Received: February 28, 2016
Accepted: May 17, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016028, DOI 10.4084/MJHID.2016.028
This article is available on PDF format at:


| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background: Concurrent
infection with multiple pathogens is common in tropics, posing
diagnostic and treatment challenges. Although co-infections of dengue,
malaria, leptospirosis and typhoid in various combinations have been
described, data on dengue and scrub typhus co-infection is distinctly
limited. Methodology: This study was a retrospective analysis of dengue and scrub typhus co-infection diagnosed between January 2010 and July 2014 at a tertiary care center. Clinical and laboratory features of these cases were compared with age and gender-matched patients with isolated dengue fever and isolated scrub typhus. Positive test for dengue non-structural 1 (NS1) antigen was considered diagnostic of dengue whereas scrub typhus was diagnosed by IgM scrub antibodies demonstrated by ELISA. Results: There were 6 cases of dengue-scrub co-infection during the review period which fitted clinical and laboratory profile with a mean age of 42.5 years. Fever, headache, and arthralgia were common. Normal hemoglobin, significant thrombocytopenia, transaminitis, and hypoalbuminemia were identified in these patients. Compared to patients with isolated dengue, those with co-infection had higher pulse rate, lower systolic blood pressure, normal leucocyte counts, higher levels of liver enzymes, greater prolongation of partial thromboplastin time (aPTT) and lower serum albumin. Co-infection was characterized by a lower nadir platelet count compared to scrub typhus, and lesser time to nadir platelet count and longer duration of hospital stay compared to either isolated dengue or scrub typhus. Conclusion: Dengue-scrub typhus co-infection may be under-diagnosed in tropics, particularly confounded during dengue epidemics. Normal leukocyte counts, early drop in platelets and hypoalbuminemia in dengue patients could be clues to concurrent scrub typhus infection. Prompt recognition and treatment of scrub typhus in such cases may reduce unnecessary hospital stay and cost. |
Introduction
Dengue, a mosquito-borne viral infection and scrub typhus, a mite-borne rickettsial infection are common causes of acute febrile illness in the tropics. During the seasonal increase in dengue cases, concomitant scrub typhus infection in endemic areas can cause a diagnostic dilemma. Both diseases have several clinical and laboratory features in common, including rash, thrombocytopenia, and hepatic dysfunction. However, concurrent infection with both pathogens is exceedingly rare, primarily due to the different vectors involved. We aim to describe the clinical and laboratory features of dengue-scrub typhus co-infection from a tertiary hospital along the southern coast of India.
Methods
We reviewed medical records from January 2010 to July 2014 for
concomitant diagnosis of dengue fever and scrub typhus. Fourteen
records were identified with this combined diagnosis. However, in 6 of
them, the diagnosis of scrub typhus was based on clinical features and
the presence of an eschar. ELISA for IgM antibodies to Orientia tsutsugamushi (O. tsutsugamushi)
was not done in these cases. Of the remaining 8 cases, 2 lacked
complete clinical and laboratory details. Finally, 6 cases of
dengue-scrub typhus co-infection fitted the criteria of inclusion for
the analysis. In all these cases, dengue fever had been confirmed by a
positive test for Non-structural 1 (NS1) antigen (J Mitra & Co, New
Delhi) with or without IgM antibodies to the virus. Scrub typhus had
been diagnosed based on compatible clinico-laboratory features and a
positive test for IgM antibodies to Orientia tsutsugamushi
by ELISA (Pan Bio-USA). The cut off had been calculated based on the
standard curve for the kit determined by the Receiver Operative
Characteristic (ROC) curves obtained from testing sera of three groups
of individuals. Testing was done on 100 serum samples each, of the
known scrub typhus positive group, sera from patients with other
febrile illnesses (malaria, enteric fever, dengue and urinary tract
infection) and sera from normal healthy volunteers. The results thus
obtained were used to determine the cutoff to detect positive and
negative results. The clinical protocol was fever, with or without an
eschar, respiratory involvement in the form of pneumonitis and a strong
history of outdoor activities in the recent past.
Eighteen age and
gender-matched patients with a diagnosis of isolated dengue fever were
also analyzed. Clinical characteristics including duration of fever,
headache, presence of rash, arthralgia and organomegaly were noted.
Laboratory findings such as blood counts, liver functions, renal
functions and coagulation profile were also determined. Serial blood
counts were available in all cases till discharge and these were used
to identify lowest platelet count and time to attainment of nadir
count. Other investigation results were collected based on tests
ordered at or within 24 hours of admission. Similarly, clinical and
laboratory characteristics of another eighteen age and gender matched
individuals diagnosed with isolated scrub typhus were also determined.
These were compared with patients having dengue-scrub typhus
co-infection.
Statistical analysis:
Descriptive methods were used to present data related to clinical
features of patients. One way ANOVA was done to compare means of the
three groups for normally distributed variables and Tukey HSD method
for multiple comparisons. For non-normally distributed variables the
medians were compared using Kruskal-Wallis test with post hoc test for
multiple comparisons. Chi-square test was performed to determine the
association between categorical variables. All tests were two-sided.
Differences were considered significant if the p-value was less than
0.05.
Ethics statement:
The Institute Ethics Committee (IEC) of the Pondicherry Institute of
Medical Sciences approved and permitted waiver of consent for the study
as this was a retrospective data analysis and did not directly involve
patients. All data analyzed were anonymized.
Results
During the period between January 2010 and July 2014, there were 6 cases with a confirmed diagnosis of dengue and scrub typhus co-infection. The mean age of the patients was 42.5 years, and 67% were males. Headache was the most common symptom apart from fever. Fever was present in all patients at admission and median duration of fever at the time of admission was 5.5 days. Arthralgia was seen in a significant number of patients (50%). Rash was observed in 2 (33%) patients, and it appeared as a diffuse blanching erythematous rash with no documentation of petechiae or purpura. Eschar was noted in 33% patients (one in the groin and other in the left axilla). Hepatosplenomegaly was uncommon (Table 1).
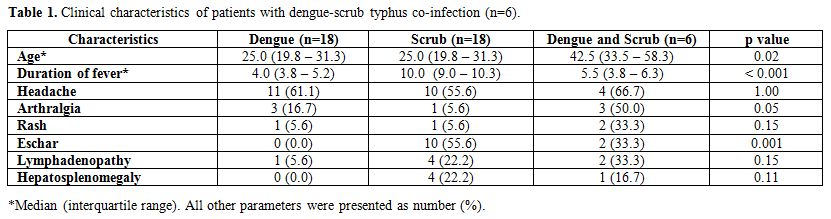 |
Table 1. Clinical characteristics of patients with dengue-scrub typhus co-infection (n=6). |
The mean hemoglobin of the patients was 10.6 g/dL. Only one
patient had elevated total leukocyte counts, whereas all others had
normal counts or leucopenia. The median lowest (nadir) platelet count
was 21.7x109 cells/L. Liver functions
revealed transaminitis in all patients, and elevation of AST and ALT
was more than three times the upper limit of normal in 83% of patients.
AST/ALT ratio was more than one in all patients. Mild hypoalbuminemia
was observed (mean serum albumin – 3g/dl).
Comparison of clinical and laboratory parameters:
We compared the clinical and laboratory characteristics of patients
with dengue-scrub co-infection, with the isolated forms of dengue
fever, and scrub typhus. Among the clinical variables, patients with
co-infection had a significantly higher pulse rate compared to those
with only dengue fever (97.0±7.6 vs 81.2±11.9; p=0.01). Median systolic
blood pressure was lower in patients with co-infection [99 (94–107) vs
118 (110–130) mm Hg; p=0.01].
Patients with co-infection had normal mean leucocyte counts (8.8x109 cells/L) compared to leucopenia (3.9x109/L; p<0.001) in dengue fever and mild leukocytosis in scrub typhus (11.6x109/L;
p=0.01). Aspartate aminotransferase (AST) and alanine aminotransferase
(ALT) were considerably higher in the co-infection group compared to
isolated dengue fever and isolated scrub typhus (Table 2).
The serum albumin levels were significantly lower in co-infection as
compared to isolated dengue (3.0 vs 3.9 g/dl; p<0.001) but not with
isolated scrub typhus (3.0 vs 2.9 g/dl; p=0.95).
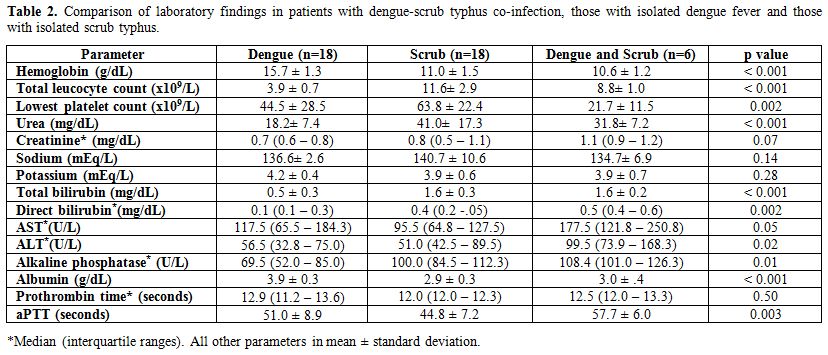 |
Table 2. Comparison of laboratory findings in patients with dengue-scrub typhus co-infection, those with isolated dengue fever and those with isolated scrub typhus. |
Hemoconcentration was conspicuous in the isolated dengue group whereas that with co-infection had lower mean hemoglobin. The drop in platelet counts during illness was most marked in co-infection and was significantly lower compared to those with isolated scrub typhus (Table 2). The nadir platelet count occurred much earlier in patients with co-infection compared to those with isolated dengue and isolated scrub typhus (Table 3).
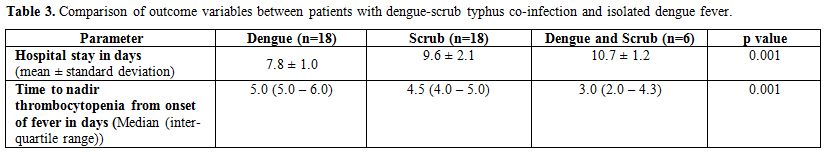 |
Table 3. Comparison of outcome variables between patients with dengue-scrub typhus co-infection and isolated dengue fever. |
All six patients with co-infection received antibiotics;
four of them were prescribed oral doxycycline and the others
azithromycin. Three patients were started on antibiotics before the
results of IgM for scrub were available based on clinical suspicion,
whereas the other three received antibiotics after laboratory
confirmation of scrub typhus co-infection. All of them responded within
48 hours of antibiotic initiation with temperature touching baseline.
Discussion
Scrub typhus is a rickettsia infection that is being increasingly
diagnosed in the tropics. In fact, it is estimated that scrub typhus
accounts for 40-50% of cases of undiagnosed acute febrile illnesses.[1]
On the other hand, dengue fever has become endemic in most parts of
Southeast Asia with outbreaks occurring with clockwork precision every
year in and around the rainy seasons. Of late the seasonal variation of
occurrence of dengue is slowly getting obliterated, with isolated cases
being reported throughout the year. It is not uncommon to detect
concurrent infections with multiple pathogens in the same individual in
developing countries, especially during periods of the outbreak. Dengue
co-infection with leptospirosis and malaria has been described in the
literature.[2] Recent studies also report a high incidence of concurrent infection with dengue and Salmonella Typhi.[3]
However, only one study from North India and a case report from this
institute has been reported, on dengue-scrub typhus co-infection to the
best of our knowledge.[4,5] Clinical and laboratory
features of both the diseases have been extensively studied and
described separately. We describe the clinical and laboratory features
of patients diagnosed with this rare combination of infections and
compare with those of patients with isolated dengue fever and isolated
scrub typhus.
Duration of fever, mean age of presentation and male to female ratio in our study were similar to the study by Ahmed et al.[4]
Abdominal pain was a common clinical manifestation in the above study,
whereas headache was the common clinical feature apart from fever in
our study. Patients with co-infection had symptoms indistinguishable
from isolated dengue fever. Eschar, the pathognomonic feature of scrub
typhus was found in only one-third of our patients. Comparison of
co-infection cases with isolated dengue revealed certain interesting
observations. A higher pulse rate and lower systolic blood pressure
were findings that suggested co-infection whereas other clinical
findings differed little between patients with co-infection and those
with dengue fever. In contrast to patients with dengue fever, those
with co-infection were likely to have normal leukocyte counts.
Interestingly, although the nadir platelet count did not differ, time
taken for the maximum drop in platelet count differed significantly
between these two groups of patients, with nadir attained earlier in
those with co-infection. In contrast, co-infection patients had both
lower nadir platelet counts and earlier nadir compared to patients with
isolated scrub typhus.
Thrombocytopenia is typically associated with dengue fever but has also been reported in cases of scrub typhus.[6,7]
In this study, we found thrombocytopenia in all three groups. Platelet
count was found to be lower in patients with co-infection compared to
those with isolated dengue fever as well as scrub typhus.
Thrombocytopenia in both infections is believed to be immune-mediated;
hence, co-infected patients probably had synergistic immune suppression
of platelets leading to lower platelet counts.[8,9] The lowest platelet counts are observed in dengue fever between days 5 and 8.[10]
This study suggests that a trend towards earlier fall and attainment of
nadir in platelet count in Dengue patients might be an indicator of
co-infection with scrub typhus in endemic areas. Conversely in a
patient with scrub typhus, marked thrombocytopenia may be a clue to the
presence of co-infection by dengue virus, especially during an
outbreak. The study by Ahmad et al. had estimated the time to recovery
of thrombocytopenia, unlike this study where we sought to determine
time to attain lowest platelet count.
Elevation of transaminases has also been described in both dengue fever and scrub typhus.[11,12]
In both these tropical illnesses aspartate aminotransferase (AST) is
found to be elevated more than alanine aminotransferase (ALT), a
finding that we noted in this study as well. In the case of
co-infection, both AST and ALT were significantly higher compared to
dengue or scrub typhus alone. A low serum albumin was more likely in
co-infection compared to isolated dengue (p<0.001) and may,
therefore, serve as a predictor of associated scrub typhus in dengue
patients. Hypoalbuminemia and leukocytosis have been reported as
independent predictors of severe scrub typhus.[13] In
our study patients with co-infection had hypoalbuminemia, but none of
them fulfilled the criteria for severe scrub typhus. Similar to
findings of the present study, elevated transaminases (AST > ALT),
bilirubin and alkaline phosphatase were also noted in the study by
Ahmed et al. and a case of dengue – scrub co-infection reported by
Iqbal et al.[4,5]
Prolonged activated partial
thromboplastin time (aPTT) is a well-known association in case of
dengue fever. The combination of prolonged aPTT, normal prothrombin
time, leukopenia, thrombocytopenia and elevated transaminases is highly
predictive of acute dengue infection.[14] In this
study, we found isolated aPTT prolongation in co-infection and isolated
dengue fever. We observed that patients with co–infection had
significant prolongation of aPTT compared to isolated scrub typhus but
not dengue fever. Hence, a prolonged aPTT in patients with scrub typhus
needs to be evaluated to rule out a co-existing dengue infection.
Among
outcome-related variables, co-infection led to a significantly longer
stay in hospital than dengue fever. Although we could not identify the
reasons for the excess days spent in hospital by patients with
co-infection, there is no doubt that this would increase the cost of
treatment, a major concern in developing countries. Moreover, the
patients with co-infection of our study spent relatively more days in
the hospital compared to the similar co-infection cohort described by
Ahmad et al. A well designed prospective study may provide vital
information regarding these aspects. We did not observe any mortality
among our co-infection cohort unlike the study from North India where
one death was noted.
Appropriate and timely laboratory
investigations help to resolve a suspicion of mixed infection. NS1
antigen and IgM ELISA for dengue and IgM for scrub typhus with high
cutoff values detected simultaneously, are strong indicators of
concomitant infection. NS1 is presumed to be detectable from the first
day and remains positive till about a week to ten days. Hence, it is
taken as a diagnostic marker for ongoing infection. A combination of
IgM and NS1 has a greater predictive value for current dengue
infection. They have high sensitivity and specificity.[15]
Detection of these parameters indicates recent or ongoing infection. An
IgG detection alone must not be relied upon for ongoing infection.
Cross-reactivity between other flavivirus infections and a non-specific
response must be borne in mind while interpreting IgM and IgG levels. A
panel of tests, with high specificity, has a better chance to
differentiate an acute infection from a reactionary response. IgM
detection in scrub typhus depends on the type of antigen used to
capture the antibodies. Several authors have documented variable
results. The manufacturers of ELISA kits have used antigens which
detect IgM at the earliest, around 3-4 days.[16] The
ideal confirmatory tests like PCR (which has high specificity almost
100% for scrub typhus, but low sensitivity) for the detection of
specific genomes may not be feasible in resource-poor settings. In the
present study, past infection by either one was ruled out, as both
Dengue NS1 and IgM antibodies for scrub typhus were detected
simultaneously.
Apart from the differences in length of hospital
stay and the time to attain nadir platelet counts, this study found a
general trend towards comparable outcomes between co-infection and
mono-infections. One explanation is that neither the patients with
mono-infection nor those with co-infection had features to suggest
severe disease (dengue shock syndrome or severe scrub typhus). A second
possible explanation is that similar to suppression of viral
replication of HIV by scrub typhus co-infection,[17]
co-infection could have also compromised dengue viral load. However,
this has not been proven so far by in vitro studies. Lastly, prompt
initiation of antibiotics (either empirically before test results or
soon after results) in co-infected patients could have altered the
course of illness nullifying the anticipated summative effects of a
co-infection.
The major limitation of this study is its
retrospective nature. Second, the number of cases of co-infection is
small. Nonetheless, we have been able to describe a co-infection that
may easily be overlooked in tropics because of very similar clinical
and laboratory features and possibly masked by the use of empiric
antibiotics. It is also likely that many cases of co-infection could
have been missed as we only included cases of scrub typhus diagnosed by
IgM ELISA. However, this strict inclusion based on an extremely
sensitive test for scrub typhus is also the strength of our study.[18]
Although the reason for testing IgM scrub antibodies in most of the
cases appeared to be the persistence of fever, the deficiency in
records precluded further analysis. Moreover, other factors like prior
use of antibiotics, dengue serotype and severity of scrub typhus could
have confounded our results, thus limiting the applicability of our
findings to differentiate co-infection from mono-infection.
Conclusion
Dengue and scrub typhus co-infection may be an under-recognized combination of concurrent infections in the tropics, particularly India. Features such as tachycardia, low systolic blood pressure, normal leukocyte counts, early drop in platelet counts and hypoalbuminemia in a case of dengue fever should alert the clinician to order additional investigations that include a sensitive test for scrub typhus, either an IgM detection or PCR if available. Shorter time to attain nadir thrombocytopenia may be another important feature that suggests co-infection and may necessitate ordering tests to detect the same. Timely detection and treatment of concurrent infection with O. tsutsugamushi may have significant implications as it is likely to reduce the length of hospital stay and in turn the cost of therapy. Larger prospective studies are needed to estimate the exact magnitude and impact of this co-infection.
References


















[TOP]