS: 22% vs.
12%; P=0.1) (Figure 3). In a multivariate analysis including
cytogenetics (unfavorable vs. intermediate/favorable risk), age (<75
years vs. ≥75
years), de novo or secondary AML, and the type of treatment, only
cytogenetics was of prognostic value (HR, 1.93; 95% CI, 1.50-2.47; P
<0.001).
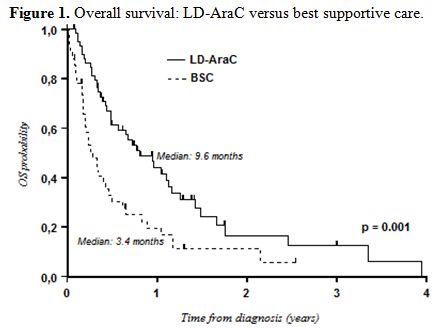 |
Figure 1. Overall survival: LD-AraC versus best supportive care. |
| Figure 2. Overall survival: LD-AraC versus intensive chemotherapy. |
| Figure 3. Overall survival: LD-AraC versus hypomethylating agents. |
There was no clear evidence that a beneficial effect of LD-AraC was restricted to any particular subtype of patients. In the univariate analysis, similar treatment effects were observed for all ages (<75 years vs ≥75 years) (median OS: 9.2 months vs 9.6 months; P=0.92), WHO PS (0-2 vs >2) (median OS: 9.6 months vs 9.2 months; P=0.63), bone marrow blastic infiltration at diagnosis (≤ 30% vs > 30%) (median OS: 17.7 months vs 9.2 months; P=0.15), initial WBC count (≤ 10x109/l vs > 10x109/l) (median OS: 11.5 months vs 4.7 months; P=0.35), and secondary AML (prior history of MDS or cancer vs no antecedents) (median OS: 5.8 months vs 13.5 months; P=0.08). There was only a significant difference regarding initial cytogenetics (favorable and intermediate-risk vs unfavorable-risk) (median OS: 11.4 months vs 4.3 months; P=0.03). In the multivariate analysis in a model taking into account all these factors, only initial cytogenetics and secondary AML appeared of prognostic value (Table 2).
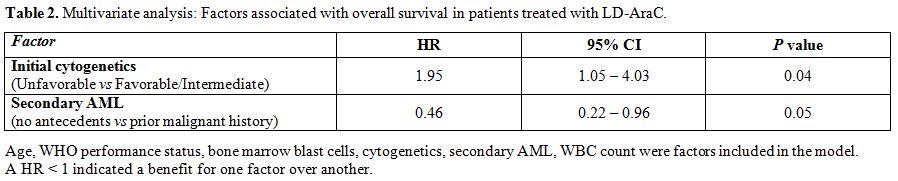 |
Table 2. Multivariate analysis: Factors associated with overall survival in patients treated with LD-AraC. |
Of the patients who received LD-AraC, 24 patients were
treated inside clinical trials, while 36 patients were not. There were
no substantial differences between those patients with respect to blood
product support, hospitalization, and days on antibiotics. However, the
clinical trials required significantly more day care visits for
patients. Median OS was 13.2 months (95% CI, 8.6-15.1 months) for
patients included in clinical trials vs 7.8 months (95% CI, 4.3-11.5
months) for those not included, with 3-year OS of 18% and 9%,
respectively (P=0.21) (Figure 4).
There were no significant differences in terms of survival between
patients receiving LD-AraC at 20 mg per day and those receiving 20 mg
twice a day.
Most of the patients (92%) upon failure after LD-AraC
therapy received only BSC with eventually a combination of
6-mercaptopurine with oral methotrexate. Five patients received a
second line therapy: one with azacitidine and 4 with a new drug inside
a phase 1 investigational trial.
| Figure 4. Overall survival: LD-AraC: Comparison between patients included into clinical trials and those not included into clinical trials. |
Discussion
Overall, despite a trend in favor of intensive chemotherapy and
hypomethylating agents over LD-AraC, no real significant advantage
could be demonstrated in terms of OS, while LD-AraC showed a
significant advantage comparatively to BSC. CR rates were higher with
intensive chemotherapy or treatment by hypomethylating agents than with
LD-AraC, but this did not translate into a significant benefit in terms
of OS. The old principle of achieving a CR with intensive chemotherapy
to convey a favorable outcome might not apply to this patient
population, for which OS and quality of life represent the most
relevant endpoints. However, one recent study regarding the quality of
life beyond 6 months after diagnosis in this patient population showed
that achievement of CR is associated with improvements in global
health, physical function, and role function without negatively
affecting other health domains.[21] In our series,
the prolonged OS contrasting with the low CR rate after treatment by
LD-AraC could be explained by the pursuit of treatment as long as the
disease was controlled and that the treatment was considered beneficial
for the patient and the selection of patients with the orientation of
frailer patients directly to BSC alone. This approach explained the
higher median number of treatment courses given in our study
comparatively to the median number of courses given in previous
studies.[15,19,22]
The same therapeutic behavior applied to hypomethylating agents can
also explain the differences between our findings and the recently
published studies of decitabine and azacitidine in elderly AML
patients.[15,19]
Our results with LD-AraC showed lower CR rates but a median OS better than those observed in previous studies.[4,12,23]
Differences in terms of CR rates could be explained by the different
schedules used. The response to LD-AraC appears dose dependent. Burnett
et al., who reported 18% to 21% of CR rate, used AraC at 20 mg twice
daily for 10 consecutive days,[12,22] while Rodriguez and Tilly, who observed 28%[2] to 32%[4]
of CR, used LD-AraC for a longer period of time (cycles of 21 days).
Theoretically, patients who achieved CR have a better median survival
compared with those who did not achieve CR. However, higher doses or
longer duration schedules are associated with a longer period of
hypoplasia. Actually our better results in terms of median OS could be
explained by a higher rate of severe toxicity in schedules with higher
doses and longer treatment[4,23] and also by recent improvements in terms of supportive care.[20]
Supportive care improvements over the last decade were also evidenced
by the difference in outcome between our series and that published by
the M.D. Anderson Cancer Center few years ago regarding patients
aged ≥70 years receiving intensive chemotherapy.[24]
They reported about patients treated between 1990 and 2008 and found
45% of CR and a median OS of only 4.6 months. This datum is close to
the results we previously reported during the same period of time,[20]
while our current series of patients treated with intensive
chemotherapy showed a significant improvement in higher CR rates and
longer survival, in relationship with improvements in supportive care.
An important point from our study was a tendency for better OS in
patients treated inside clinical trials comparatively to those who were
not. This datum stresses one more time on the importance of a regular
follow-up and on supportive care in this patient population, and can
explain the better survival with LD-AraC in our series as compared to
previous ones.[4,12,23]
The
strength of our study is that this is a report of treatment with
LD-AraC involving only elderly AML patients aged ≥ 70 years and,
therefore, reporting on a relatively homogeneous cohort of patients.
Our study, however, suffers from limitations. As expected, patient
characteristics varied significantly among the four groups of
treatment. Given the nature of clinical practice, it is conceivable
that relatively fit patients were treated with intensive chemotherapy
while less fit were offered LD-AraC or hypomethylating agents, and
frail patients received only BSC. Other limitations mainly concerned
the retrospective profile of the study with unbalanced distribution of
the treatment options, the small size of the cohorts, the absence of
data regarding comorbidities (such as diabetes, high blood pressure, or
cardiac pathology), absence of any quality of life questionnaire, and
the under-representation of patients who received intensive
chemotherapy during the last period of study while the hypomethylation
cohort belongs mainly to this same recent period. The main goal of our
study was to report the results of LD-AraC therapy in the real life of
one hematology center. In this setting, comparisons among treatments
used in this patient population were authorized, although involving
unbalanced groups.
Although a benefit in OS has been demonstrated
with LD-AraC compared with BSC, outcome with LD-AraC remains
unsatisfactory. A true step forward in the treatment of AML in elderly
patients can be expected from the development of more effective
therapies and the further improvement of supportive measures. Recently,
a lower-intensity, prolonged-therapy program testing clofarabine and
LD-AraC alternating with decitabine was well tolerated and highly
effective in older patients with AML.[25] Hypomethylating agents also represent a promising alternative to intensive chemotherapy in this patient population.[15,19]
On the basis of our findings, LD-AraC did not show sufficient evidence
of benefit over hypomethylating agents to be considered again as the
standard treatment for this patient population, but can still represent
a baseline against which new promising agents may be compared either
alone or in combination.[22]
Author contributions
MH interpreted the data, and drafted the manuscript; ME collected the data and provided technical support; IT was responsible for coordinating cytogenetics; AP was responsible for coordinating morphological data; FB, HL, SD, MM, FN, CP, and EW included patients; GS gave final approval; XT included patients, collected the data, conducted the statistical analysis, interpreted the data, and wrote the manuscript.
References
























[TOP]