Received: April 6, 2016
Accepted: June 12, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016030, DOI 10.4084/MJHID.2016.030
This article is available on PDF format at:

Roberto Castelli1, Antonio Gidaro1 and Giorgio Lambertenghi Deliliers2

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Background: Splenic marginal zone lymphoma (SMZL) is a chronic B-cell lymphoproliferative disorder, comprising less than 2% of non-Hodgkin’s lymphomas, and affecting mainly middle-aged and elderly patients with a median survival of >10 years. The typical clinical features of SMZL include splenomegaly. Treatment should be patient-tailored and can range from a ‘watchful waiting’ approach for asymptomatic patients without cytopenias to surgery, localized radiation therapy or immuno/chemotherapies. Recently, the combination of rituximab and Bendamustine (R-Benda) has been defined as highly active in patients with follicular lymphomas, but little is known about the efficacy of R-Benda in SMZL.Aim of the study: The purpose of this retrospective study was to report our experience on the efficacy of R-Benda as first line treatment in 23 consecutive elderly SMZL patients. Results: All patients had a complete resolution of splenomegaly along with restoration of their blood counts. Nineteen patients (83%) achieved a complete response (CR) to therapy; three patients (13%) achieved a partial response (PR).Ten patients (43%) obtained molecular remission. Toxicities were mild and mainly haematological and result in dose reductions for fourteen patients. Conclusions: Our data suggest a high activity and good tolerance of R-Benda, despite dose reduction due to potential toxicity. |
Introduction
Splenic Marginal Zone Lymphoma (SMZL) is a low-grade B-cell lymphoma
considered as a rare lymphoproliferative disease (2% of all
non-Hodgkin’s lymphomas). It is estimated that it represents for most
cases of otherwise unclassifiable chronic lymphoid B-cell CD
5-lymphoprolipherative disorders.[1] SMZL is
characterized by an almost exclusive involvement of the spleen and bone
marrow without lymphadenopathy. SMZL has a relatively indolent course
with a median survival of >10 years,[2-3] but some
patients can suffer a more rapid course. In fact about 25% of patients
pursue an aggressive course and most of them die of lymphoma
progression within 3-4 years.[4]
Lymphonode and
extra nodal involvement; high lymphocyte count; anemia; and
thrombocytopenia on the diagnosis or when they are developed during the
course of the disease are considered prognostic criteria of
progression.[5-6] The Integruppo Italiano Linfomi
(IIL) have proposed clinical score based on three parameters: anaemia,
elevated LDH values, and hypoalbuminemia to evaluate the risk of
progression and found that patients are showing two or more of the
above adverse prognostic factors have a median life expectancy of fewer
than five years.[7] The patients are grouped
into three categories of increasing severity: (1) low risk when none of
the risk factors are present (5-year lymphoma specific survival of
88%); (2) intermediate risk when one risk factor is present (5-year
lymphoma specific survival of 73%); and (3) high risk when two or more
risk factors are present (5-year lymphoma specific survival of 50%).
The patients enrolled in this study all belonged to intermediate or
high risk score. Treatment should be patient tailored and can range
from a ‘watchful waiting’ approach for asymptomatic patients without
cytopenias to splenectomy or localized radiation therapy. Recently, the
combination of rituximab and Bendamustine (R-Benda) has been defined as
highly active in patients with indolent lymphomas,[8] but little is known about the efficacy of R-Benda in SMZL, especially in elderly patients due to the rarity of the disease.
Patients and Methods
Twenty-three patients with splenic marginal zone B cells were
included in this retrospective study approved by Ethical Board of our
University Hospital. The diagnosis was obtained by a combination of
bone marrow histology, clinical findings, and immunophenotype. Lymphoma
cells were expressing positivity for CD19+, CD20+, CD79a+,
CD5-,CD10-, CD23-, BCL6-, cyclin D1-.None of the patients underwent
splenectomy. Staging procedures were performed as routinely at the time
of diagnosis and before treatment with rituximab/bendamustine.
Polymerase chain reaction (PCR) analysis of blood and/or bone marrow
mononuclear cells for the presence of IgH, rearrangement was also
performed prior to treatment initiation and at the time of remission.[9]
Clinical data were collected: patient’s demographics complete blood
cell count, albumin, plasma lactic dehydrogenase (LDH), and hepatitis B
and C serology, bone marrow findings. B symptoms, performance status
(according to Eastern Cooperative Oncology Group performance status,
ECOG), date of diagnosis, treatment, treatment response, date of
relapse, date of the last follow-up and cause of death were also
collected. Prognostication was performed according to the suggestion of
IIL.[7] The schedule of R–B was the following: six courses of Bendamustine at 90 mg/m2 or 70 mg/m2 (depending on clinician’s choice) on days 1 and 2 of each 28-day cycle and rituximab 375 mg/m2
on day 1 of each cycle. Post-treatment evaluation was performed at 8
weeks after the completion of the sixth rituximab/Bendamustine
infusion.
Response criteria were as follows: Complete
remission (CR): Complete resolution of symptoms, normalization of
peripheral blood counts, the absence of detectable disease by clinical
staging including bone marrow biopsy, and no evidence of clonal B-cell
population by blood and bone marrow immunophenotype. Molecular
remission: Absence of IgH rearrangement in bone marrow samples in
previously positive patients. Complete remission unconfirmed (CRu):
Complete resolution of symptoms and absence of detectable disease by
clinical staging in patients who did not undergo bone marrow evaluation
post-rituximab/Bendamustine. Partial remission (PR): At least 50%
decrease in the spleen size and the percentage of bone marrow
infiltration (evaluated by trephine biopsy) along with the improvement
of blood counts over baseline. Failure: Any lesser response than
described above.
Results and discussion
A total of 23 patients (15 females and 8 males) with SMZL median age 75 years (range 65- 88 years) were enrolled. Clinical and laboratory parameters are presented in table 1. Forty teen patients (61%) had haemoglobin levels below 11 g /dl, thrombocytopenia in nine patients (39%), all patients had bone marrow lymphatic involvement, neutropenia was present in eight patients (35%) of patients.
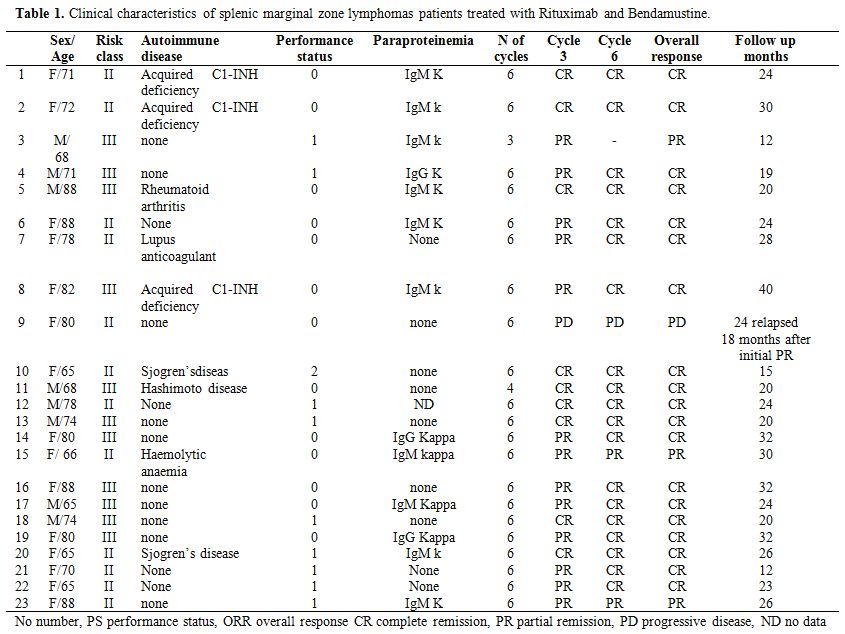 |
Table 1. Clinical characteristics of splenic marginal zone lymphomas patients treated with Rituximab and Bendamustine. |
Monoclonal serum immunoglobulin was present 13 patients (57%), and the most represented Ig Isotype was IgM. Autoimmune manifestations or concomitant autoimmune disease were present in nine patients (39%). Splenectomy was performed in none of our patients. In all patients, Rituximab-Bendamustine was given as first line treatment in a median time to diagnosis of 60 days (range 1-75) after diagnosis of SMZL. Twenty-two patients underwent 6 cycles of Bendamustine and Rituximab therapy; one patient has not completed the 6 cycle of treatment due to toxicities. Twelve patients (52%) of our study population experienced a Bendamustine dose reduction to 70 mg/m2 for two days. Responses were assessed after 3 cycles and after 6 cycles of chemotherapy. Bone marrow biopsy performed after chemotherapy is available for twenty-two patients. Nineteen patients (83%) achieved a complete response (CR) to therapy; three patients (13%) achieved a partial response (PR). One patient (4.3%) had disease progression 18 months after initial partial remission and was shift to R CHOP (Rituximab, Cyclofosfamide, Doxorubicine, Vincristine, and Prednisone) obtaining complete remission. Among patients with CR, six patients (26%) had a partial response after 3 cycles and obtained CR after 3 additional cycles of therapy. Ten patients (43%) obtained molecular remission. (Absence of IgH rearrangement in bone marrow samples in previously positive patients). The median time to clinical response in terms resolutions of clinical symptoms was 2 weeks (range 1-6) and the median time to haematological response was 4 weeks (range 2-6 weeks). Table 2 show changes in clinical and haematological parameters in 23 patients with splenic marginal zone lymphomas, prior and after treatment with Bendamustine and Rituximab. After treatment, we obtained resolution of autoimmune haemolytic anaemia and acquired deficiency of C1-INH. Patients were followed for a median time of 24 months (range 12-50 months). The treatment was generally well tolerated. One patient experienced infusion related side effects, including chills, rigors, bronchospasm and back pain treated with discontinuation of rituximab and reinfusion at a lower rate. Patients experienced hematologic toxicities: neutropenia in 16 patients (70%), one patient developed mild to moderate lasting neutropenia for 2 months, eight patients (35%) experienced thrombocytopenia. The most frequent non-haematological adverse events of any grade included nausea in 15 patients (65%), infection in 16 patients (70%). Some of the infections included three episodes of CMV infection and three episodes of herpes zoster. Eight patients (35%) presented vomiting; pyrexia was observed in eight patients (35%). Seven patients (30%) experienced grade 3 neutropenia and infection in 16%. Slight skin reactions responding to antihistaminics were seen in 3 patients (13%) during the first cycles of Bendamustine administration and disappeared thereafter.
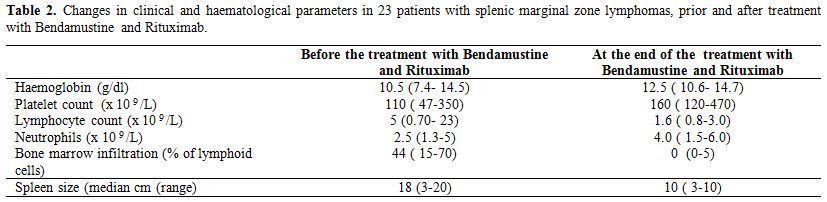 |
Table 2. Changes in clinical and haematological parameters in 23 patients with splenic marginal zone lymphomas, prior and after treatment with Bendamustine and Rituximab. |
MZL is a rare group of NHL, which is indolent in nature with
subgroups, SMZL, NMZL, and MALT lymphoma. These lymphomas share some
similarities but are different in their biological nature and hence
behave differently depending on their location. SMZL affects the
elderly with a median age of 70 years, the median survival exceeds ten
years, and most patients can be effectively managed for many years with
a watchful waiting policy. Splenectomy has been traditionally
considered the standard of care for the treatment of patients with
SMZL.[10]
However, being a major surgical
procedure, it carries a high risk of complications. For patient or
those who show a spread of the disease to lymphonode or other
extranodal sites or other adverse prognostic factors, a systemic
treatment may be appropriate. The introduction of anti-CD20 antibody
rituximab significantly improved the treatment outcomes of SMZL due to
the strong expression of CD 20 antigen in SMZL cells. Kalpadakis and
colleagues[11] showed that the use of rituximab as a
single agent yielded an overall response rate (ORR) of 100%, with 69%
complete response (CR), 19% unconfirmed response and 12% partial
response (PR). In about 25% of cases, the disease pursues an aggressive
course, and most patients die of lymphoma progression within 3-4 years.
Recently the IIL have proposed a clinical score to evaluate the risk of
lymphoma progression. The challenge of our study was to evaluate if
Rituximab Bendamustine treatment, as first line chemotherapy, may be
appropriate in high intermediate risk elderly patients with SMZL.
Nineteen
patients (83%) achieved a complete response (CR), three patients (13%)
achieved a partial response (PR). One patient (4.3%) had disease
progression, 18 months after initial partial remission and was shift to
R CHOP (Rituximab, Cyclofosfamide, Doxorubicine, Vincristine and
Prednisone) obtaining complete remission. Ten patients (43%) obtained
molecular remission. Our study suggests that R-Benda is effective
and tolerable (despite the need for dose reduction in elderlies) in
patients with SMZL.
The present study has some limitations
(retrospective, and follow be too short to asses progression free
survival and without statistical power to asses superiority of R Benda
as compared to Rituximab alone), and if these results will be
confirmed by larger and prospective studies it should be of benefits in
SMZL patients carrying risk factor for an aggressive course.
References











[TOP]