Received: May 16, 2016
Accepted: June 6, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016031, DOI 10.4084/MJHID.2016.031
This article is available on PDF format at:

Hospital de la Princesa C/Diego de León nº 62, Madrid 28006. Spain

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by-nc/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract Due to its negative impact on the
outcome of stem cell transplant (SCT) and solid organ transplant
patients (SOT) CMV has been called “the troll of transplantation”. One
of the greatest advances in the management of SCT has been the
introduction of the preemptive strategy. Since its introduction, the
incidence of the viremia, as expected, remains unchanged but there has
been a marked decline in the incidence of early CMV disease. However,
in spite of the advances in prevention of CMV disease, CMV is still
today an important cause of morbidity and mortality. Late CMV disease
is still occurring in a significant proportion of patients and the
so-called indirect effects of CMV are causing significant morbidity and
mortality. Fortunately there have been several advances in the
development of new antivirals, adoptive immunotherapy and DNA-CMV
vaccines that might transform the management of CMV in the near future. |
Introduction
Today it is widely known that CMV is a very important pathogen in the transplant setting, but, curiously, it has not always has been considered this way. It is surprising to know that the first article that identified CMV as a major pathogen in transplant patients[1] was rejected when it was first submitted for publication; the author was told that it was common knowledge that CMV does not cause disease.[2] Unfortunately, we learned that this is not true, and CMV disease was for a long time the first cause of transplant-related mortality. Due to its negative impact on the clinical outcome of SCT and SOT it has been called “the troll of transplantation” by Prof Balfour in a very graphic description:[3] “Cytomegalovirus is the troll under the bridge, hidden in shadows and often undetectable even by the most sophisticated diagnostic techniques. As we immunosuppress patients to help them cross the bridge, the troll comes out and threatens to devour them”. Now the incidence of CMV disease is pretty low (5%), so It could be logical to think that, today, CMV is not a big problem. As we will see, unfortunately this is not the case and CMV is still today an important cause of morbidity and mortality.
a) Past and Present Situation
a1) CMV disease. Mortality due to CMV-disease has decreased dramatically over time. In the 70’ and 80’, one every 5 patients died due to CMV disease, in the majority of cases due to CMV pneumonitis (figure 1). Today, the figure is around <2%. The control of CMV in stem cell transplantation (SCT) is probably the single advance with the highest impact in transplant survival in the last 25 years. What were the causes/reasons for this improvement? Certainly, there have been the advances in CMV prevention based on the development of diagnostic methods, such as antigenemia and PCR (both developed at the same time, 1988), and the development of anti-CMV antivirals such as ganciclovir (1989). Both developments allow the use of preventive strategies starting in the nineties that changed the CMV mortality dramatically. Today the incidence of CMV disease is <5%, according to the latest randomized trials (Table 1),[4-7] and large review series.[8] However, in contrast to these big advances in prevention, there have been few advances in therapy in the last 15 or 20 years (see later).
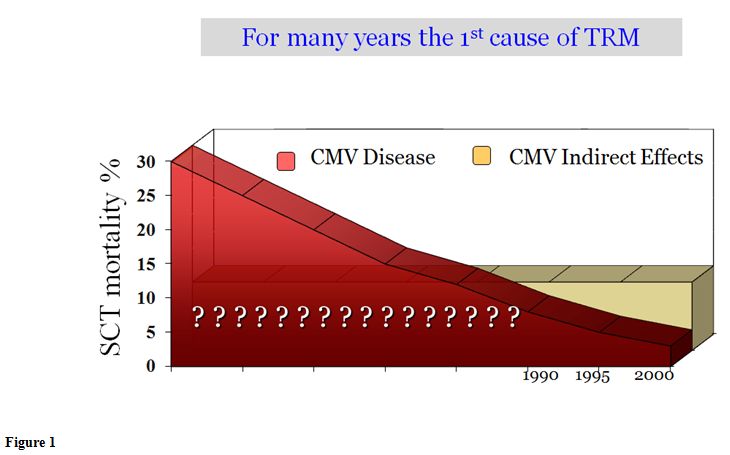 |
Figure 1 |
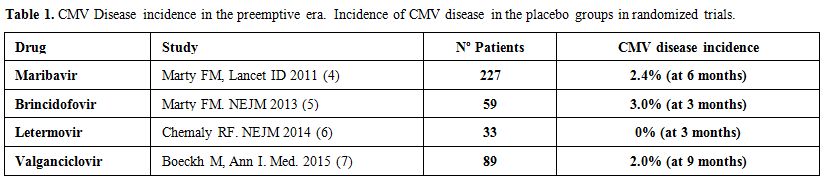 |
Table 1. CMV Disease incidence in the preemptive era. Incidence of CMV disease in the placebo groups in randomized trials. |
Another
important change over time has been the type and time of presentation
of prevalent type CMV disease, clearly related to the strategies for
CMV prevention. Since the introduction of the preemptive therapy, the
incidence of the viremia, as expected, remains unchanged in SCT
recipients but there has been a significant decline in the incidence of
early onset CMV disease (within first 100 days). Classically CMV
pneumonitis was the main disease in SCT patients. The typical median
time of presentation was between 50 to 60 days after transplant. It had
a high attributed mortality (≥70%) and was the cause of the majority of
CMV-deaths. Nonetheless, now, the gastrointestinal (GI) disease is the
most frequent CMV disease in SCT (70-80%),[4,9,10]
because we are more efficient in preventing CMV pneumonitis than the
CMV-gastrointestinal disease (GI). As a consequence the mortality of
early CMV disease (within first 100 days) has decreased.[9]
The change to GI CMV disease, as the predominant form of CMV disease,
seems to be related to the utilization of the PCR screening method
instead of antigenemia[9,11] or cultures,[12] but not related to the change of the source of cells from bone marrow to blood or umbilical cord blood.[13]
It
has been shown in several studies that, in contrast to CMV pneumonitis,
antigenemia, and, to a lesser extent, also PCR have a low sensitivity
for the diagnosis of GI CMV disease, ranging between 20-50%.[9,14,15]
These data suggest that CMV viral load in plasma does not adequately
represent CMV replication in the GI mucosa probably because, in a
proportion of patients, GI CMV disease represents a local event at
least initially, in many cases associated to GVHD.
A
consequence of the widespread use of preemptive therapy has been a
switch from early to late CMV disease, so now late CMV disease, the
disease that develops after day 100 from transplant, has become the
predominant form of presentation in many transplant centers. Moreover,
this is not a good thing. The proportion of CMV pneumonitis is higher
in late disease (>50%),[16] and these pneumonitides have the same high mortality as the early cases.
Another
area of interest in the epidemiology of CMV in SCT is the possible
impact that the new drugs might have. Some new drugs that a priori were
not considered a risk for CMV have been later associated with the
development of opportunistic infections including CMV disease in
non-transplant patients, like ruxolitinib[17,18] and Idelalisib.[19]
Patients receiving these agents prior to the transplant might have an
increased risk of CMV, an issue that should be investigated. Moreover
ruxolitinib is being used successfully for the treatment of refractory
GVHD in an SCT patients setting of high-risk CMV infection and disease.[20]
a2) CMV definitions.
We need widely accepted definitions of what are CMV infection and CMV
disease in order to evaluate the impact of CMV in SCT across different
studies and different centers. The current international definitions in
use for CMV infection and disease were published nearly fifteen years
ago.[21] They claim that in the setting of SCT the term “CMV syndrome” should be avoided.
A
central aspect of the definitions is the use of valid diagnostic tools.
PCR alone is only sufficient for the diagnosis of a central nervous
system disease, and not for other types of diseases like pneumonia or
GI CMV disease. Detection of CMV by PCR alone may be too sensitive for
the diagnosis of CMV disease and is therefore insufficient for this
purpose. We have no data on what level of CMV DNA in bronchoalveolar
lavage fluid or tissue correlates best with CMV disease, and therefore
PCR is not recommended to make the diagnosis of CMV disease.[22] In a small series the use of CMV-PCR in bronchoalveolar lavage was not clinically useful.[23]
Nonetheless, CMV PCR can be used in the diagnostic approach of a SCT
patient with pneumonitis using a negative result to rule out CMV due to
its high sensitivity and high negative predictive value.[22,24]
The
problem now is that many centers do not have CMV culture available and
depend on PCR for viral diagnosis. As a consequence in many SCT centers
it is not possible to obtain a valid diagnosis of CMV pneumonitis
without a lung biopsy, and even in this case the detection of CMV only
by PCR will be considered not acceptable as definitive proof of CMV
pneumonia or gastrointestinal disease. So in conclusion, we need new
CMV disease definitions for a world based on PCR tools with no cultures.
a3) CMV management strategies.
There are three strategies for the management of CMV: Prophylaxis,
pre-emptive therapy and treatment of established CMV disease. The
prophylaxis strategy is aimed at preventing all infections. The
pre-emptive strategy consists in treating patients with high-risk
infections to prevent disease. Moreover, finally when CMV disease is
present, the aim of the treatment is to avoid organ damage and death.
Prevention of CMV complications: CMV prevention started in the eighties with the administration of CMV seronegative blood products[25] and after, in 1995, with filtered blood products.[26] The pre-emptive strategy era started in 1991[27,28] and prophylactic ganciclovir strategy in 1993.[29,30]
As
usual, big advances are made based on landmark studies with quite a few
patients. In the case of preemptive therapy for CMV the proof of
concept was established by the City of Hope-Stanford-Syntex CMV Study
Group in an open, randomized study of 104 allogeneic SCT patients.[27]
Asymptomatic patients underwent bronchoalveolar (BAL) on day +35
post-transplant. CMV was evaluated in BAL by classic virologic
techniques: shell-vial cell and conventional cell cultures, and
cytology. Patients found positive for CMV (40 patients) were randomized
to receive (20 patients) or not receive (20 patients) intravenous
ganciclovir. At day +120 post-transplant, 75% of patients with positive
CMV on BAL not treated developed CMV pneumonitis compared to 25% of
those treated with ganciclovir, and 20% in those who were negative for
CMV on BAL. This study proved the value of preemptive therapy for the
prevention of CMV pneumonitis and started the era of the preemptive
therapy for CMV. It is probably also the single study that has saved
more lives in allogeneic SCT. A curious fact about this breakthrough
study was the terminology used for the new strategy employed. The
authors called their approach a “Trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection”,
apparently nothing new, no mention of the word “preemptive”. It was
Robert. H. Rubin, in an editorial in the same number of the journal,[31]
who recognized the novelty of the new approach, different from
prophylaxis and therapeutic approach coining the term “preemptive”
therapy.
Although screening bronchoscopy was historically the
first sample used to guide preemptive therapy, it was abandoned many
years ago due to the clear superiority in efficacy and safety of the
much more convenient sequential blood screening. Moreover, in a
randomized trial, preemptive therapy based on antigenemia proved to be
superior to preemptive therapy based on a day 35 screening
bronchoscopy.[32] CMV cultures were also abandoned in
favour of non-culture techniques like antigenemia and PCR. In a
randomized trial done more than 20 years ago[12] PCR
proved to be better than culture: PCR was associated with a lower rate
of CMV disease and CMV-associated mortality, shorter duration of
ganciclovir therapy, lower incidence and duration of severe
neutropenia, and increased overall survival.
A randomized trial
comparing prophylactic intravenous ganciclovir until day 100
post-transplant versus the preemptive ganciclovir therapy showed no
significant difference in CMV disease by day 180 after transplantation
and afterward (16.1% vs. 20.2%), and a similar overall survival.
Nonetheless, prophylactic ganciclovir was associated with higher
incidence of bacterial and fungal infections and increased use of
ganciclovir. Thus, the preemptive use of ganciclovir guided by
monitoring CMV viremia measured by antigenemia or qPCR became the
standard of care in this setting.
Treatment of CMV disease:
The treatment of CMV disease was based on noncomparative studies
perform in the late eighties, establishing ganciclovir plus
immunoglobulin as the treatment of choice for CMV disease, that was
mainly pneumonitis at the time.[33-37] Nonetheless,
even with this treatment the mortality remain high (70%). The previous
experience with monotherapy with ganciclovir, foscarnet or
immunoglobulin did not improve the clinical outcome of CMV pneumonitis,[38-42] and reviewed in.[43,44]
There
is only one randomized trial that has compared ganciclovir with placebo
in the treatment of CMV disease in allogeneic SCT patients,[45]
and it had disappointing results. A 14-day treatment course did not
appear to influence clinical symptoms, the healing of gastrointestinal
epithelium, the subsequent development of cytomegalovirus pneumonia, or
overall mortality when compared with placebo.
In the meantime,
has the treatment improved over time? Unfortunately, according to an
extensive study, the outcome of CMV pneumonia showed only a modest
improvement over time.[46] In this study,[46]
with 421 CMV pneumonitis, the overall survival at 6 months was 30%,
similar to the historical series. Outcome improved after the year 2000
showing a significant decrease in attributable mortality (adjusted
hazard ratio, aHR, 0.6, P = 0.01), and a trend to a lower all-cause
mortality: (aHR, 0.7, P = 0.06). Nonetheless, the effect of time may be
due to changes in the prevalence of important risk factors over time,
like mechanical ventilation, lymphopenia and hyperbilirubinemia.[46]
Moreover,
what happens if the patient does well and survives CMV disease?
Unfortunately, several studies have shown a grim outcome, mainly
because a previous CMV disease is an independent risk factor for
invasive aspergillosis (Hazard ratio 7.0) [47,48] and candidemia (relative risk 16.4).[49]
In fact in these studies CMV disease was the- highest risk factor
associated with IFI, greater than severe GVHD or the use of high
dose of corticosteroids. Moreover, invasive aspergillosis was the most
frequent cause of death in patients that survive a CMV disease. Based
on these results, antifungal prophylaxis seems necessary for patients
that survive CMV disease.
Cost:
Another interesting aspect of the management of CMV today is the cost
associated with the preemptive therapy of CMV infection. This
evaluation has interest since now we can use new strategies to prevent
CMV reactivation such as vaccines and adoptive immunotherapy that also
have a high cost. A recent study compared the outcomes and
post-transplantation treatment cost in 44 patients who never required
pre-emptive CMV treatment with 90 treated patients. The treated group
incurred an extra charge of $58,000 to $74,000 per patient.[50]
a4) Indirect effects.
As previously mentioned, since the introduction of preemptive therapy a
significant decline in the incidence of CMV disease has become the
norm, now <5%, with a low CMV disease-related mortality (<2%).
Nonetheless, CMV continues to be one of the leading causes of morbidity
and mortality due to the so-called “indirect effects”. It was not until
around 1990, that these indirect effects could be identified.
Previously, the high CMV disease mortality precluded the detection of
these effects. As shown in figure 1,
these days SCT patients die more due to the indirect effects of CMV
than due to CMV pneumonitis. CMV is associated with morbidity-mortality
in 3 ways: CMV disease, the development of CMV infection, and by the
presence of a positive serology pre-transplant.
It is recognized
now that CMV causes mortality in 2 distinct ways: by the direct effects
of the virus, in the form of a recognized viral disease, for example
CMV pneumonitis; and by the so-called “indirect effects”, which are
increasingly recognized as an important part of the whole viral effect.
These indirect effects consist of clinical events associated with virus
seropositivity or the development of viral infection, but not with the
viral disease itself. These effects have been shown, not only in SCT
patients but also in recipients of SOT and HIV patients. Several
viruses have been described (different respiratory viruses, Herpesvirus
type 6) but the paradigm of these direct-indirect viral effects is CMV.
For CMV, the indirect effects outlined in the literature include:
increased incidence of acute and extensive chronic graft-versus-host
disease (GVHD),[51,52] increased risk and mortality due to bacterial and fungal infections,[47,53-55] and what is more important an increase in transplant-related mortality and a decrease in overall survival. In table 2
there is a summary of studies showing the negative impact of CMV
seropositivity on the outcome of HSCT, in more than twenty-nine
thousand patients. These adverse effects have been described mainly in
patients who received depleted transplant or transplants from unrelated
donors. However, they also occur in HLA-identical siblings transplants[51,56] as showed in a large EBMT retrospective study in more than 56.000 patients, 72% of them from HLA-identical siblings:[57] CMV seropositive patients had a higher mortality compared to transplant where both patient and donor were CMV seronegative.
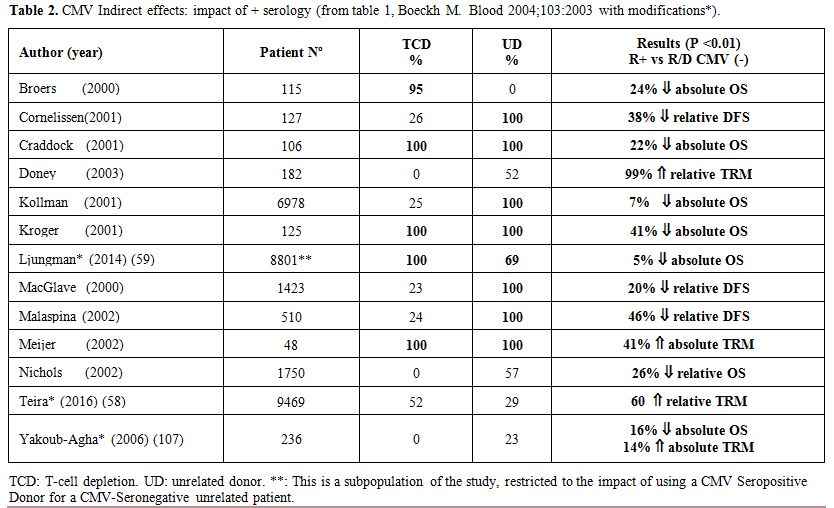 |
Table 2. CMV Indirect effects: impact of + serology (from table 1, Boeckh M. Blood 2004;103:2003 with modifications*). |
A recent CIBMTR analysis on 9,469 patients transplanted between 2003 and 2010,[58] showed that CMV reactivation was associated with inferior OS among all disease groups in multivariate analysis confirming that today, in spite of widespread preemptive therapy, CMV reactivation continues to remain a risk factor for poor post-transplant outcomes.
a5) Trends in Allogeneic Transplantation: an increase of patients at risk of CMV complications.
Changes in the age and origin of the patients and the increasing use of
unrelated donors from countries with a very low HCMV prevalence are
causing an increasing proportion of transplants in CMV-seropositive
patients from CMV-seronegative donors, a combination that has been
associated with a worse outcome than a +/+ combination and of course
-/- pairs of receptor-donors.[59] This is due to 2
facts. First, there are an increasing proportion of seropositive
patients due to the clear increase in their age, and second, there is a
decrease in the percentage of CMV seronegative donors.[60]
It
is well known that CMV seroprevalence has a strong correlation with
age. In the US population, for example, 54% of patients younger than 40
are CMV seropositive compared to 83% in those of ≥60 years.[61]
One of the most significant tendencies in allogeneic SCT is the
increase in the age of the recipients. During the decade 2002-2011,
patients older than 60 years doubled from 8% to 17%, increasing to 22%
of allogeneic transplant recipients in 2007-2013.[62]
This implies that more allogeneic transplant patients are CMV
seropositive. Moreover, allogeneic SCT are increasing in parts of the
world that previously had a low rate of activity. This is the case of
Latin America, where the seroprevalence of the population is higher
than in Europe of North America. This translates into greater resources
for the management of CMV for Latin American transplant centers.[63]
a6) CMV: a troll or a warrior of transplantation? As previously mentioned, CMV has been called the troll of transplantation mainly due to the high mortality associated with CMV pneumonia. Nonetheless, CMV has also been associated, almost three decades ago, with a decrease in leukemic relapses after SCT, particularly in acute myeloid leukemia and chronic myeloid leukemia (CML), but to a lesser extent in myelodysplastic syndrome, acute lymphoblastic leukemia and Non-Hodgkin lymphoma patients. It was first reported by Lönnqvist et al, in a small cohort study, that CMV infections were associated with a decrease in relapses in leukemic patients.[64] More recently an impressive study by Elmaagacli et al reactivated the interest on the role of CMV in decreasing relapses after SCT, defining this association as “virus-versus-leukemia” effect.[65] In this study early CMV replication was associated, in the univariate and multivariate analysis, with a marked decrease in relapse (at 10 years: 9% vs. 42%, P 0.0001) and with an increase in survival (at 10 years: 62% vs. 37%, P 0.005). Based on these results CMV can also be seen as a warrior of transplantation. Nonetheless the relation between CMV infection and leukemia recurrence in patients with hematologic malignancies after allogeneic SCT has been a highly controversial issue for many years. Contradictory results have been obtained in different studies, based on CMV serology, CMV infection, and even with both techniques. There are more than 30 studies that have evaluated the role of CMV in relapse, but their detailed analyses are outside the scope of this review. It can be said that usually multicentric studies do not find a protective effect of CMV on relapse, being unicentric studies those who find it. In the 5 largest studies, with more than 96,000 patients, no effect of CMV (serology or infection) on relapse was found.[57-59,66,67] Nonetheless, it is an interesting aspect that requires more studies.
b) Management Today
b1) Guidelines for CMV management in SCT. There are several guidelines for CMV management although the two most widely accepted are the 2008 European Conference on Infections in Leukemia (ECIL) guidelines[68] and the 2009 international consensus guidelines,[69] both quite similar.
Prevention of CMV complications:
What do we want to prevent: infection or CMV disease? This is an
important question as it has an impact on the strategy we select. A
preemptive strategy can only decrease CMV disease incidence, but
prophylaxis may have an effect on the complications produced by CMV
infection and even on those associated with CMV positive serology.
Until now, for SCT patients and with the available antivirals, the aim
has been to prevent CMV disease, and the strategy of choice for the
majority of the patients is preemptive therapy.[68,69]
A summary of the diagnosis and prevention recommendations appears in Table 3.
Ganciclovir is often used as a first-line drug for preemptive therapy.
Although foscarnet showed in a randomized trial to be as effective as
ganciclovir and with a lower incidence of severe neutropenia,[70] it is currently more commonly used as a second-line drug, because of practical reasons.
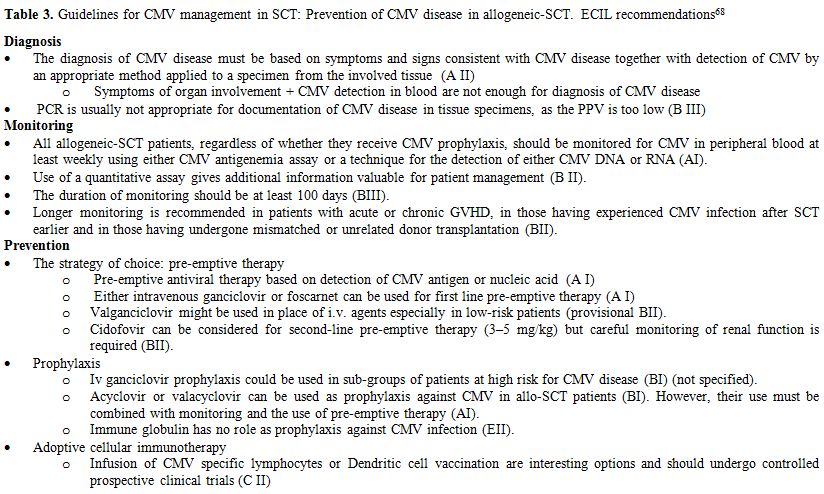 |
Table 3. Guidelines for CMV management in SCT: Prevention of CMV disease in allogeneic-SCT. ECIL recommendations[68] |
Those
patients that have only one reactivation episode after SCT are usually
treated successfully with the available antivirals. Problematic
patients are those that suffer repetitive replicative episodes,
typically in the setting of GVHD treated with intensive
immunosuppression, or transplants done with profound T cell impaired
immune reconstitution, due to T-cell depletion (in vivo or in vitro) or
a low T-cell dose on the graft (cords). These patients are prone to
devastating CMV complications, like CMV encephalitis, a form of CMV
disease with >90% mortality today.[71]
For
patients with profound T cell impaired immune reconstitution the use of
cell adoptive immunotherapy is being applied with increasing success.
For patients with intensive immunosuppression, particularly high dose
of corticosteroid, adoptive cell therapy needs further technical
refinements that are still experimental.[72] These
technologies seem not to be associated with significant toxicity but
their effectiveness needs to be further assessed in controlled trials[68] and cannot be recommended.[69]
Nonetheless, this interesting approach is not reviewed here in more
detail. There are several good recent updates for the interested
reader.[72,73]
Treatment of CMV disease: The standard treatment for CMV pneumonia, based on small studies in the late 80’ is the combination of intravenous ganciclovir plus immune globulin as recommended by the ECIL group.[68] For other types of CMV disease monotherapy with ganciclovir or foscarnet with immune globulin is recommended (Table 4).
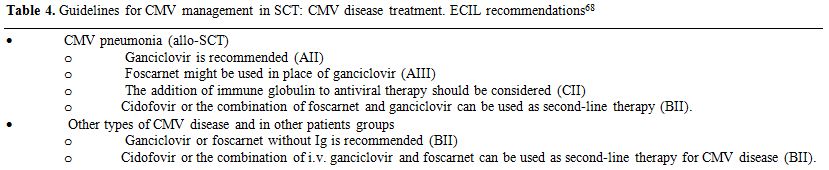 |
Table 4. Guidelines for CMV management in SCT: CMV disease treatment. ECIL recommendations[68] |
Although
recommended in the guidelines, do we need combined therapy with immune
globulin for CMV pneumonitis? Based on a recent publication[46]
the answer is probably no. In this large retrospective study on 421 CMV
pneumonia episodes in SCT patients the use of combined therapy
(ganciclovir or foscarnet) with immunoglobulins, compared with
antiviral monotherapy did not show an impact on CMV pneumonia mortality
(global or CMV-related). Contrary to early studies of ganciclovir as
monotherapy this study is the best evidence to date that monotherapy
with ganciclovir or foscarnet had a beneficial effect vs. no therapy.
The effect of antiviral monotherapy on overall survival compared with
no treatment was significant in both univariate and adjusted models.
Another smaller study did not see an impact on the association of
intravenous immune globulin with the response of CMV pneumonitis.[74]
As the role of immune globulin for the treatment of CMV is
questionable, if it is used, an unspecific immunoglobulin is
recommended over the CMV-specific, that is much more expensive and
harder to obtain.
So
inclusion, even today, it is better to prevent than to treat CMV
disease, and combined therapy with intravenous immune globulin seems to
be no better than monotherapy with antivirals (ganciclovir or
foscarnet).
b2) Today antivirals. For the management of CMV we have both high and low potency antivirals (Table 5). Low potency antivirals can be used for prophylaxis but have no role in the treatment of infections or disease by CMV. For these CMV complications high potency antivirals are needed.
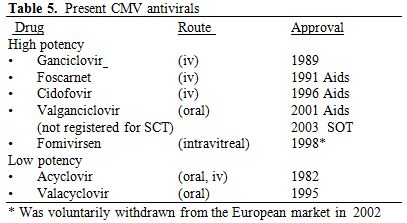 |
Table 5. Present CMV antivirals |
Two
drugs have been key in antiviral development: acyclovir and
ganciclovir. Acyclovir was discovered by Gertrude Elion and George
Hitchings in 1977 at the Wellcome Research Laboratories, which was one
of the discoveries that won them the Noble Prize in 1988. The discovery
of acyclovir, the first efficient and safe drug for the treatment of
herpes virus infections, opened a new era of antiviral therapy.
Ganciclovir was discovered in 1980 by Dr. Kelvin Ogilvie and his
research team at McGill University. Ganciclovir, the first high potency
anti-CMV agent was first used in a human in 1984 to treat a bone marrow
transplant patient. It was launched in 1989. Later came foscarnet,
cidofovir and Valganciclovir.
The
mechanism of action of all the classical anti-herpesvirus agents is the
inhibition of viral DNA polymerase. All of these antivirals, except
foscarnet, require phosphorylation by cellular kinases. Ganciclovir,
acyclovir, penciclovir, and brivudine also require an initial
phosphorylation by a virus-encoded kinase. In the case of ganciclovir
the encoded protein kinase pUL97 of the virus performs the first
phosphorylation. Foscarnet directly binds to the pyrophosphate binding
site of the viral DNA polymerase without any intracellular chemical
modification.
The
efficacy and safety profile are two essential aspects of the antivirals
for the management of CMV. The low potency antivirals, aciclovir and
valaciclovir, have a good safety profile and can be given orally or by
intravenous route (aciclovir). In contrast the available high potency
antivirals for CMV (ganciclovir, foscarnet and cidofovir) have several
and significant drawbacks:
a)
There is no approved oral agent for SCT. Valganciclovir, although
widely used in the clinic has no phase III study in SCT patients and it
is not registered for this population.
b)
The available anti-CMV agents have substantial toxicities, and this is
one of the major reasons that make preemptive therapy the strategy of
choice. The main toxicities of these drugs are nephrotoxicity,
myelosuppression and delay immune reconstitution.[75]
Ganciclovir cause neutropenia in at least 20-30% of the cases, which is
an independent negative risk factor for overall survival, disease-free
survival and transplant related mortality.[76]
Therefore, ganciclovir/valganciclovir are not adequate for neutropenic
patients, and in prophylaxis they have to start late after full
engraftment. Moreover, the prolonged use of ganciclovir (more than 4
weeks) is a risk factor for the development of late CMV disease and
invasive Aspergillosis.[77] With each week of ganciclovir treatment the risk of invasive aspergillosis increases by a factor of 1.4.[78]
Ganciclovir produces a significant increase of aspergillosis that is
independent of secondary neutropenia and proven CMV infection.[78] Both ganciclovir/valganciclovir and foscarnet are nephrotoxic and require dose adjustment with renal impairment.
c)
The use of these drugs in the prophylaxis or as preemptive treatment do
not prevent the indirect effects of CMV, with only one exception. In a
retrospective study preemptive treatment significantly decreased the
risk of extensive chronic GVHD.[79]
The
different toxicities and activity of anti-CMV antivirals have different
clinical implications according to the results of several prophylactic
studies. High-dose aciclovir, a low potency but low toxic antiviral,
compared to placebo, decreased CMV viremia by 40%, had no impact on CMV
disease incidence, but was associated with a 20% increase in overall
survival.[80] Curiously, high dose of acyclovir was
associated with a near 20% decrease in deaths due to infection (11% vs.
28%). A previous no randomized study showed that high-dose prophylactic
acyclovir reduced CMV infection by 30%, and increased overall survival
from 46% to 71%.[81] In a retrospective study, the
use of 1 year of acyclovir or valacyclovir prophylaxis was associated
with improved overall survival in allogeneic SCT.[82]
In a later randomized study that compared high dose of valaciclovir
with a high dose of aciclovir, valaciclovir proved to be more effective
than acyclovir, decreasing by 40% the rate of CMV infection and the use
of ganciclovir/foscarnet.[83]
In
contrast, ganciclovir, a high potency and higher toxic antiviral,
compared to placebo, in two randomised trials produced a greater
decrease in CMV infection (70-90%), a decrease in early CMV disease, an
increase in the incidence of severe neutropenia, but was not associated
with survival increase.[29,30] Although not
statistically significantly more patients died of infections in the
prophylactic ganciclovir group in both studies (50% vs. 25%; and 17%
vs. 6%). Moreover, in a randomized trial, intravenous ganciclovir until
day 100 after transplantation was no better than high-dose valacyclovir
in the prevention of CMV infection (12% vs. 19%) or CMV disease (1/85
vs. 2/83).[84] Nonetheless, the ganciclovir group had
a significantly higher incidence of neutropenia (32% vs. 13%), and a
non-significant increased incidence of infections after engraftment.
b3) CMV management in SCT: an “art” that is center-dependent.
Preemptive antiviral therapy has been adopted by most centers as the
strategy of choice for the prevention of CMV disease in the allogeneic
STC. However, it is a fact that the strategies apply at the centers
differ notably. Although quantitative real-time PCR assays (qRT-PCRs)
have largely replaced the pp65 antigenemia assay for the guidance of
preemptive antiviral therapy, many differences exist among centers.
There are variations in the type of sample used (plasma vs. whole
blood), in the method of DNA extraction (manual vs. automated
techniques), type of technique (commercial vs. homemade), and in the
quantitative viral load interpretation, the latter being one of the
most important factors. Some centers use the same value of PCR for all
patients and others that use different PCR cut-offs for different risk
populations.[22] Moreover, finally, the threshold
used to start preemptive therapy shows an enormous variation from
center to center (100, 200, 500, 600, 1000, 10,000, 50,000 copies /ml,
to put some examples).[22,85-90]
A
recent national survey revealed striking differences among centers in
CMV surveillance practices for the prevention of CMV disease,
especially regarding the criteria that triggered the start of
preemptive antiviral therapy, yet the overall incidence of CMV
end-organ disease was reported to be <3%, with minor variations
among centers.[91] In this study, all centers used a
no-risk adapted preemptive strategy, and all used a qRT-PCR with fully
automated DNA extraction (except 1 center). The threshold for start
therapy showed considerable variation among centers: 100 - 5000
copies/ml for plasma, and 400 - 1000 copies/ml for whole blood.
Curiously, 9 centers using the same commercial technique and the same
DNA extraction method showed variation in the threshold to start
preemptive therapy (150-5000 copies/ml).
One
of the problems with the different qRT-PCRs used was that they were not
directly comparable because of intrinsic differences in the performance
of the assays and the nature of the calibrator. The same sample with an
expected result of 1000 copies/ml studied at different centers gave
different results that vary from 0 to 20,000 copies/ml.[92]
In 2010 the first international standard for CMV QNAT was established
by the World Health Organization (WHO) Expert Committee on Biological
Standardization.[93] PCR viral loads are expressed in
international units/ ml. Will the use of normalized values to IU/ml
decrease these variations? The answer is no, at least at a significant
level. For example in the above mentioned national survey commented,[91]
the above-described differences even stood out when CMV DNA loads (in
copies/ml) were normalized to IU/ml, according to the conversion factor
recommended by the respective manufacturer.
So,
we have to conclude that the preemptive strategies employed at
different centers are probably unique to each center, and CMV
management in SCT is therefore an “art” that is center-dependent.
c) What is coming?
c1) New antivirals.
In spite of the successful prevention of early CMV disease with the
present preemptive or prophylactic strategies, CMV still causes
important morbidity and mortality. Until now, due to the toxic effects
associated with the anti-CMV drugs available, the use of prophylaxis
has not been associated with improved outcome. In contrast the use of
low prophylactic potency but low toxic antivirals (acyclovir,
valacyclovir) was associated with an increase in survival in one
randomized study[80] and 2 controlled studies.[81,82]
The development of new potent antivirals with an excellent safety
profile is an unmet need in SCT. These and other evidence strongly
support the idea that a high potency but low toxic anti-CMV drug has a
good rationale for improving the outcome of SCT patients.
Anti-CMV
agents have been difficult to develop. The last new compound was
approved in 1995 (cidofovir) and the latest new formulation in 2001
(valganciclovir). Quite unexpectedly, three new antivirals have been
developed recently more or less at the same time. As Prof. Griffiths
graphically express in an excellent editorial comparing new antivirals
and commuters in London: “you wait ages for a bus and then three come
along at the same time”.[94] The same has happened for new anti-CMV agents. These three new antivirals are: maribavir,[95] brincidofovir[96] and letermovir (Table 6).[97]
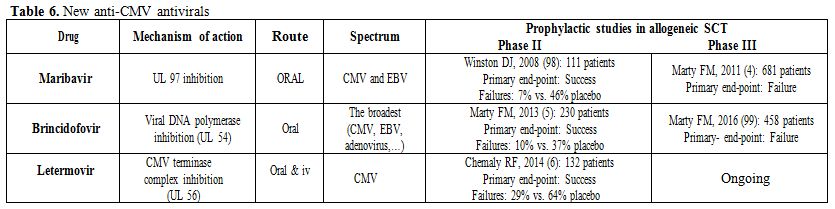 |
Table 6. New anti-CMV antivirals |
Summary of the new anti-CMV antivirals: None is approved for any indication and none of them are nephrotoxic or myelotoxic.
The antiviral spectrum is quite different (Table 6).
Letermovir is a pure anti-CMV agent; maribavir shows activity against
CMV and EBV; and brincidofovir has the broadest spectrum of all
anti-DNA viruses, broader than cidofovir.
All have a convenient posology: maribavir once every 12h; letermovir once a day; and brincidofovir twice weekly.
Maribavir
and letermovir have an entirely different mechanism of action compared
with the traditional anti-herpes agents. Maribavir binds to UL97 and
inhibits the assembly, encapsidation and nuclear egress. Letermovir
inhibits CMV terminase complex. (UL56), but not DNA-polymerase.
Moreover, brincidofovir, a lipid conjugate of cidofovir, inhibits viral
DNA-polymerase as does cidofovir.
All showed positive results in prophylactic phase II trials in allogeneic SCT (table 6).[5,6,98]
They were more effective than placebo and showed a good safety profile.
Maribavir is practically atoxic with taste disturbance as the most
frequent adverse event. Brincidofovir causes diarrhea with
dose-limiting toxicity at a dose of 200 mg twice a week or higher.
However, unfortunately, and quite unexpectedly, two of them, maribavir[4] and brincidofovir,[99]
failed primary efficacy end-point in prophylactic phase III trials in
SCT patients. Several reasons have been given for the failure of
maribavir, with the low suboptimal dose being one of the main causes
(100 mg bid).[100] A note of caution has to be made
about the phase III trial of brincidofovir because only a preliminary
presentation is available[99] and not the final
publication. Nonetheless, it appears that one of the major drivers of
the failure might be the diagnosis and management of brincidofovir
induced diarrhea as GI-GVHD in many cases. The phase III trial of
prophylactic letermovir in allogeneic SCT is ongoing and no results are
available.
The
clinical use of maribavir at high doses has shown a 50-66% response
rate in several refractory or resistant cytomegalovirus infections the
majority with CMV disease.[101,102] These are
encouraging results that merit further study. Maribavir has completed 2
Phase II randomized studies of treatment of CMV infection with high
doses (400-800-1200 mg BID), as first-line treatment (EudraCT Number:
2010-024247-32) or as salvage therapy for CMV infections that are
resistant or refractory to ganciclovir/valganciclovir or foscarnet
(NCT01611974). These doses are higher than those employed in the phase
III trial (100 mg bid).[4]
Due
to its broad spectrum of activity, brincidofovir is being studied in
other non-CMV infections. Of these, adenovirus is the infection in the
most advanced phase of development with a phase III trial running now.[103]
We
have to admit that the development of a successful anti-CMV drug is
becoming harder than expected. We now have more questions than answers:
Would they finally be effective in prophylaxis, preemptive or directed
therapy for CMV? Would they change the paradigm from preemptive to
prophylactic strategy? What impact will they have on relapse? Will they
have an impact on the indirect effects? Hopefully, one or more of these
new three antivirals will be approved for use in SCT patients. As
previously mentioned the development of new antivirals with high
anti-CMV potency, and a good safety profile is an unmet need in SCT.
The
knowledge of the biology of CMV could bring new routes for the
development of new drugs or a new use of old ones. It has recently been
reported that the inhibition of mitochondrial translation with
chloramphenicol abolished the HCMV-mediated increase in
mitochondrially-encoded proteins and significantly impaired viral
growth.[104]
c2) CMV vaccine. The prevention of CMV complications by CMV vaccine has a long unsuccessful history.[105] In allogeneic SCT patients, in a randomized, double-blind, placebo-controlled trial, a plasmid DNA vaccine that contains two plasmids that encode gB y pp65, showed to be effective (50% decrease in viremia), well tolerated and able to induce serologic and specific T cell responses against CMV.[106] Based on this good result the vaccine is now undergoing a Phase III trial for CMV prophylaxis in allogeneic SCT patients.
Unmet Medical Needs Regarding CMV in SCT Recipients
To end this review I would like to point out what are, in my opinion, the unmet medical needs regarding CMV in SCT recipients:
- New CMV disease definitions for the world based on PCRs tools with no cultures.
-The
development of potent, non-toxic anti-CMV drugs, particularly with no
toxicity to the kidneys, haematopoiesis and immunologic recovery.
-A better preemptive therapy with consistent thresholds across centers for the beginning and end of preemptive therapy.
-Improvement in the treatment of CMV disease, a field without clear progress in the last 25 years.
-An effective strategy for prevention of late CMV disease.
-And last, but not least, the reduction of the impact of the indirect effects in CMV seropositive patients.
References
 .
. 






































































































[TOP]