Received: August 29, 2016
Accepted: September 21, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016057, DOI 10.4084/MJHID.2016.057
This article is available on PDF format at:

Franco Aversa, Lucia Prezioso, Ilenia Manfra, Federica Galaverna, Angelica Spolzino and Alessandro Monti
Hematology and BMT Unit, Department of Clinical and Experimental Medicine, University of Parma, Parma, Italy.

| This is an Open Access article distributed
under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. |
|
Abstract The advantage of using a Human
Leukocyte Antigen (HLA)-mismatched related donor is that almost every
patient who does not have an HLA-identical donor or who urgently needs
hematopoietic stem cell transplantation (HSCT) has at least one family
member with whom shares one haplotype (haploidentical) and who is
promptly available as a donor. The major challenge of haplo-HSCT is
intense bi-directional alloreactivity leading to high incidences of
graft rejection and graft-versus-host disease (GVHD). Advances in graft
processing and pharmacologic prophylaxis of GVHD have reduced these
risks and have made haplo-HSCT a viable alternative for patients
lacking a matched donor. Indeed, the haplo-HSCT has spread to
centers worldwide even though some centers have preferred an approach
based on T cell depletion of G-CSF-mobilized peripheral blood
progenitor cells (PBPCs), others have focused on new strategies for
GvHD prevention, such as G-CSF priming of bone marrow and robust
post-transplant immune suppression or post-transplant cyclophosphamide
(PTCY). Today, the graft can be a megadose of T-cell depleted PBPCs or
a standard dose of unmanipulated bone marrow and/or PBPCs.
Although haplo-HSCT modalities are based mainly on high intensity
conditioning regimens, recently introduced reduced intensity regimens
(RIC) showed promise in decreasing early transplant-related
mortality (TRM), and extending the opportunity of HSCT to an elderly
population with more comorbidities. Infections are still mostly
responsible for toxicity and non-relapse mortality due to prolonged
immunosuppression related, or not, to GVHD. Future challenges lie in
determining the safest preparative conditioning regimen, minimizing
GvHD and promoting rapid and more robust immune reconstitution. |
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is a valuable treatment option for patients with various hematological disorders who lack a suitable HLA matched donor or for whom an HSCT is urgently required.[1-3] Actually, the road to full maturity of haplo-HSCT was beset by clinical problems. Until the early 1990s, haplo-HSCT was associated with a high incidence of graft rejection in T-cell–depleted transplants and severe graft-vs-host disease (GVHD) in unmanipulated transplants because of the high frequency of T cells that recognized major class I or II HLA disparities between donor and recipient.[3-5] To overcome these problems, two approaches were developed: a megadose of T-cell–depleted hematopoietic progenitor cells without any post-transplant immunosuppression[4,6,8,9] and unmanipulated grafts with innovative pharmacological immunosuppression for GVHD prophylaxis.[3,5,10-12]
Whereas the use of reduced intensity conditioning (RIC), infusion of mega doses of CD34+ cells, and graft manipulations such as selective T cell depletion were helpful to achieve engraftment with lower rates of GvHD and toxicity, delayed immune reconstitution and infectious complications remain outstanding issues for haplo-HSCT and are important causes of morbidity and mortality.[3,7,10,12,13] In the early post-transplant period, neutropenia is the principal risk factor for infections while, once engrafted, the capacity to mount an adaptive immune response to pathogens is a key factor for protecting from severe and recurrent infectious complications.
This review describes the most common infectious complication of the haploidentical HSCT and the mechanisms that may have a role in the incidence and severity of these complications.
Post-Transplant Immunological Reconstitution and Infections
Reconstitution of the T-cell pool after HSCT is achieved both through peripheral expansion of naïve and memory T-cells,[14] and de novo differentiation of hematopoietic stem cells in the thymus.[15] T-cells originating from peripheral expansion would most likely have a more limited TCR repertoire. They could also, at least in theory, be more allo-reactive, not having gone through the process of negative selection in the recipient. In adults, due to the decay in thymus function, post-grafting immune recovery depends, for months, on the expansion of the mature T cells infused with the graft. Naıve T cells are produced months after transplantation because conditioning induced tissue damage prevents T cell homing to peripheral lymphoid tissues, where T cell memory is generated and maintained.[17] Furthermore, the post-HSCT adaptive immune response is influenced by the strategy used to prevent GvHD.[3,6,13] In unmanipulated haplo-HSCT, peripheral T-cell expansion is antagonized by the immune suppressive therapy for GVHD prophylaxis. In T cell depleted haplo-HSCT the T-cell repertoire is very narrow since the number of T lymphocytes in the graft has to be particularly low to prevent GvHD, and anti-thymocyte globulin (ATG) in the conditioning exerts an additional in vivo T-cell depletion.[13,18] Even in the absence of pharmacologic agents, GVHD itself is known to have deleterious effects on immune function and can cause profound lymphoid hypoplasia, B cell defects and damage to thymic stroma, resulting in impaired T cell development.[19] Thus, the immune recovery is slow, and patients tend to remain susceptible to opportunistic infections for several months after HSCT. In a recent review, Fabricius and Ramanathan[3] reported data obtained from retrospective comparative studies by Raiola et al.,[20] Ciurea et al.,[21] and Gragert et al.[1] and a prospective study by Wang et al..[22] The incidence of infection complications after haploHSCT compares favorably with that reported after any other HSCT from alternative donors (Table 1). The rate of fatal infections was 11% in haplo, 14% in MUD, 17% in UCB and 4% in MSD.
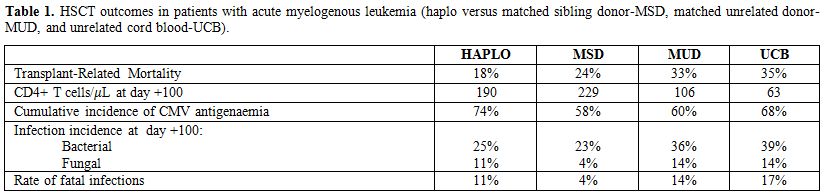 |
Table 1. HSCT outcomes in patients with acute myelogenous leukemia (haplo versus matched sibling donor-MSD, matched unrelated donor-MUD, and unrelated cord blood-UCB). |
Bacterial infections.
The incidence of bacterial infections varies according to transplant
phase, preparative conditioning regimens, underlying disease, disease
status, patient’s age and comorbidities.[23] Severity
and duration of the neutropenia, the presence of central venous
catheters and mucositis are still the most important risk factors for
bacterial infections during the pre- and peri-engraftment periods. On
the other hand, the incidence of bacterial infections in the
post-engraftment phases is low. The intensity of the conditioning
regimen plays a role in the risk of bacterial infections. Using a
reduced intensity conditioning (RIC) regimen instead of a myeloablative
(MA) protocol significantly reduces the severity of the mucosal damage
as well as the length and the severity of the neutropenic phase and
consequently the risk of the translocation of bacteria into systemic
circulation. A recent review by Balletto and Mikulska on epidemiology
and incidence of bacterial infections after haplo-HSCT confirmed that
bloodstream infections (BSIs) are the most frequent documented
bacterial infections followed by pneumonia and gastrointestinal
infections, including typhlitis and infections due to Clostridium
difficile.[23] However, defined data about the
cumulative incidence of bacterial infections in haplo-HSCT are lacking,
due to the presence of various haplo-HSCT platforms that differ
substantially from each other.
Gut microbiota.
Microbial communities in the gut are important in protecting the host
against pathogenic microbes. Notably, gut microbiota plays important
roles in the normal architecture of secondary lymphoid organs,
differentiation of induced regulatory T cells and generation of
IgA-secreting B cells as well as of memory alloreactive T cells.[24,25]
In physiological condition the gut microbiota is essential to promote
mucosa integrity and downregulate pro-inflammatory cytokines[26,27] while disruption of microbiota is potentially responsible for other infections.[28]
During allo-HSCT, the diversity, and stability of the intestinal
microbiota are disrupted and became dominated by bacteria associated
with subsequent bacteremia.[29] Several studies have
shown that gut microbial dysbiosis may have a link with complications
after HSCT, including GVHD. In mouse models and patients with GVHD
after BMT, Jenq et al.[30] observed a loss of
microbial diversity and Clostridiales and expansion of Lactobacillales
in intestinal microbiota. Eliminating Lactobacillales from the gut
flora in mice before BMT could cause GVHD. When reintroducing a
predominant species of Lactobacillus, GVHD was alleviated. After HSCT,
a relative shift toward Enterococci in intestinal microbial communities
was also found. Specifically, the shift was prominent in patients who
subsequently developed or suffered from active gastrointestinal GVHD.[31]
Interesting evidence of intestinal dysbiosis in HSCT recipients could
be exemplified by increasing the incidence of Clostridium difficile
infection (CDI) after allo-HSCT. A better understanding of the
relationship between gut microbiota and complications after allogeneic
HSCT may make gut microbiota as a therapeutic target in the future.[25]
Invasive fungal infection. Haplo-HSCT recipients are at high risk of invasive fungal infection (IFI) involving Candida and Aspergillus species.[32,33]
In studies focusing on IFI, alternative transplants are described
together with other allogeneic transplants, but the incidence rate of
IFI may vary considerably from 7.3% in HLA-identical sibling to 27% in
alternative HSCT recipients.[32-34] The
epidemiological findings of IFI have been longer reported in
retrospective studies, and only few prospective series have been
recently published.[32] The Italian Cooperative Group
[Gruppo Italiano Trapianto Midollo Osseo (GITMO)] conducted a large,
multicenter, prospective epidemiological study on 1858 patients.[33]
The cumulative incidence of proven or probable IFI was 5.1, 6.7 and
8.8% at 40 days, 100 days and 12 months from transplant, respectively.
Invasive aspergillosis was the most common infection (81.1%), followed
by invasive candidiasis (11.0%), zygomycosis (3.7%) and fusariosis
(1.8%). This study demonstrated that patient populations with different
types and severity of GVHD and with different donors are at
significantly different infectious risks. The cumulative incidence of
IFD in patients with acute GVHD, not followed by a chronic GVHD, was
2.3% and 10% after transplant from matched related donor and
alternative donors, respectively. The infectious risk became higher
when acute GVHD was followed by chronic GVHD, as 10% of transplants
from matched-related donor and 25% of those grafted from alternative
donors were complicated by an IFD. Of interest, the cumulative
incidence of IFDs was relatively low (<4%) in patients with a
chronic GVHD not preceded by an acute GVHD, also named ‘de-novo’
chronic GVHD, regardless of the type of donor. As expected, the risk
for IFD was significantly higher in patients with grade III–IV as
compared with those with grade II acute GVHD.[33]
Actually, the most recent data on unmanipulated haplo-HSCT show that
IFI does not represent a relevant posttransplant complication as
compared to MUD and UCB transplants that remain at higher risk for IFI,
particularly invasive aspergillosis (Table 2).[35-39]
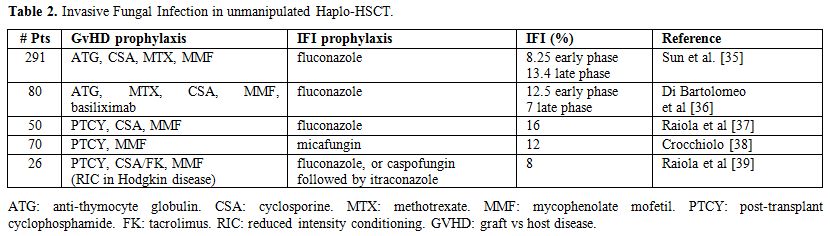 |
Table 2. Invasive Fungal Infection in unmanipulated Haplo-HSCT. |
Viral infection.
Reactivation of latent viruses such as cytomegalovirus (CMV), Epstein
Barr virus (EBV), herpes simplex virus (HSV) and varicella zoster virus
(VZV) commonly causes symptomatic disease. CMV and EBV may cause
pneumonia and post-transplantation lymphoproliferative disease (PTLD),
respectively.[41,42] Despite the use of prophylactic
and preemptive treatment, CMV-related infection continues to affect
outcome of haplo-HSCT and represents one of the main causes of
morbidity and mortality.[43-45] The combination CMV
donor-negative into CMV recipient-positive is associated with the
highest risk for CMV infection and disease.[45] In a
retrospective study based on the EBMT registry database, CMV
seropositive patients receiving seropositive unrelated donor grafts had
reduced NRM and improved survival compared to those receiving
seronegative grafts.[46]
Although surveillance
and therapeutic strategies have been established for several viral
infections (including CMV infection), such strategies are not yet
established for adenovirus (AdV) infection. AdV infection, usually
resulting from reactivation of the virus,[47] can be
divided into three categories: asymptomatic AdV infection, localized
AdV disease (e.g., hemorrhagic cystitis (HC), colitis, and pneumonia),
and disseminated AdV disease. The reported mortality of patients with
disseminated AdV disease is remarkably high, varying from 20% to 80% in
different studies.[47-49] Due to the low incidence of
AdV viremia in the absence of clinical symptoms no consensus exists on
whether systematic surveillance for AdV viremia should be performed for
adult patients at high risk.[50] Taniguchi and coll[48]
in Japan observed a high incidence 85.8%) of disseminated AdV disease
in adult patients undergoing unmanipulated haplo-SCT whose immunologic
reconstitution was compromised by the use of ATG and steroid as GVHD
prophylaxis. Disseminated AdV disease was always associated with HC.
Ethnicity appears to have an influence on these results because
Japanese patients have a higher incidence of latent infection of group
B AdV, which can translate into a higher incidence of AdV-associated
HC. Overall, AdV commonly causes severe morbidity and mortality in
children while the incidence of ADV infection in adult patients is low
and so the usefulness of routine monitoring of AdV DNA in asymptomatic
patients remains uncertain.[51] Other viral infections, such as the polyoma BK virus (BKPyV) contribute to the occurrence of HC [52].
HC is associated with significant and prolonged morbidity, especially
in highly immune compromised patients, increasing hospitalization
duration and, consequently, the cost of transplant procedures.
Infections
due to HHV-6 are generally encountered earlier than CMV. HHV-6 may lead
to engraftment delays or graft failure. It may also cause a prominent
disease with a facial rash and confused with encephalitis or acute
GvHD.[53,54]
Parasitic Infections.
The incidence of toxoplasmosis in allo-SCT recipients is high in areas
of endemicity, ranging from 6% in Europe and 3% in Brazil compared to
50.5% in USA or Japan.[55] Patients with
toxoplasmosis can present with fever, lymphadenopathy,
hepatosplenomegaly, meningitis, brain abscess, chorioretinitis,
pneumonitis, myocarditis, hepatitis, pancytopenia or disseminated
disease. Symptoms often present within 3 months post-transplant, but
later presentations may occur, particularly after discontinuation of
chemoprophylaxis, or even interruption of the anti-pneumocystis
prophylaxis with cotrimoxazole, which may exert a protective role.[56,57]
Diagnosis can be difficult in the absence of typical ring enhancing
brain lesions on computerised tomography or magnetic resonance imaging
scans in some cases. The highest risk patients are seropositive
allo-SCT recipients who have received cord blood or unrelated donor
transplant, T cell depleted transplants.
T Cell Depleted HaploHSCT
In general, TCD techniques can be classified as in vitro if the stem
cell manipulation is performed ex vivo exclusively, normally by column
adsorption. In contrast, in vivo techniques are based on a partial or
complete depletion of donor lymphocytes in the patient after
transplanting the stem cell product using ATG or alemtuzumab. In the
early ‘1990s, Aversa et al. exploited the principle of a megadose T
cell depleted HaploHSCT in patients with acute leukemia and showed that
an extensive ex vivo T-cell depletion followed by the infusion of a
mega-dose of immune-selected CD34+ cells prevents both graft rejection
and GvHD even in the absence of post-transplant immunosuppression.[8,9]
However, due to the delayed immunological reconstitution, infectious
complications remained an issue, in particular with a focus on viral
infections in the early and intermediate posttransplantation phase. In
their analysis of 103 patients with high-risk acute leukemia, 27 of the
38 non-relapsing deaths were caused by infections, whether viral (14
CMV, 1 HHV6, 1 adenovirus, 1 EBV), fungal (4 Aspergillus fumigatus, 1
Candida albicans), or bacterial (2 Pseudomonas aeruginosa, 2
Streptococcus viridans, 1 Escherichia coli).[58] The Swiss Blood Stem
Cell Transplantation group retrospectively reviewed the haplo-HSCT
performed in Switzerland from 1998 to 2010 with the aim of analyzing
the effect of in vitro TCD with graft engineering (CD34 selection or
CD3/CD19 depletion, 74%) or in vivo TCD using alemtuzumab (26%) on
immune reconstitution and infections.[59] Despite anti-infective
prophylaxis, most patients (94%) experienced post-transplant infectious
complications, 11 patients had four infections. The overall incidence
of bacterial infections was 56/69 (81%), most frequently due to
gram-negative bacteria (26%) and staphylococcal infections (16%).
Fungal infections occurred in 22/69 patients (32%) and viral infections
in 45/69 (65%) patients, mostly CMV (35%), herpes (24%) and BK (23%)
virus infections. Patients with in vivo TCD had a higher incidence of
CMV reactivations (54% vs. 28%, p= 0.015), but this did not result in a
higher NRM. The main reasons for NRM were acute respiratory distress
syndrome (n=4), viral infections (n=3), pulmonary aspergillosis (n=2),
Pneumocystosis (n=1), sepsis (n=2), toxoplasmosis (n=1), GVHD (n=1) and
myelitis (n=1). This study confirms that alemtuzumab represents an
important risk factor for CMV reactivation in patients receiving
haplo-HSCT.[60]
As the Achilles heel of T cell depleted haploHSCT
was linked to the paucity of T lymphocytes in the graft, over the past
decade, various strategies of adoptive donor T-cell immunotherapy have
been investigated to improve immune recovery and reduce non-relapse
mortality (NRM) from infectious complications.
Infusion of Pathogen-Specific T Cells.
Some groups have focused on the adoptive transfer of pathogen-specific T
lymphocytes against CMV, aspergillus, adenovirus and EBV. In the
original study by Perruccio et al.,[61] large numbers of donor
pathogen-specific T-cell clones were generated, then screened
individually for alloreactivity against recipient cells, deleted of
those cross-reacting against recipient alloantigens, and infused soon
after haplo-HSC. Infusion of Aspergillus-specific type-1 CD4+ clones
controlled Aspergillus antigenemia and helped to clear invasive
aspergillosis in 9 of 10 patients. Similarly, infusion of CMV-specific
CD4+ clones largely prevented CMV reactivation and reduced CMV
mortality. Since clearance of virally infected cells is mediated by
specific CD8+ cytotoxic cells, the infused CD4+ cells might have
conditioned APCs to stimulate the CMV-specific CD8+ T cells transferred
with the graft, thus promoting their clonal expansion. In fact, unlike
non-infused control patients, CMV-specific CD8+ cells were detected
shortly after infusing CMV-specific CD4+ clones. Among patients
receiving T-cell therapy, total CD4+ and CD8+ T-cell counts were
significantly higher. The successful transfer of immunity to
Aspergillus and CMV triggered neither acute nor chronic GvHD.[61]
An
alternative to pathogen-specific therapy is adoptive T-cell
immunotherapy, which provides large numbers of wide repertoire cells,
mirroring the physiologic immune system. The key challenge is to infuse
sufficient T cells without causing GVHD. Strategies include broad
repertoire T cells depleted of alloreactive T lymphocytes or engineered
with a suicide gene.
Ex Vivo Photodepletion of Alloreactive Donor T Cells.
Photodynamic purging appears to be an effective strategy for
selectively depleting donor alloantigen-specific T cells, thus
preventing GvHD and preserving the T cell anti-leukemia function. In a
mixed lymphocyte reaction, alloantigen-stimulated T cells uptake
4,5-dibromorhodamine methyl ester (TH9402), a compound that is
structurally similar to Rhodamine.[62] The study by Perruccio et al.,[63]
investigated a range of parameters, and combinations thereof, with the
aim of achieving optimal T cell allodepletion and preservation of
pathogen-specific responses. The remarkable drop in frequency of
alloreactive T cells is expected to allow safe infusion of relatively
large numbers of T cells across histocompatibility barriers for
adoptive transfer of donor immunity. Patients up to age 62 years with
high-risk hematologic malignancies were enrolled in a phase-1 dose
escalating study.[64] All patients engrafted rapidly, and no severe acute
GVHD occurred in the absence of immune suppressors. Higher doses were
associated with lower TRM and improved survival. This effect was mainly
attributed to a decrease in infectious complications and low relapse
rates. These findings led to the initiation of an international
multicenter phase II clinical trial and, at interim analysis, patients
receiving 2x106/kg photodepleted CD3+ T cells did not have severe GVHD
and demonstrate a high overall survival (69% at 12 months after HSCT).[10]
Infusion of T Cells Engineered to Express Suicide Genes.
Polyclonal T cells were engineered to express suicide genes, eg, the
herpes simplex thymidine kinase (HSV-TK) gene, to guarantee engineered
cell lysis if they triggered GvHD [65-68]. Ciceri et al. reported the
results in a cohort of 50 high-risk leukemia patients enrolled in phase
I–II, multicentre, non-randomised trial.[67] Overall, there were 196
infectious events (median four events per patient, range 0-14), 161 of
which occurred with 130 days. In immune reconstituted patients,
progressive normalization of antiviral responses was associated with a
decline in the number of infectious events, while patients who failed
immune reconstruction continued to have frequent infectious
complications. After 130 days, median peaks in blood titres of CMV
antigen were 0 nuclei per 10⁵ peripheral blood mononuclear cells (PBMC)
(range 0–20) in immune reconstituted patients and 21 nuclei per 10⁵
PBMC (range 14–58) in patients without immune reconstitution
(p<0·0155); and median length of antiviral treatment was 0 days
(range 0–44) in immune reconstituted patients and 47 days (range
33–105) in patients without immune reconstitution (p<0·0052). The
conditional benefit of immune reconstitution obtained by TK-cell
infusion was assessed by the cumulative incidence of non-relapse
mortality for patients alive 100 days after transplant; non relapse
mortality was 14% (infectious mortality 9%) in TK-treated
immune-reconstituted patients and 60% in non-immune-reconstituted
patients. A randomized phase III trial to address the role of HSV-TK
donor lymphocyte addbacks for recipients of haplo-HSCT is ongoing at
present.
Other researchers devised an inducible T-cell safety
switch based on the fusion of human caspase 9 to a modified human
FK-binding protein, allowing conditional dimerization and cell suicide
following administration of the small molecule dimerizing drug
AP1903.[69] Since preliminary interesting results, the Rome group has
recently launched a phase I/II study enrolling children with either
malignant or nonmalignant disorders who will receive TCR-αβ/B cell
depleted HaploSCT, followed by the infusion of titrated numbers of iC9
T cells on day 14±4. These iC9-modified T cells can contribute to T
cell immune reconstitution after T cell depleted HaploSCT and are
eliminated by the administration of AP1903, if aGVHD occurs.[70]
Regulatory T Cells.
More recently, a pioneer experience of the Perugia group has clearly
demonstrated that naturally occurring Tregs harvested from healthy
donors efficiently control the alloreactivity of a large number of
otherwise lethal, conventional T cells.[71-73] Using this strategy, there
was a rapid, sustained increase in peripheral blood T-cell
subpopulations. A wide T-cell repertoire developed rapidly. Naïve and
memory T-cell subsets increased significantly over the first year after
transplantation, demonstrating sustained immune recovery over time.
B-cell reconstitution was rapid and sustained and immunoglobulin serum
levels normalized within 3 months. Compared with standard haplo-HSCT,
specific CD4+ and CD8+ for opportunistic pathogens such as Aspergillus
fumigatus, Candida albicans, CMV, ADV, HSV, and toxoplasma emerged
significantly earlier, fewer episodes of CMV reactivation occurred, and
no patient developed CMV disease. Nevertheless, 8 of the 13 non-relapse
deaths were due to infections: adenoviral infection (n=2), bacterial
sepsis (n=1), toxoplasmosis (n=1), fungal pneumonia (n=3) or central
nervous system aspergillosis (n=1).
Selective T cell
depletion. Other attempts to improve post-transplant immune recovery
focused on improving graft content by shifting from CD34-positive
selection to negative selection of PBPCs so as to include other immune
cells.[10,74] Selective T-cell removal means depletion of a given subset
of the whole T-cell population. The aim is to reduce the incidence of
GvHD while preserving other beneficial cell functions carried out by
the residual T-cell subsets. In an innovative approach, Handgretinger’s
group in Tubingen depleted the leukapheresis product of only TCR αβ+ T
cells, thus retaining large numbers of effector cells such as TCRγδ+ T
cells and NK cells.[75,76] TcRγδ+ T cells combine conventional adaptive
features with direct, rapid responses against sterile stresses and many
pathogens. They participated in the anti-CMV response in the early
period of post-transplant immune recovery. They are not expected to
initiate GVHD because they do not recognize specifically processed
peptide antigens as presented on major histocompatibility complex (MHC)
molecules. First clinical results of these new T-depletion strategies
are encouraging and interestingly none of the studies reported a
significantly increased incidence of infections, even using MAC
regimens.[75-78] This could be partially explained by the high number of
γδ T cells in donor’s graft. Indeed, γδ T cells are considered as a
bridge between adaptive and innate immunity. γδ T cells receptors
detect unconventional antigens such as phosphorylated microbial
metabolites and lipids, non-classical MHC-I molecules and unprocessed
proteins.[79] They are concentrated within epithelial and mucosal
surfaces to maintain the epidermal integrity of the skin and intestinal
epithelium.[80] It has been hypothesized that tissue-specific antigens
are recognized by γδ T-cells resulting in immune responses protecting
potential sites of pathogen entry into the body.[81]
In two cohorts
of children transplanted either in Tubingen[75,76] or in Roma,[77,78] no
post-transplant GVHD prophylaxis was given. Engraftment was very rapid
in all patients. Few had acute grade I-II GVHD, and none developed
chronic GVHD. Immune reconstitution was fast. Locatelli et al.[77]
prospectively assessed functional and phenotypic characteristics of γδ
T lymphocytes up to 7 months after haplo-HSCT depleted of αβ+ T cells
and CD19+ B cells in 27 children with either malignant (n=15) or
nonmalignant disorders. Notably, in patients that experienced CMV
reactivation they observed a significant expansion of Vδ1 T-cell
subset; these subsets display a cytotoxic phenotype and degranulate
when challenged with primary acute myeloid and lymphoid leukemia
blasts. These results have been recently confirmed in 23 children with
non-malignant disorders.[82] The cumulative incidence of grade 1 to 2
acute GVHD was 13.1%. None of the 21 patients at risk developed chronic
GVHD. The 2-year DFS was 91%. Two died of infectious complications
(one CMV-related pneumonia and one disseminated adenovirus infection)
120 and 116 days after HSCT, respectively. Overall, 9 children
experienced viral infections and/or reactivations, the cumulative
incidence of CMV and adenovirus infection being 38%. Nevertheless, the
cumulative incidence of TRM was 9%.
Perko et al.[83] recently
investigated immunological reconstitution of 102 pediatric patients
with acute leukemia who underwent HSCT in first complete remission,
focusing on the potential role of γδ T-cells. They found that γδ T cell
recovering during the first year after HSCT correlated with a reduced
incidence of infection. Indeed, patients with an elevated number of γδ
T cell experienced only viral infection, while low/normal γδ T cell
group had viral, bacterial and fungal infections; cumulative incidence
of bacterial infection was 0% vs. 26.4%, respectively. Enhanced γδ T
cell recovery resulted in higher EFS rate at 1 year. A possible reason
to explain these results could include faster reconstitution of
intestinal mucosa integrity, or prompt anti-infective function of γδ T
cell, and possibly a better balance within gut microbiota.
To
determine whether the post-transplant immunological reconstitution can
be improved even in adult patients, this approach was recently tested
in 38 adult patients, median age 35 years (range 19-73), with acute
leukemia (n=28), lymphoma (n=4) or others diseases (n=6) [84].
Conditioning included ATG, Treosulfan, Fludarabine, and Thiotepa. No
pharmacologic prophylaxis for GvHD was given after transplantation.
Grafts contained a median of 11,6 x106/kg (range 5-19) CD34+ cells, 4
x106 CD3+ T cells/kg (range 1-35), 4,4 x104/kg (range 0.4-62) αβ+T
cells/kg, 3,85 x106 γδ+ Tcells/kg (range1-34), 4,9 x104 B cells/kg
(range 1.8-32) and 23,40 x108 CD56+NK cells/kg (range 8-91). All
patients but one achieved rapid, sustained full donor-type engraftment,
with a median time to reach 500 neutrophils and 20,000 platelets of 12
(range 10-18) and 11 days (range 6-16), respectively. Overall, acute
GvHD occurred in 6 patients. Only one patient, who had received the
highest dose of αβ+ T cells (3.7x105/kg), developed and died from grade
III-IV acute GVHD. Skin limited acute GvHD occurred in the remaining 5
patients. Only one patient progressed to moderate chronic GvHD. Tending
to confirm the working hypothesis, there was a rapid, sustained
increase in peripheral blood T-cell subpopulations. The CD4 and CD8
counts reached 200/μL medianly on, respectively, days 45 (range, 19-98)
and 38 (range, 13-69). Naïve and memory T-cell subsets increased
significantly over the first year after transplantation. B-cell
reconstitution was rapid and sustained and immunoglobulin serum levels
normalized within 3 months. A single CMV reactivation occurred in 4
cases (in only one with unfavorable CMV-serology, CMV recurrence was
documented 3 times), no patient has so far developed CMV disease. In
two patients, CMV reactivation was associated with a significant
expansion of pathogen-specific CD8+ T cells and both cleared viral load
spontaneously. No patient had Epstein-Barr virus-related
post-transplantation lymphoproliferative disease, and no invasive
fungal disease occurred. The cumulative incidence of NRM was 20% even
though 11 patients were in the upper age for transplantation (between
61 and 73 years). Overall, six of the 11 non-relapse deaths were due to
infections: 1 EBV, 1 adenovirus, 1 RSV, 1 miliary tuberculosis, 2
gram-negative sepsis.
All these recent experiences confirm that
current T cell-depleted HSCT strategies (either Treg/Tcon
immuno-therapy or αβ T cell depletion) offer the unique opportunity to
harness both natural and adaptive immunity to control leukemia relapse
and infections in the absence of GvHD.
Unmanipulated haploHSCT
Crossing the histoincompatibility barrier in HSCT is today feasible
without ex vivo T- cell depletion. Two major approaches have been so
far used: the GIAC-based strategy and the posttransplant CY-based
protocol.
The “GIAC” Strategy. This modality is based on the
following four elements: (G) donor treatment with recombinant
granulocyte colony-stimulating factor (rhG-CSF); (I), intensified
immunologic suppression; (A), (ATG; (C), a combination of PBPCs and
bone marrow cells. In the original study, Huang et al.[85] reported the
results in 171 patients who had received a myeloablative conditioning
and intensive posttransplant immunosuppression that included ATG,
cyclosporine, methotrexate, mycophenolate mofetil, and anti-CD25
antibody (basiliximab). All patients achieved sustained, full donor
chimerism. The 2-year incidence of opportunistic infections was 40%. In
their most recent update including 250 acute leukemia patients, a total
of 120 occurrences of opportunistic infections were recorded in 106
patients during the duration of follow-up.[86,87] The median time for an
opportunistic infection to develop was 280 days (range, 5-1120) after
transplantation. The infected loci included lungs (74 occurrences),
skin (28 occurrences), gastrointestinal tract (24 occurrences), and
central nervous system (6 occurrences). Causes of infections of the
skin were varicella-zoster (16 cases) and herpes simplex virus (12
cases). Pneumonia was due to bacteria in 13 cases, Aspergillus in 18
cases, Candida albicans in 1 case, Pneumocystis carinii in 5 cases, and
CMV in 16 cases. In the other 21 cases, no pathogen could be
documented, and 9 of them responded to antibiotics. At 3 years after
transplantation, the cumulative incidence of opportunistic infections
was 49.1%. The cumulative incidence of grade III-IV aGVHD was 13.4%,
the incidence of cGvHD and extensive cGVHD at 2 years was 54% and
22.6%, respectively. Even though a higher disease-free survival was
achieved –partly due to the inclusion of standard and good risk
patients - the concern remains that a higher incidence of GVHD is
usually associated with a higher treatment-related mortality and higher
cost of care for these patients.
Consistent with their previous
work, the Benjing group showed that high-dose ATG was associated with
delayed recoveries of CD19+ B cells, CD3+ T cells, and CD4+ T cells
during the first month after haploHSCT.[88] Furthermore, they also showed
that high-dose ATG delayed the recoveries of CD4+, CD4+CD45RA+, and
CD4+CD45RO+ T cells for 2 months, delayed the recovery of CD4−CD8− T
cells for 6 months, and delayed the recovery of CD8+CD28+ T cells for
12 months after transplantation. The persistent delay in CD4−CD8− T
cell recovery was closely related to an increased risk of EBV infection
post-haploHSCT. The study showed that the schedule based on 6 mg/kg ATG
was associated with a faster recovery of T cell subsets and a lower
incidence of EBV infection compared to the schedule of 10 mg/kg ATG.
Using
the Peking-based strategy, Di Bartolomeo et al.[36] yielded promising
results in 80 acute leukemia patients (median age of 37 years, range,
5-71). A myeloablative conditioning (MAC) regimen was used in 64 (80%)
patients and a reduced intensity conditioning (RIC) in the other 16
(20%). They achieved a 91% engraftment rate, with a median of 21 days
(range, 12-38) for the absolute neutrophil count and 28 days (range,
14-185) for platelets. The cumulative incidences of grade 2-4 aGVHD and
cGVHD were 24% and 17%, respectively. Twenty-seven patients (34%), 13
in the standard-risk group and 14 in the high-risk group, respectively,
died from transplantation related complications at a median time of 76
days (range, 6-369). Causes of death included infections in 11 patients
(14%), pneumonia in 5 (6%), multiorgan failure in 5 (6%), acute GVHD in
3 (4%), liver failure in 1 (1%), veno-occlusive disease in 1 (1%), and
CNS disease complications in 1 (1%). TRM was 32% at 6 months and 36% at
1 and 3 years. In the first 6 months after transplantation, 56 patients
developed CMV reactivation (C.I. 70%), 38 bacterial septicemia (C.I.
47%), 25 hemorrhagic cystitis (C.I. 31%), 13 CNS complications (C.I.,
16%), 10 fungal infections (C.I. 14%), and 5 veno-occlusive disease
(C.I., 6%). The 3-year probability of OS for all patients was 45% (54%
for the standard-risk group and 33% for high-risk group (P=06).
Arcese
et al. [89] have recently updated the results of 97 patients who
received a single conditioning regimen, even though with different
intensity according to age and comorbidity (TBF-MAC=68; TBF-RIC=29),
before the infusion of an unmanipulated G-CSF-primed BM from a
haploidentical donor. Regardless of the conditioning regimen, the GvHD
prophylaxis was identical for all the patients and included five drugs:
ATG, CSA, MTX, MMF and the anti-CD25 monoclonal antibody (basiliximab).
Neutrophil and platelet engraftment rates were 94% and 84%,
respectively. The cumulative incidence of grade II-IV acute and
extensive chronic GvHD was 31% and 12%, respectively. Overall, 31
patients (32%) died of transplant-related complications at a median of
76 days (range 9–527). The infections were the leading cause of NRM
accounting for 48% of all deaths. At 1 and 5 years, NRM was 31% and
34%, respectively.
Post-transplantation Cyclophosphamide.
The most commonly used new strategy for GvHD prevention is in vivo
depletion of bidirectional alloreactive T lymphocytes by means of high
doses of post-transplant CY (PTCY).[2,5,10-12] Indeed, PTCY
preferentially targets proliferating alloreactive T cells that are
activated in vivo after recognition of alloantigen, thus reducing the
risk of both GvHD and graft rejection in a combination of additional
post-transplant immune suppression with tacrolimus and MMF. The optimal
scenario in this setting would be to spare non-alloreactive donor naïve
and memory T cells, both to guarantee primary responses to newly
encountered antigens and, simultaneously, to confer adaptive immunity
to the recipient.[90] Using a non-myeloablative conditioning regimen of
CY, fludarabine, and 2 Gy TBI and GVHD prophylaxis with PTCY (50 mg/kg
days 3 and 4), MMF (days 5-35), and tacrolimus (days 5-180), a
sustained engraftment was achieved in 87% of 210 acute leukemia
patients treated by the Johns Hopkins group.[11] Grade II-IV and III-IV
aGVHD occurred in 27% and 5% of patients, respectively; cGVHD in 15%.
The cumulative incidences of relapse and NRM were 55% and 18%,
respectively. A total of 113 patients died of relapse (n=79),
infections (n=15), pulmonary complications (n=7), GVHD (n=5), or other
causes (n=7). Overall survival and event-free survival (EFS) at 3 years
were 41% and 32%, respectively. The high relapse rate, which was
probably the result of poor disease debulking by the nonmyeloablative
conditioning and lack of GVHD-related GVL effect, dampened the
advantage of a relatively low NRM. Switching to myeloablative
conditioning regimens (MAC) reduces the risk of relapse but increases
the NRM.[91] In an attempt to reduce the risk of relapse, they adopted,
in Genoa, a MAC regimen that consisted of either thiotepa, busulfan,
fludarabine (TBF) or TBI and fludarabine (F-TBI).[37] The choice of the
conditioning was based on the patient’s age, restricting TBI to
patients under the age of 56, and on whether they had already received
a previous allogeneic graft with TBI in the conditioning. Three
patients died before engraftment could be evaluated (one from
Legionella pneumonia); the cumulative incidence of engraftment was 90%
for neutrophils on day +32 and 86% for platelets on day +60. The CI
rate of grade II-III aGVHD was 12%, and no patient developed grade IV
aGVHD, and there was a 26% incidence of chronic GvHD. The cumulative
incidence of TRM at 6 months was 18%, most of the events occurring
early (median day +68), with 1 outlier on day +168. The CI rate of TRM
was 9% for patients in remission and 26% for patients in relapse
(P=0.10). Transplantation-related complications and infections were:
hepatic sinusoidal obstructive syndrome in 1 patient (2%), severe
mucositis in 3 patients (6%), hemorrhagic cystitis in 20 (40%),
pericarditis in 4 (9%). BK virus was detected in 13 patients (65%). CMV
reactivation occurred in 25 patients (50%) and CMV disease in 2 (1
colitis and 1 pneumonia) with a median time of CMV reactivation of 39
days (range, 3-60). EBV DNAemia was detected in 4 patients, HHV6
viremia in 1, adenovirus pneumonia in 1. Nineteen patients developed
sepsis, occasionally with more than one causative agent: 9
gram-negative sepsis (6 E. coli, 1 Enterobacteriacae, 1
Corynebacterium, and 1 Pseudomonas aeruginosa); 13 gram-positive
sepsis, most Streptococcus viridans and Enterococcus faecium. IFI were
seen in 9 patients (5 Aspergillus pneumonia, 2 Candida krusei sepsis,
and 1 Fusarium). Infections were the primary cause of death in 6
patients (12%), 3 sepsis and 3 pneumonia (Legionella, adenovirus, and
Invasive aspergillosis). With a median follow-up of over 8 months
(range, 4-22 months), the actuarial overall survival and DFS at 18
months from transplantation was 62% and 51%, respectively.
Crocchiolo
et al.[38] described infectious complications after unmanipulated
haploHSCT using PTCY in 70 consecutive adult patients with lymphoma and
found, aside from a high incidence of viral infections/reactivations,
especially in the early post-transplant period, a quite low incidence
of late bacterial infections, together with a very low incidence of
IFIs after day +180. At last follow-up, a total of 224 documented
infectious events occurred among 67 of 70 patients, with a median of 3
events/patient (range 1–10); 55% were of viral origin (n=123), 40%
bacterial (n = 89), 5% fungal (n = 11). Cumulative incidence of first
viral infection was 70% and 77% at day +100 and +365, respectively; at
one year, the incidence of bacterial infections and IFI were 63% and
12%, respectively. In 54% (35 of 65 patients at risk) at least one CMV
reactivation developed; two non-fatal (1 colitis, 1 pneumonia) and 1
fatal (pneumonia) CMV diseases occurred. No primary CMV infections
occurred in the 5 CMV seronegative patient/donor pairs.
Polyomavirus-related hemorrhagic cystitis was observed in 13 patients
(19%): 10 were caused by BK virus and 3 by JC virus. No EBV-related
lymphoproliferative disorders occurred. Forty-five patients (64%)
presented with at least one documented bacterial infection: 10 (14%),
21 (30%), and 14 (20%) patients had an infection by gram-positive,
gram-negative, or both types of bacteria, respectively. Eleven IFIs
were detected in 9 patients: n=6 probable invasive aspergilloses
(pneumonia in 5 patients and sinusitis in 1), n=5 invasive
candidiasis, all by non-albicans Candida (2 candidemias, 2 colitis, and
1 hepatosplenic candidiasis); median of occurrence was 62 days from
haplo-HSCT (range 0–739). Notably, no cases occurred under active GVHD,
and only 2 IFI occurred beyond day +180. When considering the timing of
all episodes, bacterial infections occurred mostly between day 0 and
+30, whereas viral infections/ reactivations between days +31 and +100,
with 11.08 bacterial events/1000 pt-days between day 0 and +30, and
15.15 viral events/1000 pt-days between days +31 and +100. The overall
incidence of viral events between day 0 and day +180 was 8.8
events/1000 pt days. A total of 13 bacterial and 13 viral infections
were observed after 1 year from transplant. These results are in line
with that reported usually in the setting of unmanipulated haplo-HSCT.
Ciurea et al.[92] analyzed the early results of haplo-HSCT in adult
recipients treated on 2 successive studies, and report improved
outcomes with T cell replete (TCR) grafts and PTCY. Viruses were the
most common cause of infection in both groups. During the first 180
days posttransplantation, there were 22 episodes per 1000 patient-days
in the TCD group and 11 episodes per 1000 patient-days in the TCR
group. Patients in the TCD group were 1.5 times more likely to develop
a viral infection (P=0.035) during this time period. Among the viral
infections, CMV reactivation and human polyomavirus BK cystitis were
the most frequent. Fourteen of 33 patients (42.2%) had CMV reactivation
with a total of 30 episodes in the TCD group compared with 15 of 32
patients (46.8%) with 24 episodes in TCR group. For human polyomavirus
BK cystitis, 15 of 33 cases (45.4%) were found in the TCD group versus
11 of 32 (34.4%) in the TCR group. There was no significant difference
in the incidence of bacterial infections between the 2 groups.
Thirty-two bacterial infections occurred in the TCR group compared with
38 episodes in the TCD group. On average, 7 and 10 episodes occurred
per 1000 patient-days for the TCR and TCD group, respectively. Invasive
fungal infections were third in frequency, with 12 episodes in 11 of 33
patients (33%) in the TCD group, and only 3 episodes in 3 of 32
patients (9%) in the TCR group. Patients in the TCD group were 5.6
times more likely to have an IFI within 6 months post-transplantation
than those in the TCR group. Survival analysis revealed a significantly
lower probability of death from an infection in the TCR group. The NRM
attributed to infections was 24% in the TCD group and 9% in the TCR
group (P = 0.01).
Similarly, Tischer et al.[93]
retrospectively
compared the incidence of virus infections and outcome in the context
of immune reconstitution in two different haplo-HSCT settings. The
first was a combined T-cell-replete and T-cell-depleted approach using
ATG over the conditioning (cTCR/TCD group, 28 patients; median age 31
years). The second was a T-cell-replete (TCR) approach using PTCY (TCR/
PTCY group, 27 patients; median age 43 years). The incidence of
herpesvirus infection was markedly lower in the TCR/PTCY (22%) than in
the cTCR/TCD group (93%). Recovery of CD4+ T cells on day +100 was
faster in the TCR/PTCY group. CMV reactivation was 30% in the TCR/ PTCY
compared to 57% in the cTCR/TCD group, and control with antiviral
treatment was superior after TCR/PTCY transplantation (100 vs. 50%
cTCR/TCD). Furthermore, EBV-related lymphomas were observed only in the
cTCR/TCD group (25% vs. 0%). Although the incidence of aGvHD
grades
II–IV and 1-yr overall survival were comparable in both groups, virus
infection-related mortality was significantly lower after TCR/PTCY
transplantation (0% vs 29%; p=0.009). Cumulatively, 139 occurrences
of virus infection were observed, 71 of them asymptomatic, 68
symptomatic, and 20 associated with disease. Virus infections affected
46 of 55 patients, whereas no virus infection was detected in nine
patients only. Cumulatively, 87 occurrences of viral pathogens were
identified in 27 patients (96%) of the cTCR/TCD group. Within these
occurrences, 51 patients were symptomatic or developed disease (n=17).
In the TCR/PTCY group, 52 occurrences were seen in 19 patients (70%),
17 symptomatic or suffering from disease (n=3). The most
frequently observed viral pathogens across both groups were HHV-6,
polyomavirus JC/BK, EBV, CMV, HSV, and ADV. In particular, symptomatic
virus infection and disease were induced by the Herpesviridae HSV, VZV,
CMV, EBV, and HHV-6 in 26 patients (93%) of the cTCR/TCD group, but
only in six patients (22%) of the TCR/PTCY group. Besides the
occurrence of HHV-6, for each of these Herpesviridae, the incidence of
virus infection-related symptoms and disease was distinctly higher in
the cTCD/TCR than in the TCR/PTCY group. On day +100, predictors of
better OS were lymphocytes >300/μl, CD3+ T cells >200/μl, and
CD4+ T cells >150/μl, whereas the application of steroids >1
mg/kg was correlated with worse outcome.
These results suggest
the strategy based on the infusion of unmanipulated graft followed by
PTCY by preserving antiviral immunity and allowing fast immune recovery
of CD4+ T cells is well suited to handle the important issue of
infection after haplo-HSCT.
Conclusion
Much progress has been made over the past 20 years in the clinical, biological and technical aspects of the T cell-depleted as well as of unmanipulated full-haplotype-mismatched HSCT. Haplo-HSCT has evolved from a last-attempt option for end-stage patients, to an established form of treatment that must be considered for selected patients with high-risk hematological disorders. Today, a high rate of engraftment can be achieved without severe GvHD and with low regimen-related toxicity and mortality. Although haplo-HSCT strategies differ according to each center and clinician, the current options include in vitro selective T cell-depleted “megadose” stem cell graft with no pharmacologic prophylaxis of GVHD or in vivo T cell depletion using the GIAC strategy or PTCY strategy (Table 3).
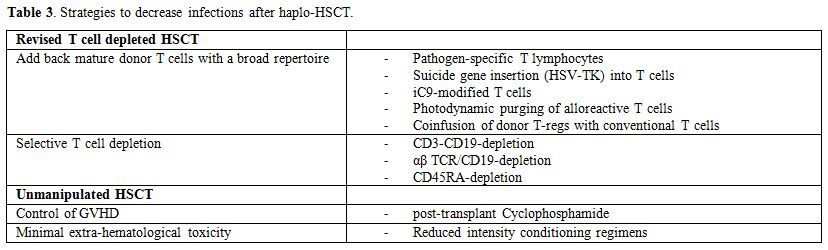 |
Table 3. Strategies to decrease infections after haplo-HSCT. |
Improving immunologic reconstitution remains a major issue,
as it represents the key to further decreasing toxicity and NRM in any
form of transplantation. In the last decade, numerous advances in graft
engineering and pharmacologic management of alloreactivity have
decreased the incidences of GVHD and improved immune reconstitution
offering the unique opportunity to harness both natural and adaptive
immunity to control infections making this graft source an acceptable
option for patients without a suitable matched donor.
References
























































































 .
. 


 .
. [TOP]