Vincenzo De Sanctis1, Ashraf T. Soliman2, Heba Elsedfy3, Saif AL Yaarubi4, Nicos Skordis5, Doaa Khater6, Mohamed El Kholy3, Iva Stoeva7, Bernadette Fiscina8, Michael Angastiniotis9, Shahina Daar10 and Christos Kattamis11
1 Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy
2 Department of Pediatrics, Division of Endocrinology, Alexandria University Children's Hospital, Alexandria, Egypt
3 Department of Pediatrics, Ain Shams University, Cairo, Egypt
4 Pediatric Endocrine Unit, Department of Child Health, Sultan Qaboos University Hospital, Al-Khoud, Sultanate of Oman
5 Division
of Pediatric and Adolescent Endocrinology, Paedi Center for Specialized
Pediatrics, St. George's University Medical School at the University of
Nicosia, Cyprus
6 Department of Pediatrics,
Endocrinology Unit, Alexandria University Children's Hospital, Egypt,
and Child Health Department, Sultan Qaboos University Hospital, Muscat,
Oman.
7 Paediatric Endocrinologist,“Screening and
Functional Endocrine Diagnostics” SBALDB. Professor Ivan Mitev, Medical
University Sofia, Bulgaria
8 Department of Pediatrics, NYU School of Medicine, New York, USA
9 Medical Advisor, Thalassemia International Federation (TIF), Nicosia, Cyprus
10
Department of Hematology, College of Medicine and Health Sciences
Sultan Qaboos University Oman, Sultanate of Oman & Visiting
Scholar, Stellenbosch Institute for Advanced Study (STIAS), Wallenberg
Research Centre at Stellenbosch University, Stellenbosch 7600, South
Africa.
11 First Department of Paediatrics, University of Athens, Athens, Greece.
Corresponding
author: Vincenzo De Sanctis MD, Pediatric and Adolescent Outpatient
Clinic, Quisisana Hospital, 44100 Ferrara, Italy; Tel.: +39 0532
770243; E-mail:
vdesanctis@libero.it
Published: October 28, 2016
Received: July 9, 2016
Accepted: September 20, 2016
Mediterr J Hematol Infect Dis 2016, 8(1): e2016058, DOI
10.4084/MJHID.2016.058
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Iron overload in patients with
thalassemia major (TM) affects glucose regulation and is mediated by
several mechanisms. The pathogenesis of glycaemic abnormalities in TM
is complex and multifactorial. It has been predominantly attributed to
a combination of reduced insulin secretory capacity and insulin
resistance. The exact mechanisms responsible for progression from norm
glycaemia to overt diabetes in these patients are still poorly
understood but are attributed mainly to insulin deficiency resulting
from the toxic effects of iron deposited in the pancreas and insulin
resistance. A group of endocrinologists, haematologists and
paediatricians, members of the International Network of Clinicians for
Endocrinopathies in Thalassemia and Adolescence Medicine (ICET-A)
convened to formulate recommendations for the diagnosis and management
of abnormalities of glucose homeostasis in thalassemia major patients
on the basis of available evidence from clinical and laboratory data
and consensus practice. The results of their work and discussions are
described in this article.
|
Introduction
β
Thalassemias are a group of inherited chronic hemolytic anemias
characterized by reduced (β +) or absent (β 0) synthesis of the β
globin chains of the haemoglobin A tetramer. They are particularly
common in people of Mediterranean, African, and Southeast Asian
ancestry. More than 30,000 babies are born with homozygous β-
thalassaemia worldwide each year and there are 100 million individuals
who are asymptomatic β-thalassaemia carriers. Three clinical and
haematological conditions of increasing severity are recognized: the β
-thalassemia carrier state, β- thalassemia intermedia (Non Transfusion
Dependent Thalassemia; NTDT) and β-thalassaemia major (TM).[1,2]
Today,
nearly all subjects with TM survive into adult life, and many patients
who have access to excellent care with proper chelation survive beyond
50 years of age. The improved survival of patients with TM results in
an increasing prevalence of complications of iron overload, including
abnormalities of glucose homeostasis. Disturbances of glucose
homeostasis range from increased insulin resistance and mild glucose
intolerance to overt diabetes mellitus. Patients with mild disorders
are usually asymptomatic; impaired glucose tolerance (IGT) is common,
occurring in up to 24.1%.[3-5] Unfortunately, this represents an
additional potential risk to their cardiac function.[6]
Although
iron overload induced DM shares certain characteristics with both type
1 diabetes and type 2 diabetes, it appears to be a separate entity with
a unique pathophysiology. As in type 1 DM, insulin deficiency is a
primary defect; however, it is usually relative rather than absolute.
Similar to type 2 DM, the onset of the disease is usually gradual and
insidious and insulin resistance is detected in some patients.[3,4]
Therefore,
patients with TM and health professionals should be aware of the high
incidence of glucose abnormalities in patients with thalassemia
syndromes.[5,7] Detecting the pre-diabetes stage is critical because
prediabetes and clinical diabetes can potentially be reversed or
prevented with optimum chelation treatment.[5,7]
Aims
The
International Network of Clinicians for Endocrinopathies in Thalassemia
and Adolescent Medicine (ICET-A)[8] planned the current project to
formulate recommendations for accurate diagnosis and effective
management of abnormalities of glucose homeostasis in patients with TM.
A brief
review and description of the clinical management of these patients is
also provided with particular attention to the assessment, prevention,
and treatment of iron overload. The
aim of this project is to support the clinical practice of
paediatricians, internists, haematologists and other physicians who
care for patients with TM. Diagnostic Criteria Used for the Assessment of Glucose Abnormalities
The
diagnosis of IGT and DM is currently made during a period of stable
baseline health according to standard American Diabetes Association
(ADA criteria).[9]
Criteria for diagnosis of diabetes mellitus (DM):
o
With classic symptoms of hyperglycemia or hyperglycemic crisis; a
random plasma glucose ≥ 11.1 mmol/L (≥ 200 mg/dL).
o
Fasting plasma glucose (FPG) ≥ 7.0 mmol/l (≥ 126 mg/dl) or 2-hour
plasma glucose ≥ 11.1 mmol/l (≥ 200 mg/dl). Fasting is defined as no
caloric intake for at least 8 h.
o Hemoglobin A1c (HbA1c) ≥ 6.5%.
The
test should be performed in a laboratory using a method that is
National Glycohemoglobin Standardization Program (NGSP) certified and
standardized to the Diabetes Control and Complications Trial (DCCT)
assay.
Criteria for increased risk for diabetes (prediabetes):
o Fasting plasma glucose (FPG) between 100 mg/di (5.6 mmol/L) to 125 mg/dl (6.9 mmol/l).
o 2-h PG, in the 75-g OGTT, between 140 mg/dl (7.8 mmol/L) to 199 mg/dL
(11.0 mmol/L). The test should be performed as described by the WHO,
using a glucose load containing the equivalent of 75 g anhydrous
glucose dissolved in water. In the absence of unequivocal
hyperglycemia, results should be confirmed by repeat testing.
o HbA1c between 5.7 and 6.4%
The
Canadian Diabetes Association Clinical Practice Guidelines Expert
Committee[10] recommends that the decision of which test to use for
diabetes diagnosis is left to the clinician’s judgement. Each
diagnostic test has advantages and disadvantages. In the absence of
symptomatic hyperglycemia, if the result of a single laboratory test is
in the diabetes range, a repeat confirmatory test must be done on
another day. It is preferable that the same test is repeated (in a
timely fashion) for confirmation.
In the case of symptomatic
hyperglycemia, the diagnosis has been made, and a confirmatory test is
not required before treatment is initiated. If results of 2 different
tests are available and both are above the diagnostic cut-off points,
the diagnosis of diabetes is confirmed. When the results of more than
one test are available, and the results are discordant, the test whose
result is abnormal should be repeated and the diagnosis made on the
basis of the repeat test.[10]
Formulation of Recommendations for the Diagnosis
and Management of Disturbances of Glucose Homeostasis in Thalassemia
Major Patients
A
systematic search of PubMed and Google Scholar from May 2006 through
September 2016 was performed. Searches were prospectively limited to
publications in the English language. MeSH terms and strings used in
various combinations in the literature search included: thalassemia
combined with diabetes (507 references), impaired glucose tolerance (83
references), anemia (380 references), iron overload (144 references),
chronic liver disease (352 references), chelation therapy (94
references), zinc (204 references), treatment of diabetes (34
references), diabetes and complications (264 references).
Recommendations from published guidelines were also used when available
and appropriate (11 references).
Organization and Evidence Levels
Two
chairmen (VDS and ATS) appointed an expert panel of pediatricians,
endocrinologists, and haematologists, selected for their expertise in
research and the clinical treatment of thalassemia. This advisory
committee, chaired by nine clinicians to support the systematic review
of the literature and to guarantee the accuracy of the process,
suggested the use of a unified system called the Strength of
Recommendation Taxonomy (SORT) developed by editors of the US family
medicine and primary care journals (ie, American Family Physician,
Family Medicine, Journal of Family Practice, and BMJ USA).[11-14]
Evidence was graded using a 3-point scale based on the quality of
methodology (e.g., randomized control trial, case control,
prospective/retrospective cohort, case series, etc.) and the overall
focus of the study as follows:I. Good-quality patient-oriented II. Limited-quality patient-oriented evidenceIII. Other evidence, including consensus guidelines, opinion, case studies, or disease oriented evidence.The strength of recommendation was ranked as follows:A. Recommendation based on consistent and good-quality patient-oriented evidence.B. Recommendation based on inconsistent or limited-quality patient-oriented evidence.C. Recommendation based on consensus, opinion, case studies, or disease-oriented evidence. Process Followed for the Preparation of Manuscript
The
two chairmen, pediatric endocrinologists (VDS and ATS), prepared the
draft article which was subjected to scrutiny by a panel of experts
consisting of six pediatric endocrinologists (HE, SA, NS, DK,MEK and
IS), one paediatrician (BF), and three pediatricians/thalassemiologists
(MA,SD and CK) with at least four decades of experience in this field.
During the preparation of the draft, comments from members of the
ICET-A Network (hematologists, thalassemiologists, paediatricians, and
endocrinologists) were also considered. Final clinical recommendations
and observations were prepared by the Steering Committee and approved
by the ICET-A Network Board for use by any healthcare professional
managing TM patients. The interpretation and application of clinical
practice recommendations will remain the responsibility of the
individual clinician. The recommendations will be considered current
for a period of 3 years from the date of publication unless reaffirmed
or updated before that time.
Due
to the large number of references reported in the literature, the
Steering Committee decided to cite only the scientific publications on
which our work is based.
In
those situations where documented evidence based data were not
available or were showing inconsistent or limited conclusions, expert
ICET-A opinion and the medical consensus was used to generate clinical
recommendations.
Management of β-Thalassemia Major
a.Transfusions.Clinical
management of TM consists of regular life-long red blood cell
transfusions (RBCs) and iron chelation therapy to remove the excess
transfusional iron. Current guidelines for the treatment of anemia in
TM recommend transfusions at a hemoglobin (Hb) level of more than 9.0%
g/dl, which is associated with adequate inhibition of bone marrow
expansion. In patients with TM, the rate of transfusional iron loading
should be monitored and considered when choosing the appropriate dose
of an iron-chelating agent.[15]b. Iron overload and its management. The
characteristic pattern of iron deposition with regular transfusions
initially involves iron storage as ferritin and hemosiderin in
reticuloendothelial cells such as macrophages of the spleen, liver, and
bone marrow. This is followed by iron accumulation elsewhere, mainly in
hepatocytes, but also in endocrine glands, anterior pituitary, and
myocardium. Three
iron chelator drugs are currently approved: deferoxamine (DFO),
deferiprone (DFP) and deferasirox (DFX). Iron excretion induced by
chelators is the sum of urinary and faecal iron excretions. For
deferoxamine, urinary iron excretion represents around 50% of the
total, for deferiprone 80-98% and for deferasirox less than 5%.[16-23]A
variety of factors differentiate the currently available iron
chelators, including the mode of administration, the dosing schedule,
the chelator’s ability to remove iron from different organs (i.e.,
heart, liver) and the adverse effects. These factors should be
carefully considered when choosing a chelation regimen.[18]The
availability of more than one iron chelating drug stimulated the
studies for benefits from combination therapy. Combination treatment
(two drugs daily taken at full doses and simultaneously or alternating
the two drugs during the week) may be considered every time there is a
need to look for an additive or synergistic effect in patients with
severe iron overload and heart disease.[22,23] A single uncontrolled
study suggests that combination therapy (DFO plus DFP) may reverse
endocrine complications such as glucose intolerance in patients with
TM.[24] The same holds true with optimum monotherapy preserving low
iron load and iron negative balance. Assessment of iron overload
The
accurate evaluation of iron overload is crucial in order to plan and
monitor iron chelation therapy. Multiple methods of assessing the
degree of iron overload exist and each method has benefits and
limitations. In clinical practice, combinations of the different
techniques and serial measurements are used to assess the iron burden
and to adjust chelation therapy.[25,26] Invasive methods include liver
and heart biopsies. In general, ubiquitous access to non-invasive
methods has replaced biopsies as the standard method for measuring
tissue iron concentrations in most centres. The
non-invasive methods of measuring iron overload include serum ferritin
(SF), non-transferrin bound iron (NTBI), labile plasma iron (LPI) and
liver iron concentration (LIC) as determined by MRI R2*, liver
superconducting quantum interference device (SQUID) and cardiac T2*
MRI.[27]a. Serum ferritin (SF).In
the majority of clinical centres, the standard method of evaluating the
total amount of body iron is a measurement of SF concentration in the
blood.In
the absence of confounding factors, such as inflammation, vitamin C
deficiency, oxidative stress, liver dysfunction and increased cell
death, SF is proportional to the degree of cellular iron stores.
Therefore, serial assessments are recommended.[2]b. Liver iron concentration (LIC).The
liver contains most of the body iron stores (70-80%) and is the main
crossroads of iron trafficking (storage from intestinal absorption and
from red-cell catabolism, chelation by iron chelating drugs and
excretion through bile). Several studies have linked very high LIC
(> 15 mg/g dry weight) to worsening prognosis, liver fibrosis
progression and hepatocellular carcinoma. Levels above 7 mg/g dry
weight are indications to increase chelation since a major risk of
complications occur at levels 7-14.[28]LIC
can also be measured accurately using SQUID and MRI. SQUID has been
validated but has limited availability and cannot measure iron in the
heart. MRI is widely available, robust and reproducible. Inter-observer
variability is insignificant and inter-study variability is
approximately 5%-7%. Variability among scanners is also small.[29,30]
c. Iron load of other tissues and organs and MRI.The
introduction of MRI for the assessment of tissue iron in the early
2000s completely changed our understanding of iron overload and its
management. This method is non-invasive, cost-effective, with no
radiation exposure, and of widespread availability.[31-40]MRI
has proven effective in detecting and accurately quantifying iron in
the heart, liver and other organs, including endocrine glands
(pancreas, pituitary and adrenal). Pituitary R2 correlated
significantly with serum ferritin as well as liver, pancreatic, and
cardiac iron deposition.[34-36]
One significant advantage of cardiac MRI is its ability to recognize
preclinical cardiac iron deposition, allowing effective early treatment
and so preventing progression to heart failure.T2*
is the time needed for the organ to lose approximately two-thirds of
its signal and is measured in milliseconds (ms). T2* shortens as iron
concentration increases. Its reciprocal, 1000/T2*, is known as R2* and
is measured in units of inverse seconds (S-1).However,
pancreas R2* measurements have several limitations: (a) they have not
gained widespread use, (b) functional correlates require further
investigation and (c) the pancreas may be difficult to locate in older,
splenectomized thalassemia major patients because of glandular
apoptosis, fatty replacement, and loss of normal anatomic
landmarks.[37-39]Recommendations:• Current
practice is to start chelation therapy after transfusion of 5-10 units
of blood (approximately 1-2 gr/Fe), or when the ferritin level rises
above 1,000 μg/l. (I,C)•
Serum ferritin has been used to start, formulate and monitor
chelation therapy, but it is now known to be an imprecise indicator of
total body iron burden since it can yield inappropriate results in the
presence of inflammation, abnormal liver function or ascorbate
deficiency. Despite these reservations, trends in serum ferritin
concentrations serve as a reasonable, cost efficient and readily
applicable surrogate marker for the iron load. (I,C)•
LIC estimation using MRI shows excellent correlation with that
obtained from liver biopsy and is an accurate method to assess liver
iron content and proportional iron stores.(I,A)•
Pancreatic imaging has a potential role in the assessment of iron
deposition and for the prediction of the development of glycemic
abnormalities. (I,B)•
Prospective data are needed to prove the validity of pancreatic
MRI imaging for the assessment of effects of different chelators as
well as their doses; more evaluation is required before this
measurement can be recommended for routine use. (II,C) Prevalence of Glucose Abnormalities in Patients with TM
Glucose tolerance abnormalities and DM are common complications in patients with TM. Pancreatic
iron loading in these patients begins after the first decade of life
and the incidence of complications increases with age. The rate of iron
accumulation is directly related to the annual blood consumption, the
delay in starting chelation and to low compliance and/or inadequate
chelator doses. While glucose intolerance occurs at an early stage of
adolescence, DM frequently occurs at later stages and is usually
secondary to iron overload and subsequent chronic liver disease.Depending
on the age composition of cohorts, up to 25% of patients with TM may
have isolated impaired fasting plasma glucose (FPG), a condition in
which the fasting blood glucose is elevated above what is considered
normal, but is not high enough to be classified as DM.[39-41] FPG has a
good correlation with other glycemic indices such as fasting insulin,
insulin resistance index and beta cell function index. Impaired FPG is
considered a pre-diabetic state. However, it is not known how many
patients with TM with impaired FPG progress over the years to
diabetes.[41]The
prevalence of DM and IGT in adolescents and young adults with TM
conventionally treated with DFO varies in different series (up to 10.5% and 24%, in different series.[3,7]Glucose,
insulin, and C-peptide levels during oral glucose tolerance tests
(OGTT) from 36 thalassaemic patients with normal (n=23), impaired
(n=6), or diabetic glucose tolerance (n=7) and 32 control subjects were
examined. Patients with impaired glucose tolerance presented
hyperinsulinemia and delayed peak insulin during OGTT. The
C-peptide/insulin ratio was decreased in patients with abnormal glucose
tolerance compared to controls. Insulin sensitivity was significantly
reduced in patients with impaired glucose tolerance or diabetes
compared to controls.[42]The
considerable variation in the occurrence of glycemic abnormalities can
be partially explained by the marked differences in the age composition
of cohorts, their genetic background, transfusion regimens, degree of
chelation and the screening method used. Pathogenic Mechanisms
The
pathogenesis of glycaemic abnormalities in TM is complex and
multifactorial. The initial insult appears to affect iron-mediated
insulin resistance rather than defective insulin production;
subsequently, pancreatic ß-cell damage and insulin deficiency develop
as a result of direct toxic damage by the non-transferrin bound iron to
pancreatic ß-cells. Pancreatic islets have an extreme susceptibility to
oxidative damage and to low expression of the antioxidant defence
system. Moreover, a high expression of divalent metal transporter
predisposes further pancreatic islets to greater accumulation of iron
than other cells, potentiating the danger of iron-catalyzed oxidative
stress.[43-47]These
patients are a very heterogeneous group with some individuals
exhibiting mainly insulin deficiency and others predominantly insulin
resistance. The traditional concept has been that the initial insult is
insulin resistance compensated by hyperinsulinemia, related to liver
dysfunction (due to iron deposition), that may interfere with insulin’s
ability to suppress hepatic glucose uptake. Also,
at the level of the muscle, iron deposits may decrease glucose uptake.
With advancing age, persistent insulin resistance along with a
progressive reduction in circulating insulin levels (due to declining
β-cell function), with a concomitant reduction in insulin sensitivity,
aggravates glucose disturbances leading to glucose intolerance and DM.
Then, pancreatic damage and insulin deficiency subsequently develop
leading to DM.[4,48-56]
However,
this is not always the sequence of events leading to the development of
DM. It has been shown that a defect in β-cell insulin secretion can be
present early, before the development of glucose intolerance, resulting
from the toxic effect of iron deposition in the pancreas.[54]In
addition, impaired liver function, hepatitis C infection, family
history of diabetes mellitus and genetic factor(s) and triggered
autoimmune response may also play a role.[4,53] Assessment of Insulin Resistance/Sensitivity
Various indices of insulin resistance/sensitivity using the data from OGTT have been proposed in the last 20 years.HOMA-IR
has been widely utilized for the estimation of IR. It is calculated by
multiplying fasting plasma insulin (FPI) by FPG, then dividing by the
constant 22.5, i.e. HOMA-IR = (FPI x FPG)/ 22.5. The spectrum of
HOMA-IR indices in populations is ethnic dependent, and specific
cut-off values should be established to allow its use in
differentiating normal from impaired insulin sensitivity. However,
there is significant variability in defining the threshold of
HOMA-IR.[57-59]
A
summary of reports on HOMA-IR cut-off in different adult populations
has been reported by Pilar Gayoso-Diz et al.[57] The threshold value (66th-90th
percentile) reported in 9 studies varied from >1.55 to
>3.8, mean 2.31 ± 0.66. In 140 subjects aged 7-16 years the
threshold
value was 3.[56]Hyperinsulinemic-
euglycemic clamp is known to be the "gold standard" for estimating
insulin sensitivity. However, its time and financially consuming
realization led to a simplified approach to the quantification of
insulin sensitivity. In thalassemic subjects insulin sensitivity
(ISI-0,120) is evaluated by a relatively new index derived from OGTT,
using the fasting (0 min) and 120 min post-oral glucose (OGTT) insulin
and glucose concentrations.[56]Recommendations:•
The homeostatic model assessment (HOMA-IR) is a validated method
to measure insulin resistance from fasting glucose and insulin.
However, there is a lack of reference values for subjects with
thalassemia (II,B) Correlation of Abnormalities of Glucose Homeostasis with iron Overload and Chronic Liver Disease
Elevated
serum ferritin concentrations and hepatitis C infection have long been
considered as important factors associated with the development of
abnormal glucose tolerance in patients with TM.[3-5,24,60,61]a. Pancreas and iron loadThe
pancreatic ß-cell function is most closely correlated with pancreatic
iron (R2*), while insulin resistance is more strongly associated with
somatic iron balance indices (serum ferritin, LIC). Normal
pancreas R2* is < 30 Hz: values of 30-100 Hz constitute mild
pancreatic siderosis, 100-400 Hz moderate, and values >400 Hz severe
siderosis. Both pancreatic and cardiac R2* are correlated with glucose
intolerance and diabetes. The presence of detectable cardiac iron is a
relatively good predictor of overt diabetes but lacks sensitivity for
milder glucose dysregulation.[38]b. Chronic liver disease and iron loadOlder
patients (>25 years) with hemoglobinopathies are at high risk of
hepatitis C virus (HCV) infection, as they were transfused before
the introduction of HCV donor screening. Despite antiviral therapy,
liver disease represents an important cause of mortality in these
patients. Chronic HCV infection is the leading cause of liver
cirrhosis, hepatocellular carcinoma, and metabolic disorders. Insulin
resistance is a representative of the metabolic disorders that leads to
the development of diabetes and also affects the outcome of antiviral
treatment with interferon.[62-64]De
Sanctis et al. studied 29 patients with TM who received intensive
subcutaneous (SC) chelation with DFO for periods of 6.2 to 8.8 years.
All patients had normal oral glucose tolerance tests before SC
chelation therapy was introduced and 22 of 29 patients had normal liver
function tests. At the end of the intensive chelation period 12
patients still had normal oral glucose tolerance (7 with normal liver
function and 5 with chronic active hepatitis), 11 patients developed
impaired glucose tolerance tests (3 patients had normal liver function,
5 had chronic active hepatitis and 3 had cirrhosis), and 6 patients
developed frank DM (one with chronic active hepatitis and 5 with
cirrhosis).[61] Risk Factors Associated with FPG, IGT, and DM
Elevated
fasting plasma glucose (FPG) is considered an index of a pre-diabetic
state. However, the rate of progression from abnormal FPG to overt
diabetes in TM patients is not known.[41]Risk
factors associated with IGT were male sex, poor compliance and/or
inefficient dose of chelator(s), increased liver iron concentration
(above 7 mg/g dry weight), splenectomy and lower insulin secretion
(area under curve) after OGTT, and all factors contributing to high
transfusional iron accumulation.[3,7,63]The
main risk factors associated with DM in addition to the above are
advanced age at the start of chelation therapy and liver cirrhosis or
severe fibrosis. In some studies, the strongest predictor for the
development of diabetes was the duration of transfusion therapy and
inefficiency of chelation, with every decade of transfusion exposure
increasing the odds of developing diabetes by 2.5 times.[3,7,65]Zinc
deficiency might lead to a suppression of the ability of the pancreas
to secrete sufficient amounts of insulin in response to oral glucose
load in patients with TM. Serum zinc levels should be monitored to
possibly provide useful complementary information regarding glucose
metabolism.[66,67]Recommendations:•
Serum zinc levels should be routinely monitored in these patients
so as to provide additional valuable information regarding glucose
metabolism. Zinc levels should be measured every 6 months according to
the TIF 2014 guidelines. (I,B) Natural History of Glycometabolic Status and Risk Factors in TM
The
natural history of the glycometabolic state in TM adults is
characterized by a deterioration of glucose tolerance (GT) over
time.[68,69]Messina
et al. studied the evolution of GT, insulin secretion, and peripheral
insulin sensitivity during a 3-yr follow-up in a homogeneous population
consisting of fourteen non-diabetic adults with TM. GT deterioration
over time was accompanied by a reduction of insulin sensitivity, with
no concomitant change in insulin secretion. No patient developed
diabetes mellitus (DM) during follow-up.[70]Kattamis
et al. reported that the prevalence of IGT increased progressively from
13.4% to 39% over the first 4 years of observation but remained
constant during the following 6 years of observation after the
intensification of chelation. In contrast, DM had a very low
prevalence, beginning with 0.5% at 13-16 years, increasing to 2.4% by
the age of 21-24 years.[71]Recommendations:•
Understanding the sequence of abnormalities in the progression
from normal glucose homeostasis to IGT/DM and identifying the risk
factors for glycometabolic disturbances in thalassemic patients
facilitates the formulation of interventions. (II,B)•
Diagnosis of impaired IFG or IGT indicates a pre-diabetic state
which, if not managed appropriately, could progress in TM patients to
diabetes (II,B)
Screening Strategy for Diagnosis of Glucose Abnormalities in Patients with TM
Annual
random plasma glucose or fasting plasma glucose measurement as well as
the performance of OGTT for all patients with TM aged > 10 years
have been used.[3,7]
The international guidelines recommend a
fasting glucose semi-annually, and if this is greater than 6.1 mmol/L,
OGTT is indicated. In addition, USA (Standards of Care Guidelines for
Thalassemia, 2012) and the ICET-A standards of care 2013
guidelines recommend a two-hour OGTT at 10, 12, 14, and 16 years of age
and annually thereafter (Table 1).
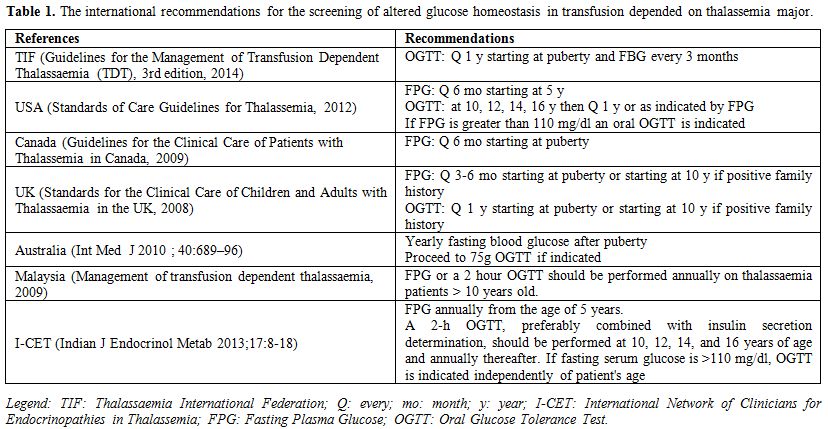 |
Table
1. The international recommendations for the screening of altered
glucose homeostasis in transfusion depended on thalassemia major. |
Nevertheless,
the most accurate method for assessing glucose metabolism in patients
with TM is still controversial. Even if the annual OGTT at the age of
10 years is the recommended method, a diagnosis of normal glucose
tolerance during OGTT does not exclude abnormal postprandial glucose
levels at home. There is now evidence that the OGTT may miss episodes
of hyperglycaemia.[39,72,73] Noetzli et al. found that fasting glucose
> 97 mg/dL and insulin > 9 µU/mL accurately identified an
abnormal OGTT result (89% sensitivity, 90% specificity).[38]
Furthermore,
some patients with TM and normal fasting and 2-h glucose levels have
elevations in the middle of the OGTT (indeterminate glycemia [INDET])
or when assessed randomly or by continuous glucose monitoring.[72-75]
The clinical significance of INDET in TM is not known.
The use of
continuous glucose monitoring system (CGMS) appears to diagnose early
more glycemic abnormalities compared to using HbA1c, fasting glucose
and OGTT.[41,72-75] The benefit of CGMS, as opposed to other diabetes
screening methods, is that it shows a glucose trend, with readings
every minute, rather than single-point measurement. This enables
capture of increased blood glucose levels over a 24-hour period, which
reflects the variable nature of DM.
Recommendations:
•
The most accurate method to evaluate altered glucose metabolism in
patients with TM is still controversial.(I,A)
• We recommend fasting blood glucose, insulin and calculation of HOMA-IR index.(I,C)
•
OGTT in subjects with high serum ferritin can identify patients at high
risk of glucose dysregulation and is recommended at 10, 12, 14, and 16
years of age and annually thereafter. (II,B)
•
Up to now, little is known about the efficacy of continuous glucose
monitoring system (CGMS) as a useful measure for detecting the
variability of glucose fluctuations in 24 hours and for assessment of
glucose homeostasis in transfusion-dependent beta thalassemia patients,
especially due to the lack of clear guidelines. (I,C)
•
If a patient with TM develops symptoms of hyperglycaemia (polyuria,
polydipsia, weight loss), a blood glucose should be performed.(I,A) Clinical Characteristics and Management of IGT and DM in Thalassemia Major
The
usual symptoms of polyuria, polydipsia, and weight loss, have been
reported to occur in 94.5% of patients with TM and diabetic
ketoacidosis (DKA) has been reported to be the presenting manifestation
of diabetes in 13.8% to 31.1% of patients.[76] However, in
our personal experience, diabetic ketoacidosis is rare. There was a
broad range of symptoms at the clinical onset of diabetes from
asymptomatic glycosuria (12 cases) to ketosis (13 cases), or
ketoacidosis (four cases). The mean age at diagnosis was 17 years
(range 11-24). This may be due to an early detection of mild glucose
disturbances.[76]
The mean daily insulin requirement in this
series was 0.98 U/kg body weight (range 0.15-1.72). In general
terms the metabolic control was good in 4 patients, poor in 8, and very
poor in 17. There was a negative correlation between insulin dose and
metabolic control. The determination of C-peptide concentrations in 10
patients showed a variation in pancreatic β-cell function: it was
increased in one, normal in three, and reduced in 6 cases.[76] The
majority of patients had iron chelation treatment with desferrioxamine
on average for 4-9 years.
The onset of diabetes is often
associated with the presentation of cardiac dysfunction. Moreover,
these patients with clinical diabetes are at a high risk for additional
complications such as thyroid dysfunction or hypogonadism and should be
strictly monitored.[3,4,6,7,76]
Management of DM should be
individualised. The first line treatment in all TM patients with
glucose disturbances should be an intensification of iron chelation
therapy to achieve a negative iron balance. Platis et al. obtained a
reversal in one-third of glucose metabolism disorder cases by using
combination therapy (DFO and DFP).[77] Intensive iron chelation therapy
with DFO plus DFP seems to be associated with an improvement in glucose
intolerance in terms of glucose and insulin secretion, particularly in
patients in early stages of glucose intolerance.[24] Christoforidis et
al. showed that patients receiving combined therapy (DFO plus DFP) had
an average reduction of insulin resistance index (IRI), accompanied by
an average increase in the ß-cell function index and a slight decrease
in the insulin sensitivity index (ISI 0–120). In contrast, patients
receiving monotherapy either with DFO or DFP showed deterioration in
glucose tolerance, indicated by an average reduction of ß-cell function
index, a concomitant increase in average IRI and a reduction of ISI
0–120.[78]
There is very limited published data on the efficacy
and safety of oral antidiabetic agents in patients with TM. The only
drugs used in small studies in this context with good effect were
metformin, glibenclamide, and acarbose.[79-82]
In established
diabetes, the medical treatment depends on the severity of β-cell
damage and subsequent insulin deficiency. Introducing oral hypoglycemic
drugs in the early stage of diabetes before dependence on insulin
proved beneficial in preliminary studies.
Metformin is considered
first choice in patients with type 2 diabetes. There is little research
in thalassaemia except on one case report in a 25-year-old Tunisian
patient.
Insulin resistance also plays a part in the
pathogenesis of diabetes in thalassaemia. Since metformin reduces
insulin resistance, it could be promising and indeed can be considered
in early stages.[79]
The efficacy of glibenclamide administration
in the management of glucose disturbances was evaluated in 33 patients
with thalassemia, aged 12-30 years (mean 17.4 ± 3.7), in whom diet and
exercise failed to regulate hyperglycemia. Improvement of OGTT was
observed in 73% of TM patients treated with glibenclamide versus 43% of
the control group for a mean period of 59 months. Deterioration of OGTT
occurred more rapidly (33.7 ± 26.1 vs. 40.7 ± 34.5 mos), and in more
patients of the untreated group (57%) than in treated patients (27%).
Among treated patients, the effectiveness of oral hypoglycemic agents
lasted longer in patients with diabetes (64.1 ± 40.3 mos) than in
patients with impaired curves (54.2 ± 31 mos).[80]
Seventeen TM
patients with impaired glucose tolerance (IGT) or non-insulin dependent
diabetes mellitus (NIDDM) and hyperinsulinism were treated for 12
months with acarbose (100 mg. orally with breakfast, lunch and evening
meals). An improvement in glucose tolerance was observed in 2 out of 11
TM patients with IGT and in all TM patients with NIDDM. Acarbose does
not appear to improve insulin resistance directly but may have an
indirect effect delaying the absorption of glucose of complex
carbohydrates and disaccharides.[81,82]
Overall there is limited data on the effect of oral antidiabetic drugs in thalassaemia.
Compared
to the general diabetic population, there is no marked difference in
the monitoring of glycaemic control in thalassaemic patients. When
overt DM develops, patients require daily subcutaneous injections of
insulin to normalise blood sugar levels. Since treatment of diabetes in
patients with TM is an additional burden, support from doctors and
psychologists is needed. Typically, a basal/bolus dose or a combination
of both is used to treat DM. Short acting rapid insulin before meals
remains the insulin of choice for those without fasting hyperglycaemia.
Self-monitoring of blood glucose (SMBG) is recommended, at least
three times a day in patients on insulin therapy. The use of
carbohydrate counting and insulin-to-carbohydrate ratios in conjunction
with the usual diet to guide insulin therapy can help to optimize
glycemic control. Exercise is beneficial and is known to play a vital
role in overall health.[4]
Overall, TM patients with diabetes
should strive to attain plasma glucose goals as per the ADA
recommendations for people with diabetes.[4,7,83,84]
All patients
with DM should regularly be monitored for the development of
complications. Kidney function and imaging of the fundi should be
carried out to evaluate the presence and degree of diabetic
complications. However, the incidence of retinopathy and nephropathy in
patients with diabetes and thalassaemia is lower than in patients
affected by juvenile diabetes.[4,7] This may be due to normal or below
normal serum levels of cholesterol and triglycerides, to the frequent
presence of hypogonadism and low insulin growth factor 1 (IGF-1)[85,86]
as well as comparable shorter period of observation. With regard to
macrovascular complications of diabetes, they include ischaemic heart
disease, cerebrovascular disease, and peripheral vascular disease. A
recent study by Pepe et al. showed that DM in patients with TM
significantly increases the risk for cardiac complications, heart
failure, hyperkinetic arrhythmias and myocardial fibrosis.[6]
The
credibility of Hb A1c as a gold standard for the measurement of control
of diabetes in TM patients has been questioned because the hemoglobin
composition of patients’ erythrocytes is considerably modified, due to
regular and frequent transfusions. As a rule, the patient’s
erythrocytes are a mixture of transfused red cells from donors with a
normal Hb composition, with Hb A of around 95%, and Hb F of 2-3%.
Storage erythrocytes have functional and metabolic differences as well
as a considerably shorter life span compared to healthy red
cells.[3,4,74,87-89] On the other hand, the results of a recent study
showed that assessment of HbA1c prior to transfusion is a reliable
index of the average glucose concentration for the period between
transfusions ranging from 2-4 weeks and up to 40 days. In the Kattamis
et al. study a cut off value of 6.8-7% was suggestive of diabetes and
values between 6 and 7% of prediabetes.[90] Further studies are needed
to confirm these observations.
Serum fructosamine levels have been
proposed as an appropriate laboratory measurement when monitoring
long-term glycemic control in patients with TM and diabetes
mellitus.[4,7] A single measurement with this assay provides an
assessment of glycemic control over the preceding 2–3 weeks. Some
limitations with the use of the fructosamine assay have been noted,
including the short half-life of fructosamine which might result in
fructosamine being more susceptible to rapid changes in blood glucose,
and difficulty with standardization of the assay because albumin can be
profoundly affected by disease states and drugs.[74,91]
Diabetes
in pregnancy is associated with risks to the woman and to the
developing foetus. The International clinical guideline contains
recommendations for the management of diabetes and its complications in
women who wish to conceive and those who are already pregnant.[90,91]
Women
with TM are potentially at high risk for development of hyperglycemia
during pregnancy (gestational diabetes mellitus). Therefore, those who
are contemplating pregnancy should be evaluated prior to conception to
rule out any impairment of glucose homeostasis. Specific criteria for
the diagnosis of gestational diabetes should be used.[92,93]
Recommendations:
• Intensive
iron-chelation therapy and prevention and treatment of chronic
hepatitis C are now the most important issues in managing impairment of
glucose homeostasis in patients with transfusion dependent
β-thalassemia. (II,A)
• Management of DM should be individualised.(II,C)
•
During initiation of insulin, blood glucose monitoring both
pre- and post-prandially as well as at bedtime and overnight may help
to determine dosage requirements.(II,A)
• Patients with diabetes who are on insulin should perform self-monitored
blood glucose testing at least three times a day.(II,A)
• Continuous glucose monitoring (CGMS) is under investigation as a
potential new measure of prandial glucose control, especially in the
more difficult cases. (II,A)
• Patients with TM should strive to attain plasma glucose goals as per
the ADA recommendations for all people with diabetes.(I,A)
• There is limited published data on the efficacy and safety of oral antidiabetic agents. (II,A)
• Glycated hemoglobin A1c reflects a mean glycemia over the preceding 3
months (erythrocyte life span). In diabetes management, the target
value is set below 6.5%, to reduce the risk of chronic complications.
However, HbA1c is a poor marker in subjects with diabetes and
hemoglopinopathies.(I,A) Fructosamine determination is useful for
monitoring diabetes in these patients.(I,A)
• TM women with normal glucose tolerance pre-pregnancy should still be
advised that they may develop glucose intolerance later in
pregnancy, and that repeat OGTT should be performed at both 12–16 and
24–28 weeks gestation with measures at 0, 1 and 2 h using the specific
gestational diabetes criteria.(I,A)
• Women
with diabetes who are planning to become pregnant should be informed
that establishing good glycaemic control before conception and
continuing this throughout pregnancy will reduce the risk of
miscarriage, congenital malformation, stillbirth and neonatal death. It
is important to explain that risks can be reduced but not eliminated.
(I,A)
• TM
women with pre-existing diabetes should have pre-pregnancy counselling
and planning to aim for optimal glycemic control before and throughout
pregnancy to minimize adverse pregnancy outcomes. (I,A)
• All pregnant patients with DM should regularly be monitored for the development of complications. (I,A)
• Plasma glucose levels should be monitored closely during the
peri-partum period and until hospital discharge. (II,C)
• Chelation treatment should be interrupted during pregnancy.(I,C)
• Diabetic
patients with TM should regularly be seen by a specialized
multidisciplinary team with expertise in both diabetes and TM,
including ongoing diabetes self-management education.(I,A) The team
should include an endocrinologist and dietician with experience in TM.(I,C)
On Behalf of ICET-A Participants:
Valeria Kaleva
- Head of Department of Paediatrics, Medical University, Varna
& Head of Division of Pediatric Hematology Oncology, University
Hospital "St. Marina", Varna, Bulgaria; Nada A. Soliman - Primary Health Care, Ministry of Health, Alexandria, Egypt; Praveen Sobti - Professor Pediatric Hemato-Oncology, Christian Medical College and Hospital, Ludhiana Punjab, India; Su Han Lum - Department of Paediatrics, University Malaya Medical Center, Malaysia; Mehran Karimi - Hematology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran; Maria Concetta Galati - Pediatric Pediatric Oncohematology Unit, Pugliese-Ciaccio Hospital, Catanzaro, Italy; Giuseppe Raiola - Pediatric Unit, Pugliese-Ciaccio Hospital Catanzaro, Italy; Rania Elalaily - Department of Primary Health Care, Abu Nakhla Hospital, Doha, Qatar; Mohamed Yassin - National Center for Cancer Care and Research Medical Oncology Hematology Section HMC, Doha, Qatar; Soad Al Jaouni -
Head Division of Pediatric Hematology Oncology, Deputy Chair of
Hematology & Head Section of Hematology Research Lab, King Fahd
Medical Research Center Department of Hematology Faculty of Medicine,
King Abdulaziz University Jeddah, Kingdom of Saudi Arabia; Duran Canatan - Director of Thalassemia Diagnosis Center of Mediterranean Blood Diseases Foundation Antalya, Turkey; Yurdanur Kilinc - Çukurova University, Medical Faculty, Department of Pediatric Hematology, Adana, Turkey; Mohamed Elshinawy
- Department of Pediatrics Alexandria University Children's Hospital,
Egypt and Child Health Department, Sultan Qaboos University Hospital,
Muscat, Oman; Yasser Wali - Pediatric Hematology Unit, Child Health Department,
References
- Weatherall DJ. The definition and epidemiology of
non-transfusion dependent thalassemia. Blood Rev 2012;26 Suppl 1:S3-6
http://dx.doi.org/10.1016/S0268-960X(12)70003-6

- Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med 2005;353:1135-1146 http://dx.doi.org/10.1056/NEJMra050436 PMid:16162884

- De
Sanctis V, Soliman A, Yassin M. Iron overload and glucose metabolism in
subjects with ß-thalassaemia major: an overview. Curr Diabetes Rev
2013;9:332-341 http://dx.doi.org/10.2174/1573399811309040005 PMid:23687960

- Tzoulis P. Review of endocrine complications in adult patients with ß-thalassaemia major. Thalassemia Reports 2014; 4:51-56

- Hafez
M, Youssry I, El-Hamed FA, Ibrahim A. Abnormal glucose tolerance in
beta thalassemia: assessment of risk factors. Hemoglobin
2009;33:101-108 http://dx.doi.org/10.1080/03630260902817131 PMid:19373585

- Pepe
A, Meloni A, Rossi G, Caruso V, Cuccia L, Spasiano A, Gerardi C,
Zuccarelli A, D'Ascola DG, Grimaldi S, Santodirocco M, Campisi S, Lai
ME, Piraino B, Chiodi E, Ascioti C, Gulino L, Positano V, Lombardi M,
Gamberini MR. Cardiac complications and diabetes in thalassaemia major:
a large historical multicentre study. Br J Haematol 2013;163:520-527 http://dx.doi.org/10.1111/bjh.12557 PMid:24111905

- De
Sanctis V, Soliman AT, Elsedfy H, Pepe A, Kattamis C, El Kholy M,
Yassin M. Diabetes and Glucose Metabolism in Thalassemia Major: An
Update.Expert Rev Hematol. 2016;9:401-408 http://dx.doi.org/10.1586/17474086.2016.1136209 PMid:26697756

- De Sanctis V, Soliman AT. ICET-A: an Opportunity for Improving Thalassemia Management. J Blood Disord 2014;1:1-2

- American
Diabetes Association. Standards of medical care in diabetes-2015.
Diabetes Care 2015;38 Suppl 1:S8-S16 PMCid:PMC4582910

- Canadian
Diabetes Association Clinical Practice Guidelines Expert Committee,
Booth G, Cheng AY. Canadian Diabetes Association 2013 clinical practice
guidelines for the prevention and management of diabetes in Canada.
Methods. Can J Diabetes 2013;37 Suppl 1:S4-7
PMid:24070961

- Ebell
MH, Siwek J, Weiss BD, Woolf SH, Susman JL, Ewigman B, Bowman M.
Simplifying the language of evidence to improve patient care: Strength
of recommendation taxonomy (SORT): a patient-centered approach to
grading evidence in medical literature. J Fam Pract. 2004;53:111-120
PMid:14764293

- Ebell
MH, Siwek J, Weiss BD, Woolf SH, Susman J, Ewigman B, Bowman M.
Strength of recommendation taxonomy (SORT): a patient-centered approach
to grading evidence in the medical literature.J Am Board Fam Pract.
2004;17:59-67 http://dx.doi.org/10.3122/jabfm.17.1.59 PMid:15014055

- No authors listed. SORT: the strength-of-recommendation taxonomy. Am Fam Physician. 2005;71:19-20 PMid:15666558

- Weiss BD. SORT: Strength of recommendation taxonomy. Fam Med. 2004;36:141-143 PMid:14872363

- Angelucci
E, Barosi G, Camaschella C, Cappellini MD, Cazzola M, Galanello R,
Marchetti M, Piga A, Tura S. Italian Society of Hematology: practice
guidelines for the management of iron overload in thalassemia major and
related disorders. Haematologica 2008;93:741-752 http://dx.doi.org/10.3324/haematol.12413 PMid:18413891

- Cohen
A, Glimm E, Porter J. Effect of transfusional iron intake on response
to chelation therapy in beta-thalassemia major. Blood 2008; 111:583-587
http://dx.doi.org/10.1182/blood-2007-08-109306 PMid:17951527

- Hershko
CM, Link GM, Konijn AM, Cabantchik ZI. Iron chelation therapy. Curr
Hematol Rep 2005;4:110-116 PMid:15720959

- Giardina PJ, Grady RW. Chelation therapy in beta-thalassemia: an optimistic update. Semin Hematol 2001;38:360-366 http://dx.doi.org/10.1016/S0037-1963(01)90030-7

- Piga
A, Gaglioti C, Fogliacco E, Tricta F. Comparative effects of
deferiprone and deferoxamine on survival and cardiac disease in
patients with thalassemia major: a retrospective analysis.
Haematologica 2003;88:489-496 PMid:12745268

- Cohen
AR, Galanello R, Piga A, De Sanctis V, Tricta F. Safety and
effectiveness of long-term therapy with the oral iron chelator
deferiprone. Blood 2003;102:1583-1587 http://dx.doi.org/10.1182/blood-2002-10-3280 PMid:12763939

- Galanello R, Agus A, Campus S, Danjou F, Grady R. Combined iron chelation therapy. Ann N Y Acad Sci. 2010;1202:79-86 http://dx.doi.org/10.1111/j.1749-6632.2010.05591.x PMid:20712777

- Piga
A, Roggero S, Marletto F, Sacchetti L, Longo F. Combined use of oral
chelators and desferrioxamine in thalassemia. Hematology 2005; 10 Suppl
1: 89-91 http://dx.doi.org/10.1080/10245330512331389737 PMid:16188646

- Hershko
C, Cappellini M, Galanello R, Piga A, Tognoni G, Masera G. Purging iron
from the heart. Br J Haematol 2004; 125: 545-551 http://dx.doi.org/10.1111/j.1365-2141.2004.04946.x PMid:15147368

- Farmaki
K, Angelopoulos N, Anagnostopoulos G, Gotsis E, Rombopoulos G, Tolis G.
Effect of enhanced iron chelation therapy on glucose metabolism in
patients with beta-thalassaemia major. Br J Haematol 2006; 134: 438-444
http://dx.doi.org/10.1111/j.1365-2141.2006.06203.x PMid:16822284

- Belhoul
KM, Bakir ML, Saned MS, Kadhim AM, Musallam KM, Taher AT.Serum ferritin
levels and endocrinopathy in medically treated patients with ß
thalassemia major. Ann Hematol 2012;91:1107-1114 http://dx.doi.org/10.1007/s00277-012-1412-7 PMid:22281991

- Taher AT, Musallam KM, Inati A. Iron overload: consequences, assessment and monitoring. Hemoglobin 2009;33 Suppl 1:S46-S57 http://dx.doi.org/10.3109/03630260903346676 PMid:20001632

- Westwood
MA, Sheppard MN, Awogbade M, Ellis G, Stephens AD, Pennell DJ.
Myocardial biopsy and T2* magnetic resonance in heart failure due to
thalassaemia. Br J Haematol 2005;128:2 http://dx.doi.org/10.1111/j.1365-2141.2004.05234.x PMid:15606544

- Brittenham
GM, Griffith PM, Nienhuis AW, McLaren CE, Young NS, Tucker EE, Allen
CJ, Farrell DE, Harris JW. Efficacy of deferoxamine in preventing
complications of iron overload in patients with thalassemia major. N
Engl J Med 1994;331:567-573 http://dx.doi.org/10.1056/NEJM199409013310902 PMid:8047080

- Angelucci
E, Baronciani D, Lucarelli G, Baldassarri M, Galimberti M, Giardini C,
Martinelli F, Polchi P, Polizzi V, Ripalti M. Needle liver biopsy in
thalassaemia: analyses of diagnostic accuracy and safety in 1184
consecutive biopsies. Br Haematol 1995;89:757- 761 http://dx.doi.org/10.1111/j.1365-2141.1995.tb08412.x

- Jensen
PD, Jensen FT, Christensen T, Nielsen JL, Ellegaard J. Relationship
between hepatocellular injury and transfusional iron overload prior to
and during iron chelation with desferrioxamine: a study in adult
patients with acquired anemias. Blood 2003; 101:91-96 http://dx.doi.org/10.1182/blood-2002-06-1704 PMid:12393528

- Hoffbrand AV, Taher A, Cappellini MD. How I treat transfusional iron overload. Blood 2012;120:3657-3669 http://dx.doi.org/10.1182/blood-2012-05-370098 PMid:22919029

- Kirk
P, He T, Anderson LJ, Roughton M, Tanner MA, Lam WW, Au WY, Chu WC,
Chan G, Galanello R, Matta G, Fogel M, Cohen AR, Tan RS, Chen K, Ng I,
Lai A, Fucharoen S, Laothamata J, Chuncharunee S, Jongjirasiri S,
Firmin DN, Smith GC, Pennell DJ. International reproducibility of
single breathhold T2* MR for cardiac and liver iron assessment among
five thalassemia centers. J Magn Reson Imaging 2010;32:315-319 http://dx.doi.org/10.1002/jmri.22245 PMid:20677256 PMCid:PMC2946327

- Liu P, Olivieri N. Iron overload cardiomyopathies: new insights into an old disease. Cardiovasc Drugs Ther 1994;8:101-110 http://dx.doi.org/10.1007/BF00877096 PMid:8086319

- Noetzli
LJ, Carson SM, Nord AS, Coates TD, Wood JC. Longitudinal analysis of
heart and liver iron in thalassemia major. Blood 2008;112:2973-2978 http://dx.doi.org/10.1182/blood-2008-04-148767 PMid:18650452 PMCid:PMC2556627

- Noetzli
LJ, Panigrahy A, Mittelman SD, Hyderi A, Dongelyan A, Coates TD, Wood
JC. Pituitary iron and volume predict hypogonadism in transfusional
iron overload. Am J Hematol 2012;87:167-171 http://dx.doi.org/10.1002/ajh.22247 PMid:22213195

- Zamani
F, Razmjou S, Akhlaghpoor S, Eslami SM, Azarkeivan A, Amiri A. T2*
magnetic resonance imaging of the liver in thalassemic patients in
Iran. World J Gastroenterol 2011;17:522-525 http://dx.doi.org/10.3748/wjg.v17.i4.522 PMid:21274383 PMCid:PMC3027020

- de
Assis RA, Ribeiro AA, Kay FU, Rosemberg LA, Nomura CH, Loggetto SR,
Araujo AS, Fabron Junior A, de Almeida Veríssimo MP, Baldanzi GR,
Espósito BP, Baroni RH, Wood JC, Hamerschlak N. Pancreatic iron stores
assessed by magnetic resonance imaging (MRI) in beta thalassemic
patients. Eur J Radiol 2012;81:1465-1470 http://dx.doi.org/10.1016/j.ejrad.2011.03.077 PMid:21501938

- Noetzli
LJ, Papudesi J, Coates TD, Wood JC. Pancreatic iron loading predicts
cardiac iron loading in thalassemia major. Blood 2009;114:4021-4026 http://dx.doi.org/10.1182/blood-2009-06-225615 PMid:19726718 PMCid:PMC2774543

- Papakonstantinou
O, Ladis V, Kostaridou S, Maris T, Berdousi H, Kattamis C,
Gourtsoyiannis N. The pancreas in beta thalassemia major: MR imaging
features and correlation with iron stores and glucose disturbances. Eur
Radiol 2007;17:1535-1543 http://dx.doi.org/10.1007/s00330-006-0507-8 PMid:17149622

- Au
WY, Lam WM, Chu WC, Tam S, Wong WK, Pennell DJ, Lie AK, Liang R. A
magnetic resonance imaging study of iron overload in hemopoietic stem
cell transplant recipients with increased ferritin levels. Transplant
Proc 2007;39:3369-3374 http://dx.doi.org/10.1016/j.transproceed.2007.09.027 PMid:18089387

- Soliman
AT, Yasin M, El-Awwa A, De Sanctis V. Detection of glycemic
abnormalities in adolescents with beta thalassemia using continuous
glucose monitoring and oral glucose tolerance in adolescents and young
adults with ß-thalassemia major: Pilot study. Indian J Endocrinol Metab
2013;17:490-495 http://dx.doi.org/10.4103/2230-8210.111647 PMid:23869308 PMCid:PMC3712382

- Noetzli
LJ, Mittelman SD, Watanabe RM, Coates TD, Wood JC. Pancreatic iron and
glucose dysregulation in thalassemia major. Am J Hematol
2012;87:155-160 http://dx.doi.org/10.1002/ajh.22223 PMid:22120775

- Chern
JP, Lin KH, Lu MY, Lin DT, Lin KS, Chen JD, Fu CC. Abnormal glucose
tolerance in transfusion-dependent beta-thalassemic patients. Diabetes
Care 2001;24:850-854 http://dx.doi.org/10.2337/diacare.24.5.850 PMid:11347742

- Cario
H, Holl RW, Debatin KM, Kohne E. Insulin sensitivity and beta-cell
secretion in thalassaemia major with secondary haemochromatosis:
assessment by oral glucose tolerance test. Eur J Pediatrics
2003;162:139-146 PMid:12655415

- Fernandez-Real
JM, Lopez-Bermejo A, Ricart W: Iron stores, blood donation, and insulin
sensitivity and secretion. Clin Chem 2005;51:1201-1205 http://dx.doi.org/10.1373/clinchem.2004.046847 PMid:15976100

- Cooksey
RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, McClain DA:
Oxidative stress, beta-cell apoptosis, and decreased insulin secretory
capacity in mouse models of hemochromatosis. Endocrinology
2004;145:5305-5312 http://dx.doi.org/10.1210/en.2004-0392 PMid:15308612

- Tiedge
M, Lortz S, Drinkgern J, Lenzen S: Relation between antioxidant enzyme
gene expression and antioxidative defense status of insulin-producing
cells. Diabetes 1997;46:1733-1742 http://dx.doi.org/10.2337/diab.46.11.1733 PMid:9356019

- Loebstein
R, Lehotay DC, Luo X, Bartfay W, Tyler B, Sher GD: Diabetic nephropathy
in hypertransfused patients with ß-thalassemia: the role of oxidative
stress. Diabetes Care 1998; 21:1306-1309 http://dx.doi.org/10.2337/diacare.21.8.1306 PMid:9702438

- Suvarna
J, Ingle H, Deshmukh CT. Insulin resistance and beta cell function in
chronically transfused patients of thalassemia major. Indian Pediatr
2006;43:393-400 PMid:16735760

- Cavallo-Perin
P, Pacini G, Cerutti F, Bessone A, Condo C, Sacchetti L, Piga A, Pagano
G. Insulin resistance and hyperinsulinemia in homozygous
beta-thalassemia. Metabolism 1995;44:281-286 http://dx.doi.org/10.1016/0026-0495(95)90155-8

- Rimondi
F, Banin P, Gamberini MR, De Sanctis V. The continuous glucose
monitoring system (CGMS) in patients with beta-thalassemia major and
impaired glucose homeostasis: preliminary results. Pediatr Endocrinol
Rev 2008;6 Suppl 1:190-192 PMid:19337177

- Al-Futaisi
A1, Wali Y, El-Beshlawi I, Al-Riyami S, Almahrezi A. Case Study: using
a continuous glucose monitoring system in a patient with diabetes and
beta-thalassemia hemoglobinopathy. Pediatr Hematol Oncol
2009;26:515-519 http://dx.doi.org/10.1080/08880010902975892 PMid:19863207

- Monge
L, Pinach S, Caramellino L, Bertero M T, Dall’omo A, Carta Q. The
possible role of autoimmunity in the pathogenesis of diabetes in
B-thalassemia major. Diabetes Metab 2001; 27: 149-154
PMid:11353881

- Khalifa
A S, Salem M, Mounir E, El- Tawail M M, El Savvy M , Abd Al-Aziz MM.
Abnormal glucose tolerance in Egyptian Beta thalassemic patients:
Possible association in genotyping. Pediatr Diabetes 2004; 5: 126-132 http://dx.doi.org/10.1111/j.1399-543X.2004.00051.x PMid:15450007

- Saudek CD, Hemm RM, Peterson CM. Abnormal glucose tolerance in beta thalassemia major. Metabolism 1977; 26: 43-52 http://dx.doi.org/10.1016/0026-0495(77)90126-3

- Angelopoulos
NG, Zervas A, Livadas S, Adamopoulos I, Giannopoulos D, Goula A, Tolis
G. Reduced insulin secretion in normoglycaemic patients with
beta-thalassaemia major. Diabet Med. 2006;23:1327-1331 http://dx.doi.org/10.1111/j.1464-5491.2006.01988.x PMid:17116183

- Gayoso-Diz
P, Otero-González A, Rodriguez-Alvarez MX, Gude F, García F, De
Francisco A, Quintela AG. Insulin resistance (HOMA-IR) cut-off values
and the metabolic syndrome in a general adult population: effect of
gender and age: EPIRCE cross-sectional study. BMC Endocr Disord. 2013
Oct 16;13:47. doi: 10.1186/1472-6823-13-47. http://dx.doi.org/10.1186/1472-6823-13-47

- Ascaso
JF, Romero P, Real JT, Priego A, Valdecabres C, Carmena R. Insulin
resistance quantification by fasting insulin plasma values and HOMA
index in a non-diabetic population. Med Clin (Barc). 2001;117:530-533 http://dx.doi.org/10.1016/S0025-7753(01)72168-9

- Gutt
M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman
N, Skyler JS, Marks JB. Validation of the insulin sensitivity index
(ISI-0,120): comparison with other measures. Diabetes Res Clin Pract.
2000;47:177-184 http://dx.doi.org/10.1016/S0168-8227(99)00116-3

- Mowla
A, Karimi M, Afrasiabi A, De Sanctis V. Prevalence of diabetes mellitus
and impaired glucose tolerance in beta-thalassemia patients with and
without hepatitis C virus infection. Pediatr Endocrinol Rev 2004; 2
Suppl. 2: 282- 284 PMid:16462712

- De
Sanctis V, D'Ascola G, Wonke B. The development of diabetes mellitus
and chronic liver disease in long term chelated beta thalassaemic
patients. Postgrad Med J 1986;62:831-836 http://dx.doi.org/10.1136/pgmj.62.731.831 PMid:3543913 PMCid:PMC2422789

- Papadopoulos
N, Deutsch M, Georgalas A, Poulakidas H, Karnesis L. Simeprevir and
Sofosbuvir Combination Treatment in a Patient with HCV Cirrhosis and
HbS Beta 0-Thalassemia: Efficacy and Safety despite Baseline
Hyperbilirubinemia. Case Rep Hematol. 2016;2016:7635128. doi:
10.1155/2016/7635128. Epub 2016 Mar 2. http://dx.doi.org/10.1155/2016/7635128

- Eslam
M, Kawaguchi T, Del Campo JA, Sata M, Khattab MA, Romero-Gomez M. Use
of HOMA-IR in hepatitis C.J Viral Hepat. 2011;18:675-684 http://dx.doi.org/10.1111/j.1365-2893.2011.01474.x PMid:21914084

- Khattab
M, Eslam M, Sharwae MA, Shatat M, Ali A, Hamdy L. Insulin resistance
predicts rapid virologic response to peginterferon/ribavirin
combination therapy in hepatitis C genotype 4 patients. Am J
Gastroenterol. 2010;105:1970-1977 http://dx.doi.org/10.1038/ajg.2010.110 PMid:20234345

- Gamberini
MR, Fortini M, De Sanctis V, Gilli G, Testa MR. Diabetes mellitus and
impaired glucose tolerance in thalassaemia major: incidence,
prevalence, risk factors and survival in patients followed in the
Ferrara Center. Pediatr Endocrinol Rev 2004;2 Suppl 2:285-291
PMid:16462713

- Fung
EB, Gildengorin G, Talwar S, Hagar L, Lal A. Zinc status affects
glucose homeostasis and insulin secretion in patients with thalassemia.
Nutrients 2015;7:4296-4307 http://dx.doi.org/10.3390/nu7064296 PMid:26043030 PMCid:PMC4488784

- Dehshal
MH, Hooghooghi AH, Kebryaeezadeh A, Kheirabadi M, Kazemi S, Nasseh A,
Shariftabrizi A, Pasalar P. Zinc deficiency aggravates abnormal glucose
metabolism in thalassemia major patients. Med Sci Monit 2007;13:
235-239

- Cunningham MJ, Macklin EA,
Neufeld EJ, Cohen AR. Complications of beta-thalassemia major in North
America. Blood 2004;104: 34-39 http://dx.doi.org/10.1182/blood-2003-09-3167 PMid:14988152

- De
Sanctis V, Zurlo MG, Senesi E, Boffa C, Cavallo L, Di Gregorio F.
Insulin dependent diabetes in thalassemia. Arch Dis Child 1988;63:58-62
http://dx.doi.org/10.1136/adc.63.1.58 PMid:3348650 PMCid:PMC1779356

- Messina
MF, Lombardo F, Meo A, Miceli M, Wasniewska M, Valenzise M, Ruggeri C,
Arrigo T, De Luca F. Three-year prospective evaluation of glucose
tolerance, beta-cell function and peripheral insulin sensitivity in
non-diabetic patients with thalassemia major. J Endocrinol Invest
2002;25:497-501 http://dx.doi.org/10.1007/BF03345490 PMid:12109619

- Kattamis
C, Ladis V, Tsoussis D, Kaloumenou I, Theodoridis C. Evolution of
glucose intolerance and diabetes in transfused patients with
thalassemia. Pediatr Endocrinol Rev 2004;2 Suppl 2:267-271
PMid:16462709

- Albaker
WI, Yousef AA, Khamis AH, Aldilaijan AF, AlMaghlouth NK. The continuous
glucose monitoring system (CGMS) in patients with beta-thalassemia
major. Saudi J Med Med Sci.2013;1:88-93 http://dx.doi.org/10.4103/1658-631X.123654

- Soliman
A, De Sanctis V, Yassin M, Elalaily R, Eldarsy NE. Continuous glucose
monitoring system and new era of early diagnosis of diabetes in high
risk groups. Indian J Endocrinol Metab. 2014;18:274-282 http://dx.doi.org/10.4103/2230-8210.131130 PMid:24944918 PMCid:PMC4056122

- Choudhary
A, Giardina P, Antal Z, Vogiatzi M. Unreliable oral glucose tolerance
test and haemoglobin A1C in beta thalassaemia major--a case for
continuous glucose monitoring? Br J Haematol. 2013;162:132-135 http://dx.doi.org/10.1111/bjh.12322 PMid:23594287 PMCid:PMC4055036

- Soliman
AT, Yasin M, El-Awwa A, De Sanctis V. Detection of glycemic
abnormalities in adolescents with beta thalassemia using continuous
glucose monitoring and oral glucose tolerance in adolescents and young
adults with ß-thalassemia major: Pilot study. Indian J Endocrinol
Metab. 2013;17:490-495 http://dx.doi.org/10.4103/2230-8210.111647 PMid:23869308 PMCid:PMC3712382

- De
Sanctis V, Zurlo MG, Senesi E, Boffa C, Cavallo L, Di Gregorio F.
Insulin dependent diabetes in thalassemia. Arch Dis Child 1988;63:58-62
http://dx.doi.org/10.1136/adc.63.1.58 PMid:3348650 PMCid:PMC1779356

- Platis
O, Anagnostopoulos G, Farmaki K, Posantzis M, Gotsis E, Tolis G.
Glucose metabolism disorders improvement in patients with thalassaemia
major after 24-36 months of intensive chelation therapy. Pediatr
Endocrinol Rev 2004;2 Suppl. 2:279-281 PMid:16462711

- Christoforidis
A, Perifanis V, Athanassiou-Metaxa M. Combined chelation therapy
improves glucose metabolism in patients with beta-thalassaemia major.
Br J Haematol 2006;135:271-272 http://dx.doi.org/10.1111/j.1365-2141.2006.06296.x PMid:16965387

- Dhouib
N, Turki Z, Mellouli F, Ouederni M, Yahiaoui S, Nagi S, Ben Slama C,
Bejaoui M. Efficacy of metformin in the treatment of diabetes mellitus
complicating thalassemia major. Tunis Med 2010;88:136.
PMid:20415181

- Ladis
V, Theodorides C, Palamidou F, Frissiras S, Berdousi H, Kattamis C.
Glucose disturbances and regulation with glibenclamide in thalassemia.
J Pediatr Endocrinol Metab 1998;11 Suppl 3:871-877
http://dx.doi.org/10.1111/j.1749-6632.1998.tb10525.x

- Mangiagli
A, Campisi S, De Sanctis V, Nicoletti MC, Cardinale G, Galati MC,
Raiola G, Rigano P, Saviano A; Study Group of the Italian Pediatric and
Diabetes Society (SIEDP) on Endocrine Complications in Non-Endocrine
Disease. Effects of acarbose in beta-thalassaemia major patients with
normal glucose tolerance and hyperinsulinism. Pediatr Endocrinol Rev
2004;2 Suppl 2:272-275 PMid:16462710

- Mangiagli
A, Italia S, De SV, Campisi S. Impaired glucose homeostasis in young
adult thalassemic patients: a pilot study with acarbose. J Pediatr
Endocrinol Metab. 2002;15:205-210 http://dx.doi.org/10.1515/JPEM.2002.15.2.205 PMid:11874186

- Moran
A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA,
Sabadosa KA, Stecenko A, Slovis B. Clinical care guidelines for cystic
fibrosis-related diabetes: a position statement of the American
Diabetes Association and a clinical practice guideline of the Cystic
Fibrosis Foundation, endorsed by the Pediatric Endocrine Society.
Diabetes Care 2010; 33: 2697-2708 http://dx.doi.org/10.2337/dc10-1768 PMid:21115772 PMCid:PMC2992215

- O’Riordan
SM, Robinson PD, Donaghue KC, Moran A. Management of cystic
fibrosis-related diabetes in children and adolescents. Pediatr.
Diabetes 2009; 10 (Suppl. 12): 43-50 http://dx.doi.org/10.1111/j.1399-5448.2009.00587.x PMid:19754617

- Incorvaia
C, Parmeggiani F, Mingrone G, Sebastiani A, De Sanctis V. Prevalence of
retinopathy in diabetic thalassaemic patients. J Pediatr Endocrinol
Metab 1998;11 Suppl 3:879-883 PMid:10091161

- De
Sanctis V, Incorvaia C, Soliman AT, Candini G, Pepe A, Kattamis C,
Soliman NA, Elsedfy H, Kholy ME. Does Insulin Like Growth Factor-1
(IGF-1) Deficiency Have a "Protective" Role in the Development of
Diabetic Retinopathy in Thalassamia Major Patients? Mediterr J Hematol
Infect Dis 2015 May 20;7(1):e2015038. doi: 10.4084/MJHID.2015.038 .
eCollection 2015 http://dx.doi.org/10.4084/mjhid.2015.038

- Tahara
Y, Shima K. Kinetics of HbA1c, glycated albumin, and fructosamine and
analysis of their weight functions against preceding plasma glucose
level. Diabetes Care 1995;18:440-447 http://dx.doi.org/10.2337/diacare.18.4.440 PMid:7497851

- Jandric
Balen M, Lukenda V, Jandric I, Ragu A, Zukanovic S, Miškic B. HbA1C -
overall glycemia marker and hemolytic anemia indicator. Med Glas
(Zenica). 2012;9:406-408

- Debard A,
Charmion S, Ben Ameur S, Gaultier JB, Cathébras P. Inappropriate low
glycated hemoglobin and hemolysis. Rev Med Interne. 2009;30:525-527 http://dx.doi.org/10.1016/j.revmed.2008.10.010 PMid:19019499

- Kattamis
C, Delaporta P, Dracopoulou M, Paleologos G, Chrousos GP, Papassotiriou
I, Kattamis A. Credibility of HbA1c in diagnosis and management of
disturbances of glucose and diabetes in transfused patients with
thalassemia. Riv Ital Med Adolesc. 2014;12: 65-71

- Youssef
D, El Abbassi A, Jordan RM, Peiris AN.Fructosamine--an underutilized
tool in diabetes management: case report and literature review. Tenn
Med. 2008;101:31-33 PMid:19024248

- Middleton
PG, Wagenaar M, Matson AG, Craig ME, Holmes-Walker DJ, Katz T, Hameed
S. Australian standards of care for cystic fibrosis-related diabetes.
Respirology. 2014;19:185-192 http://dx.doi.org/10.1111/resp.12227 PMid:24372844

- NICE
clinical guideline 63.Diabetes in pregnancy: management of diabetes and
its complications from pre-conception to the postnatal period, National
Institute for Health and Clinical Excellence, www.nice.org.uk. 2008:
pp.1-38






























































































