Sohaib Ahmad1, Nadia Shirazi2, Nowneet K Bhat3, Minakshi Dhar1,4, Garima Mittal5, Manish Mittal1, Nidhi Kaeley1 and Manoj Kumar1
1 Department of Medicine, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun
2 Department of Pathology, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun
3 Department of Pediatrics, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun
4 Presently in Department of Medicine, All India Institute of Medical Sciences, Rishikesh
5 Department of Microbiology, Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun
Corresponding
author: Dr. Sohaib Ahmad. Professor, Department of Medicine. Himalayan
Institute of Medical Sciences. SRH University. Jolly Grant. Dehradun
(Uttarakhand). INDIA-248016. Tell: +91-9412460098. E-mail:
sohadia@hotmail.com
Published: January 1, 2017
Received: September 28, 2016
Accepted: November 21, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017006 DOI
10.4084/MJHID.2017.006
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background & Objectives: Classically associated with Plasmodium (P.) falciparum,
neurological complications in severe malaria is associated with
increased morbidity and mortality. However, reports implicate the long
considered benign P. vivax
for causing severe malaria as well. We aimed to analyse the cerebral
complications in malaria, and study if there is a species-related
difference in the presentation and outcomes.
Methods:
We retrospectively compared patients with malaria hospitalised from
2009-15, with (n=105) and without (n=1155) neurological involvement
regarding outcomes, complications, demographic attributes, clinical
features, and laboratory parameters. Subsequently, the same parameters
were studied in those with cerebral malaria due to mono-infections of P. vivax or P. falciparum and their co-infection.
Results: Cerebral malaria was observed in 8.3% (58/696), 7.4% (38/513) and 17.6% (6/51) of P. vivax, P. falciparum
and combined plasmodial infections respectively. Those with cerebral
malaria had significantly (p<0.05) longer hospitalisation, delayed
defervescence, required mechanical ventilatory support and dialysis
despite comparable levels of azotemia and renal insufficiency, and
adverse outcomes compared to non-cerebral malaria. Severe
thrombocytopenia, respiratory distress and mechanical ventilation were
significantly (p<0.05) associated with P. vivax cerebral malaria.
Conclusions:
The plasmodial species are comparable in clinical and laboratory
parameters and outcomes in cerebral malaria in isolation and
combination (p>0.05). P. vivax is emerging as the predominant cause of cerebral malaria, and its virulence is comparable to P. falciparum.
|
Introduction
Cerebral
involvement is a severe manifestation of acute malaria. In endemic
countries, the central neurological system is more frequently and more
severely affected regarding sequelae in children than in adults,
presumably, because adults acquire some degree of immunity with age
after repeated episodes of malarial infection.[1]
Cerebral malaria is a multifactorial disease-seizures, impaired
substrate delivery leading to hypoxia and hypoglycaemia, reduced
perfusion due to shock, hypovolemia and acidosis are the proposed
pathogenetic mechanisms. Cytoadhesion of parasitized red blood cells to
the brain microvessels seems to be the main histopathological finding;
immunological factors (leukocytes, cytokines and chemokines),
platelets, nitric oxide scavengers and heme, are additionally involved
in the development of the disease. These factors integrate a systemic
inflammatory response during a malarial infection that acts in the
brain and is largely responsible for the clinical features pertaining
to the nervous system. However, it is not known to what extent and
timing each factor contributes to the pathogenesis and interferes with
the prognosis of the disease.[2]
Neurological
sequelae may develop in 5-11% cases with cerebral malaria, and a
fraction of patients may suffer long-term neurological impairments.[3,4,5,6] While the mortality attributed to malaria in India[7]
is 0.05%, the figure multiplies manifold in those with cerebral
involvement. Mortality in cerebral malaria was reported as 5.5-7% in
children in Africa[3,8] and 20% in adults in South Asia.[9]
Concomitant acute renal failure and metabolic acidosis cause a 6-fold
increase in mortality suggesting associated vital organ dysfunction has
a summative effect on mortality in severe malaria.[10]
Nearly 75% of deaths in children and 20% mortality in adults with
cerebral malaria occurs within the first 24 hours before they can
benefit from the full effect of antimalarials.[1]
Lactic acidosis, severe anaemia, hypoglycaemia, retinal haemorrhages
and leucocytosis have been proven to be associated with mortality and
the development of neurological sequelae in some studies.[10,11]
The
major thrust for reducing mortality and morbidity is on vector control
and development of the yet elusive effective vaccine. Nevertheless,
recognition of the early signs of neurological involvement and
indicators of poor outcome can allow the prompt initiation of the
available therapies for the malarial infection. Although, artemisinin
combination therapy (ACT) is recommended and the drugs are freely
available and used in the urban areas, older drugs like chloroquine and
quinine are the cornerstone of treatment in the countryside given their
availability in the national malaria control programme. Infection with
chloroquine-resistant organisms and delay in initiation of ACT may also
account for complications, sequelae and increased mortality.
Myriad presentations of acute malaria due to P. vivax and P. falciparum in isolation as well as in combination have been regularly observed in this part of the world.[12] Though traditionally attributed to Plasmodium (P.) falciparum infection, the cerebral manifestations are increasingly being recognised in those infected with P. vivax.
We hypothesised that cerebral malaria due to different etiological
species is different in term of presentation, complications and
outcomes. We undertook this study to test our hypothesis and also to
compare the outcomes and complications in cerebral and non-cerebral
malaria. We also intended to identify the demographic attributes,
clinical features, and haematological and biochemical parameters
precluding cerebral involvement.
Materials and Methods
Study setting:
Uttarakhand is a hilly north Indian state with the Ganges being the
major river system. It is surrounded by Tibet in the north, Nepal to
the east and the Indian states of Uttar Pradesh and Himachal Pradesh in
the south and north-west respectively. The vegetation includes alpine
meadows, subalpine conifer and subtropical pine forests, moist
deciduous forests and grasslands. Nearly 70% of its 10 million
population resides in rural areas. Uttarakhand has two principal
divisions – Garhwal and Kumaon comprising of seven and six districts
respectively. The Himalayan Hospital is a 1000 bed tertiary care
teaching hospital affiliated with the Himalayan Institute of Medical
Sciences located 25 km from Dehradun, the capital of Uttarakhand. The
hospital caters mainly to the Garhwal division, some districts of the
Kumaon division and the densely populated adjoining districts of Uttar
Pradesh.
Patient Selection:
Ours was a retrospective hospital-based observational study approved by
the institutional research and ethics committees of the Swami Rama
Himalayan University. All patients hospitalised for acute malaria over
a period of 6 years (2009-2015) were included in the study. The
diagnosis of acute malaria was considered if the peripheral blood smear
was positive and the included subjects were categorised as having
malaria due to P. falciparum, P. vivax or both the species.
Data Collection:
Clinical information including the duration of fever, associated
symptoms (nausea, vomiting, headache, loose stools, breathlessness,
abdominal pain, bleeding manifestations and the site of bleed, if
present, seizures, unconsciousness, oliguria and swelling over the
body, etc) and signs (heart rate, blood pressure, respiratory rate,
oxygen saturation, palpable organomegaly and other abnormal clinical
findings) at the time of presentation in the hospital was retrieved
from the hospital records for all patients included in the study.
Demographic details including age, gender and occupation were also
collected and compiled. The haematological and biochemical
investigations carried out at the time of hospitalisation were also
noted. Outcomes studied were mortality and morbidity (the duration of
hospitalisation, hypoglycemic events, shock, bleeding, severe
thrombocytopenia, organ dysfunction, time to regain consciousness and
defervescence, time to recovery of platelets and creatinine, and the
need for transfusion, intensive care, mechanical ventilation and
dialysis).
Severe malaria was diagnosed as per the WHO
guidelines issued in 2012 and 2015 with minor modifications in a bid to
define organ dysfunction. Cerebral malaria was diagnosed if more than
two episodes of convulsions were reported in 24 hours or the subject
was disoriented at presentation (A Glasgow coma score < 11 in adults
or a Blantyre coma score < 3 in children) in the absence of other
biochemical abnormalities precluding neurological dysfunction. Renal
impairment was defined as a rise in blood urea nitrogen (BUN) > 20
mmol/l and serum creatinine (> 3 mg/dl). Pulmonary involvement
(respiratory distress) was defined as tachypnea (>30/min) along with
a fall in oxygen saturation to <92%. Liver dysfunction was defined
as a two-fold rise in alanine transaminases [Normal value: 10–40 IU/l];
isolated hyperbilirubinemia i.e. (1.5-6 mg/dl) was not attributed to
liver dysfunction if liver transaminases were within normal limits. The
definition of jaundice followed by us is different from that by the WHO
as the parasite count mandated by the latter was not available in most
of our patients. The increase in platelet count in consecutive samples,
or beyond 50,000/mm3 when less at
presentation was considered as recovery of platelet counts.[12]
Improvement in serum creatinine was taken into account when elevated
serum creatinine normalised to lie within the reference range.
Data Analysis:
Data was analysed using the statistical software SPSS version 22.
Qualitative data was presented in the form of frequency and percentage,
and quantitative data as a mean + standard deviation. It was observed
that the data was not normally distributed; the Kruskal-Wallis – H test
was used to compare the difference among groups followed by
Mann-Whitney U test to compute the differences between the groups.
Chi-square and Fisher’s exact tests were used to check the significance
of the differences in categorical variables. A p <0.05 was
considered as statistically significant.
Results
A
total of 1649 patients (age range: 2 months to 96 years) of acute
malaria were treated at our centre over the last six years. Of these,
389 patients were excluded due to inadequate clinical, haematological
and biochemical data and/or the analysis was performed on 1260
patients. Of those included, 63.1% hailed from rural areas. The average
delay in seeking specialized care was 6.3 days; qualified as well as
unqualified medical personnel treated 82.2% (n=1306) patients in the
periphery. Treatment was with chloroquine in 79.1%, artemisinin
compounds in 15.0% and 5.7% with quinine; duration and doses prior to
the presentation were variable. The patients aged less than 18 years
accounted for 25% of all cases and were treated by the paediatricians.
A little over half of the patients were infected with P. vivax (n= 696; 55.2%), 513 (40.7%) with P. falciparum and
51 (4.0%) with both the plasmodial species. Overall, 96.5% of all
patients were febrile at presentation with chills and rigours reported
by 74.7% patients. Nausea and/or vomiting and headache were reported in
45.7% and 26.1% patients respectively. Giddiness and hiccoughs were
experienced by 2.5% and 0.8%. The overall mortality (and loss to
follow-up taken together) was 4.9%.
Neurological manifestations were observed in 8.3% (58/696), 7.4% (38/513) and 17.6% (9/51) of P. vivax, P. falciparum
and mixed plasmodial infections respectively. Of all the patients with
cerebral malaria (n=105), 40.9% were less than 18 years of age.
Seizures were observed in 25 (40.3%) and 28 (65.1%) adults and children
respectively while the remaining presented with unconsciousness without
overt seizure activity. Symmetrical upper motor neurone signs including
increased muscle tone and brisk tendon reflexes were observed in 76.1%
cases with patellar and/or ankle clonus (20%), and extensor plantar
responses (70.4%). Abdominal reflexes were universally absent wherever
documented. Reduced oral intake and restlessness were observed in 3
patients each (2.8%) in the paediatric age group. Restlessness and
paraesthesias were noticed before unconsciousness in 3 (4.8%),
opsoclonus in 1 while focal weakness was observed in 3 adult patients.
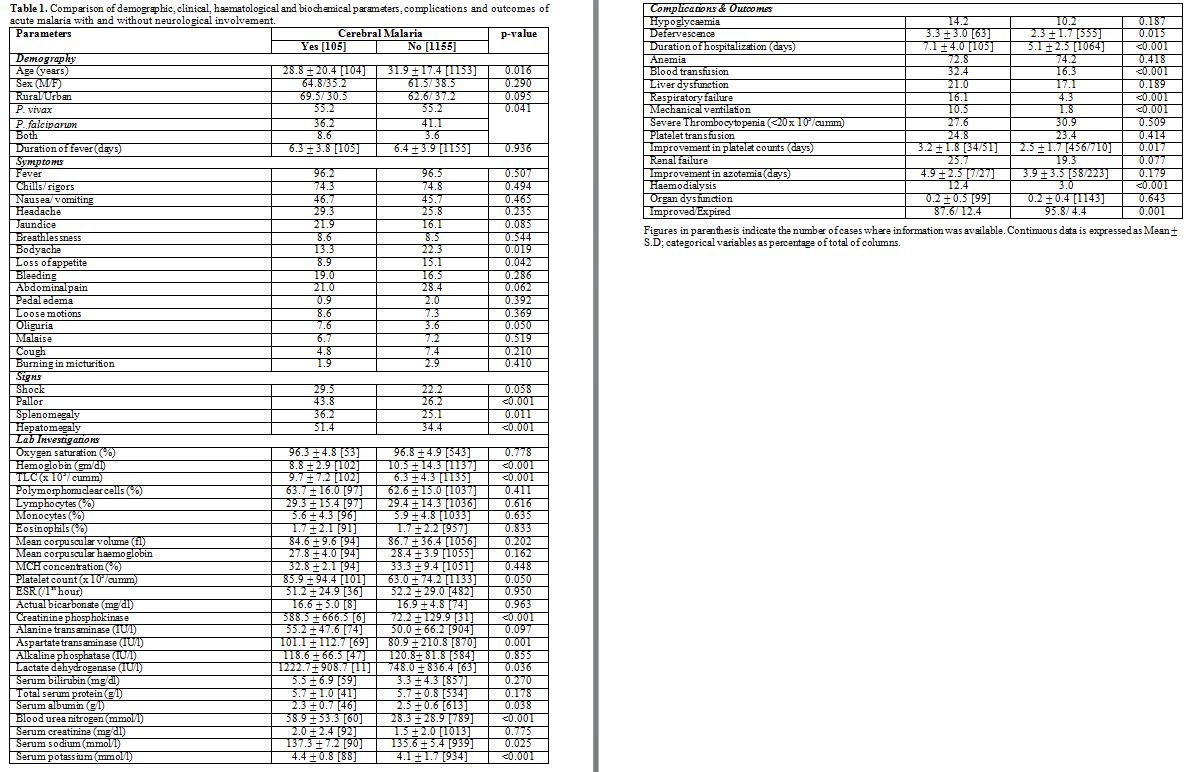
Table 1
shows the comparative analysis of patients with and without
neurological manifestations regarding demographic, clinical,
haematological and biochemical parameters, complications and the
outcomes. Those with cerebral malaria were younger in age with a
greater likelihood of presenting with enlargement of the spleen and/or
the liver, a low haemoglobin and serum albumin in comparison to those
without neurological manifestations. These patients defervesce
significantly later, had a significantly greater hospital stay, more
blood transfusions, and greater need for hemodialysis and mechanical
ventilation as compared to their counterparts without cerebral malaria.
Also, their comparative chances of deteriorating while on treatment and
succumbing to the disease 12.3% vs. 4.2%; p< 0.001) were
significantly more. Of the 13 patients who succumbed, one had pure
cerebral involvement. Four mortalities occurred in children < 5
years of age while one adolescent (15 years) died. ACT was used to
treat the patients in 95.1% (1199/1260); non-clearance of the parasite
from the blood smears and/or non-resolution of fever within three days
of proper therapy led to its replacement with quinine in 6 (0.5%)
cases. Quinine was used as the initial treatment in 61 (4.8%) cases.
 |
Table
1. Comparison of demographic, clinical, haematological and biochemical
parameters, complications and outcomes of acute malaria with and
without neurological involvement. |
In the second step,
we compared the complications and outcomes in patients with cerebral
malaria based on the etiological species. Jaundice was reported
significantly more in falciparum cerebral malaria than the other
subgroups. However, no significant difference in the etiological groups
in terms of clinical, haematological and biochemical characteristics
barring a couple of red cell parameters (mean corpuscular haemoglobin
and its concentration) was observed. The associated complications and
outcome measures are shown in Table 2. Respiratory distress and failure requiring mechanical ventilation and/or intensive care were not observed in P. falciparum mono-infections; however, the requirement rose significantly with P. vivax
co-infection (p=0.003). Two patients infected with both the Plasmodial
spp. had manifested bleeding of which 1 received platelet transfusion
despite having mild thrombocytopenia (1.0-1.5 lacs/cumm). Anaemia was
universally seen across the three etiological groups with no
significant difference between the haemoglobin and hematocrit values.
The mean serum creatinine values (mg/dl) were 1.9, 2.2 and 1.7
(p>0.05) for P. vivax, P. falciparum and co-infection groups respectively.
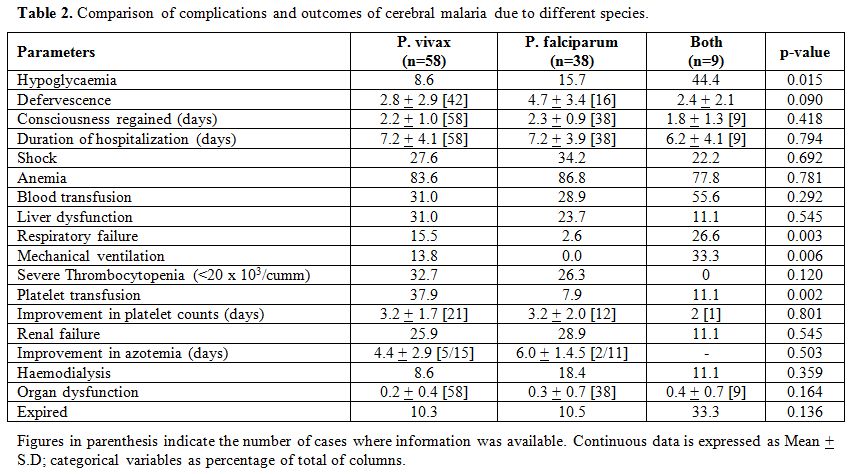 |
Table 2. Comparison of complications and outcomes of cerebral malaria due to different species. |
Of the 13 patients
who succumbed, shock (n=31), anemia (n=86), renal dysfunction (n=27),
liver dysfunction (n=28), respiratory failure (n=14) were associated in
5, 11, 7, 5 and 3 patients respectively; more than 2 complications were
observed in 9 patients. Mortality in cerebral malaria was significantly
associated with concomitant renal insufficiency (p=0.020); association
with shock, anaemia, respiratory failure and liver dysfunction was not
significant (p>0.05).
Discussion
In
the present study, greater morbidity and higher mortality were observed
in acute malaria patients with neurological complications. However,
cerebral malaria due to P. vivax or P. falciparum
in isolation or combination did not apparently differ significantly in
terms of morbidity and mortality. The predictive value of development
of cerebral complications is poor with regard to demographic, clinical
and laboratory parameters. Cerebral manifestations developed in 13.6%
of all children and adolescents, and 6.5% of all adults with malaria.
The higher incidence in children is consistent with the existing
literature and is apparently due to inadequate immune response to the
endemic infection. Another prominent observation is that P. vivax has overtaken P. falciparum
as the predominant species producing neurological manifestations over
the years in term of the number of cases although the association is
not statistically significant (p=0.596).
The neurological symptoms
and signs elicited at the time of presentation are similar in frequency
to that described in the literature.[1,9] Earlier studies suggested that surviving patients fully recover,[13]
but publications over the past 20 years highlight that many children
sustain a significant brain injury. Nearly 11% are discharged with
gross neurological deficits[4,5] though some deficits such as blindness, ataxia, and central hypotonia improve with time.[6]
Almost a quarter of these have long-term impairments about cognition,
motor function, or behaviour while epilepsy develops in 10%.[6,14,15,16]
The main risk factors for neurological sequelae identified include
repeated seizures, profound and prolonged coma, intracranial
hypertension, and hypoglycemia.[17] None of our
patients suffered neurological sequel apparently due to the absence of
the risk mentioned above factors. Hypoglycemia (plasma glucose < 40
mg/dl) occurs in about 8% of adults[18] and about 20% of children with cerebral malaria.[19] In our study, hypoglycemia was documented in 14.2% cases of cerebral malaria.
The incidence of seizures in adults has been variably described as 10-50% in studies from South Asia;[1,9,20]
seizures were reported in 40% adults with cerebral malaria in our
study. Differences in the parasite virulence characteristics and the
decreased use of chloroquine pretreatment may account for this wide
variation in the incidence of seizures. Also, the seizures are partial
motor and occasionally subtle (especially in children) such as
repetitive eye or hand movements and occult. Although more than one
seizure is frequent, status epilepticus is unusual in adults, and
consciousness after a seizure is usually obtunded.[9,10] Given the facts mentioned above, the fraction of seizures observed in our study is apparently an underestimation.
Clinical
features, namely anorexia and body ache, were reported significantly
more from those with intact consciousness for obvious reason.
Hepatosplenomegaly and pallor observed significantly more in those with
cerebral malaria were presumably associated with increased hemolysis
suggested by significantly higher (p<0.05) aspartate transaminase
and lactate dehydrogenase levels translated into significantly lower
haemoglobin and higher rates of transfusion (p<0.05). The morbidity
was significantly more (p<0.05) in those with cerebral compared to
those without non-cerebral malaria apparently due to delayed
defervescence, need for assisted mechanical ventilation, dialytic
support and transfusions.
Although bleeding manifestations and
mean haemoglobin and hematocrit levels were comparable amongst the
etiological groups, significantly more patients with vivax cerebral
malaria received platelet transfusions. This occurs mainly because of
the severe thrombocytopenia detected in nearly a third of all with
vivax cerebral malaria. Similarly, respiratory distress and incumbent
mechanical ventilation were significantly more (p<0.05) in vivax
cerebral malaria. Moreover, the incidence of renal and liver
dysfunction, occurrence of anaemia and shock, organ dysfunction and
mortality in those with cerebral malaria due to P. vivax was comparable to P. falciparum. These data imply the exponential rise of the malignant potential of P. vivax given the increasing fraction of P. vivax
as the cause of severe malaria. Although, the lower numbers in
co-infected etiological group desist us from drawing any conclusion,
evidence from existing literature lends credence to our observation of
the protective effect of co-infection of P. vivax and P. falciparum[21]
at least for severe thrombocytopenia and renal failure, though it does
not extrapolate to the other organs and mortality. The reason for this
disparity may be related to which plasmodial species pre-existed and
which superinfected. P. vivax superinfection over an existing P. falciparum infection raises P. falciparum parasitaemia thereby causing severe malaria. In contrast, P. falciparum superinfection over an existing P. vivax infection reduces P. falciparum parasitaemia and hence, protecting from the development of severe malaria.[21]
The
overall mortality of adult cerebral malaria is about 20% (10); 12.9%
(n=8) of our adults with cerebral malaria died. Mortality depends on
the associated vital organ dysfunction and rises from 8% in “pure”
cerebral malaria to 50% with associated acute renal failure and
metabolic acidosis.[10] An adverse outcome was
significantly related to renal dysfunction and metabolic acidosis in
our study too (p<0.02). One patient with concomitant respiratory
compromise succumbed in our study. Mortality may also occur for want of
intensive care facilities, renal replacement therapy and good nursing
care. Most deaths (61.5%) occurred within 24 hours of
hospitalization as was seen in earlier studies. Consciousness was
regained in our study after a median of 2 days (2.2+1.0 days) and the
maximum duration was recorded as 5 days. As per the existing
literature, those with a Glasgow coma score <11 needed a median of 2
days to regain consciousness but occasional adult patients may take
more than 1 week.[10] In contrast to earlier studies,[3,11]
hypoglycemia, deep coma, respiratory distress, circulatory failure, and
heavy parasitemia in cerebral malaria were not found to be associated
with mortality in the present study presumably due to availability of
intensive and supportive management supplemented by good nursing care.
The
limitations of our study are mainly its retrospective nature and its
attendant biases, mainly reliance on hospital records that were
incomplete at times. Neither, the level of parasitaemia was assessed
routinely nor sequential peripheral blood smears were performed as a
standard protocol. Due to this, we could not calculate the effect of
parasitaemia on the complications and the outcome. Also, the
defervescence could not be correlated to the disappearance of parasite
from the peripheral smear. The effect of adequacy of treatment received
prior to hospitalization and delay in the diagnosis was not taken into
account. Also, PCR was not used to confirm the Plasmodial specie.
Nevertheless, our reliance on objective parameters and inclusion of
only those cases with adequate clinical, hematological and / or
biochemical data circumvents this to a large extent. This is the first
study with such a large number of patients of malaria from this part of
the world to the best of our knowledge. Also, PCR is not available
routinely for assessment of the species. Blood smear examination with
trained pathologists is reliable, quite apparent from the low level of
morbidity in our study. Prospective interventional studies evaluating
the treatment practices and adequacy of treatment delivered are needed
to understand the lacunae and improving the outcomes of cerebral
malaria.
Conclusions
Neurological complications in malaria, classically caused in P. falciparum infections are increasingly being observed in P. vivax
infections. The situation is alarming with vivax constituting the major
burden of malaria in north India, little recognition of its malignant
potential compounded by the almost comparable complications and
outcomes. Our study may also prove to be an initiator for further
research into possible genetic alterations that the parasite or its
carrier may have incurred due to decades of insecticide use,
injudicious use of conventional antimalarials, industrialization and
ecological transformations and/ or possible co-infection with
unrecognized viruses.
References
- Idro R, Jenkins NE, Newton CRJC.
Pathogenesis, clinical features, and neurological outcome of cerebral
malaria. Lancet Neurol 2005; 4: 827-40. https://doi.org/10.1016/S1474-4422(05)70247-7

- Martins
YC, Carvalho LJM, Daniel-Ribeiro CT. Challenges in the Determination of
Early Predictors of Cerebral Malaria: Lessons from the Human Disease
and the Experimental Murine Models. Neuroimmunomodulation
2009;16:134-145 https://doi.org/10.1159/000180268 PMid:19212133

- Idro
R, Karamagi C, Tumwine J. Immediate outcome and prognostic factors for
cerebral malaria among children admitted to Mulago Hospital, Uganda.
Annals Trop Paedia 2004; 24: 17-24. https://doi.org/10.1179/027249304225013240 PMid:15005962

- Newton
CR, Krishna S. Severe falciparum malaria in children: current
understanding of pathophysiology and supportive treatment. Pharmacol
Ther 1998; 79:1-53 https://doi.org/10.1016/S0163-7258(98)00008-4

- Brewster DR, Kwiatkowski D, White NJ. Neurological sequelae of cerebral malaria in children. Lancet 1990; 336:1039-43 https://doi.org/10.1016/0140-6736(90)92498-7

- van
Hensbroek MB, Palmer A, Jaffar S, Schneider G, Kwiatkowski D. Residual
neurologic sequelae after childhood cerebral malaria. J Pediatr 1997;
131:125-129 https://doi.org/10.1016/S0022-3476(97)70135-5

- http://nvbdcp.gov.in/malaria3.html [accessed on 22.04.2016]
- Oluwayemi
OI, Brown BJ, Oyedeji OA, Adegoke SA, Adebami OJ, Oyedeji GA. Clinical
and laboratory predictors of outcome in cerebral malaria in suburban
Nigeria. J Infect Dev Ctries 2007; 7 (8): 600-7.

- Sattar
MA, Hoque HW, Amin MR, Faiz MA, Rahman MR. Neurological findings and
outcome in adult cerebral malaria. Bangladesh Med Res Counc Bull 2009;
35: 15-17 https://doi.org/10.3329/bmrcb.v35i1.2313 PMid:19637540

- Newton CRJC, Hien TT, White N. Cerebral malaria. J Neurol Neurosurg Psychiatry 2000 69: 433-441. https://doi.org/10.1136/jnnp.69.4.433 PMid:10990500 PMCid:PMC1737146

- Angyo
IA, Pam SO, Szlachetka R. Clinical pattern and outcome in patients with
acute severe falciparum malaria at Jos University Teaching Hospital,
Nigeria. East Afr Med J 1996; 73:823-6. PMid:9103694

- Saurabh
Srivastava, Sohaib Ahmad, Nadia Shirazi, SK Verma, Prashant Puri.
Retrospective analysis of vivax malaria patients presenting to tertiary
referral centre of Uttarakhand. Acta Tropica 2011; 117: 82-85 https://doi.org/10.1016/j.actatropica.2010.10.001 PMid:20943199

- Muntendam
AH, Jaffar S, Bleichrodt N, van Hensbroek MB. Absence of
neuropsychological sequelae following cerebral malaria in Gambian
children. Trans R Soc Trop Med Hyg 1996; 90: 391-4 https://doi.org/10.1016/S0035-9203(96)90518-0

- Ngoungou EB, Preux PM. Cerebral malaria and epilepsy. Epilepsia 2008; 49:19-24 https://doi.org/10.1111/j.1528-1167.2008.01752.x PMid:18754957

- Carter
JA, Mung’ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, Newton
CR. Persistent neurocognitive impairments associated with severe
falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry
2005; 76:476-81 https://doi.org/10.1136/jnnp.2004.043893 PMid:15774431 PMCid:PMC1739592

- John
CC, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, Wu B, Boivin
MJ. Cerebral malaria in children is associated with long-term cognitive
impairment. Pediatrics 2008; 122:e92-e99 https://doi.org/10.1542/peds.2007-3709 PMid:18541616 PMCid:PMC2607241

- Idro
R, Marsh K, John CC, Newton CRJ. Cerebral Malaria: Mechanisms of Brain
Injury and Strategies for Improved Neurocognitive Outcome. Paediat Res
2000; 68 (4): 267-74. https://doi.org/10.1203/PDR.0b013e3181eee738 PMid:20606600 PMCid:PMC3056312

- White
NJ, Warrell DA, Chanthavanich P, et al. Severe hypoglycemia and
hyperinsulinemia in falciparum malaria. N Engl J Med 1983;309:61-6. https://doi.org/10.1056/NEJM198307143090201 PMid:6343877

- Taylor
TE, Molyneux ME, Wirima JJ, et al. Blood glucose levels in Malawian
children before and during the administration of intravenous quinine
for severe falciparum malaria. N Engl J Med 1988;319:1040-7. https://doi.org/10.1056/NEJM198810203191602 PMid:3050516

- Tripathy
R, Parida S, Das L, Mishra DP, Tripathy D, Das MC, Chen H, Maguire JH,
Panigrahi P. Clinical Manifestations and Predictors of Severe Malaria
in Indian Children. Pediatrics 2007; 120 (3): e454-e460. https://doi.org/10.1542/peds.2006-3171 PMid:17766489

- Mohapatra
MK, Dash LK, Bariha PK, Karua PC: Profile of mixed species (Plasmodium
vivax and falciparum) malaria in adults. J Assoc Physicians India.
2012, 60: 20-24. PMid:23777020

[TOP]























