Mohamed Yassin1, Ashraf T Soliman2, Vincenzo De Sanctis3, Abbas Moustafa4, Sandra Abou Samaan4 and Abdulqadir Nashwan5
1 Department of Hematology, Al-Amal Hospital, Hamad Medical Center (HMC), Doha, Qatar.
2 Department of Pediatrics, Alexandria University Children Hospital, Elchatby, Alexandria, Egypt.
3 Pediatric and Adolescent Outpatient Clinic, Quisisana Hospital, Ferrara, Italy.
4 Department of Radiology HMC, Doha, Qatar.
5 Department of Nursing HMC, Doha, Qatar.
Corresponding
author: Vincenzo De Sanctis MD, Pediatric and Adolescent Outpatient
Clinic, Quisisana Hospital, 44100 Ferrara, Italy; Telephone: +39 0532
770243; E-mail:
vdesanctis@libero.it
Published: January 1, 2017
Received: October 4, 2016
Accepted: December 5, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017008 DOI
10.4084/MJHID.2017.008
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Acute
iron intoxication (FeI) in humans has not been adequately studied. The
manifestation of FeI, defined as a serum iron concentration >300
µg/dL (55 µmol/L) within 12 hours of ingestion, include various
symptoms appearing in progressive stages. Systemic toxicity is expected
with an intake of 60 mg/kg. A 27-year-old female nurse presented with
unintended acute intravenous iron intoxication (FeI) a week after
self-injecting herself with 20 ampoules of IV iron (4,000 mg elemental
iron, 60 mg/kg). She had stable vital
signs and mild hepatic tenderness. Hepatic MRI
(Ferriscan®) showed a moderate/severe liver iron content (LIC: 9 mg/g
dry tissue). Her hemogram, electrolytes, hepatic and renal functions
were normal. Based on the high dose of iron received and her elevated
LIC, chelation therapy was advised. She accepted only oral therapy and
was started on deferasirox at a dose of 30 mg /kg daily. This oral
chelation proved to be effective in clearing her hepatic iron overload
after six months (LIC: 2 mg /g dry tissue), without side effects. This
case also proved the value of Ferriscan® in diagnosing the degree of
hepatic FeI and monitoring therapy to achieve a safe level of LIC.
|
Introduction
Poisoning
from medications can happen for a variety of reasons, including
intentional overdose, inadvertently taking an extra dose, dispensing or
measuring errors, and exposure through breast milk. Iron
poisoning is more commonly seen in children than in adults.[1]
Iron
poisoning is primarily a clinical diagnosis. A combination of history,
physical examination, and laboratory features can identify patients at
risk for systemic toxicity.[2-5] The purpose of the
laboratory evaluation is to confirm the diagnosis of iron poisoning and
to monitor for clinical effects. Measurement of serum iron
concentration (SIC) is useful for establishing the diagnosis. A serum
iron level of more than 350 μg/dl between 2 and 6 h post-ingestion is
supposed to indicate a significant intoxication and levels more than
500 μg/dl suggest grave danger of acute liver failure.[3,4]
However, SIC cannot always be correlated with the severity or
the clinical phase of iron intoxication
because it measures free iron circulating in the
blood and not the intracellular iron that causes systemic toxicity. The
primary mechanism for iron-induced tissue damage is free radical
production and lipid peroxidation.[4]
The
generation of reactive oxygen species (ROS) secondary to iron
intoxication is because, as a transition metal, iron is a key
participant in both the Fenton and Haber–Weiss reactions, resulting in
the creation of hydroxyl radicals. As physiologic defenses for the
detoxification of ROS become overwhelmed, ROS causes a direct cellular
damage such as lipid membrane destruction (via hydroxyl
radical-initiated lipid peroxidation). The organ systems most affected
include the gastrointestinal tract, liver, vessels, and occasionally
pulmonary damage, renal damage and pancreatic necrosis.[4-6]
In
2005, a review of 70 patients with iron toxicity showed hepatotoxicity
in 13 patients with severe toxicity
(serum alanine transaminase >1,000 U/L) in nine patients.
Ten of these patients (all <18 years) died with one of them
requiring liver transplantation.[7-11]
We report
a young female adult with an acute unintended intravenous injection of
iron that lead to iron intoxication (60 mg/kg of elemental iron).
Because patient refused parenteral deferoxamine (DFO, Desferal ®)
therapy, oral deferasirox (Exjade ®) was used as an iron chelator.
Deferasirox (Exjade®) is the first approved oral iron chelator in the USA. It is selective for iron (as Fe3+).
It is a tridentate ligand that binds iron with high affinity in a 2:1
ratio. Its primary use is to reduce chronic iron overload in patients
who are receiving long-term blood transfusions for conditions such as
β-thalassemia.
It was approved by the United States Food and Drug
Administration (FDA) in November 2005. According to FDA (May 2007),
renal failure and cytopenias have been reported in patients receiving
deferasirox oral suspension tablets. It is approved in the European
Union by the European Medicines Agency (EMA) for children six years and
older for chronic iron overload from repeated blood transfusions.[12]
FerriScan®, a non-invasive
magnetic resonance imaging (MRI) method for
measuring the degree of body iron burden through quantification of
liver iron concentration (LIC), is used for quantifying and monitoring
hepatic (tissue) iron overload.[13]
Case Report
A
27-year-old female nurse self- referred to
hematology clinic for an unintended
exposure of 20 ampoules of a preparation
containing iron, given intravenously (IV), over a period of 20 days for
iron deficiency anemia (Hb 9 g/dl, low hematocrit and low serum
iron). Each
ampoule contained 200 mg of elemental iron,
(4,000 mg elemental iron, 60 mg/kg body
weight). Referred symptoms at the admission included
mild abdominal pain a week after the last
injection.
The clinical examination was not remarkable apart from mild hepatic tenderness. Serum
iron concentration, ferritin, electrolytes, blood urea
nitrogen (BUN), glucose, alanine and aspartate aminotransferases,
albumin, prothrombin time (PT), arterial blood gasses
and complete blood
count with differential were urgently requested.
Markers of iron overload and hepatic MRI (Ferriscan ®) showed a severe iron overload (Table 1 and Figure 1).
Serum iron and ferritin concentrations were 200 µmol/l and 1473 µg/l
respectively. Therefore, she was advised to be treated with parenteral
deferoxamine (DFO, Desferal ®). Her CBC serum electrolytes, liver and
renal functions were all normal. The treatment with DFO was refused and
a novel oral iron chelator, mainly used in patients with
transfusion-dependent β-thalassemia, was recommended (deferasirox,
Exjade ®) at the dose of 30 mg /kg daily. The duration of treatment was
advised taking into consideration the patient's clinical status, the
serum iron concentration and the LIC levels. The mild symptoms
previously reported by the patients disappeared after 4 weeks of
treatment. The iron chelation therapy with deferasirox was stopped
after 6 months. Despite LIC normalization, serum ferritin levels
remained above the normal levels (1,000 µg/l; normal levels in females:
18–160 µg/L). A periodic 3 months’ reassessment of serum ferritin was
advised.
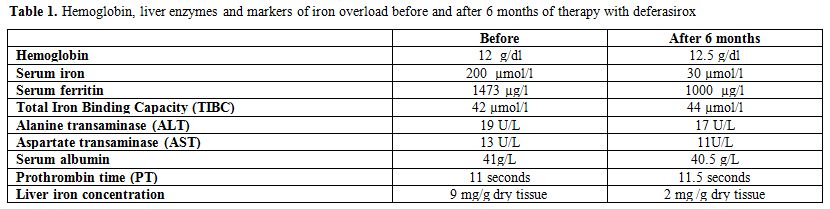 |
Table 1. Hemoglobin, liver enzymes and markers of iron overload before and after 6 months of therapy with deferasirox |
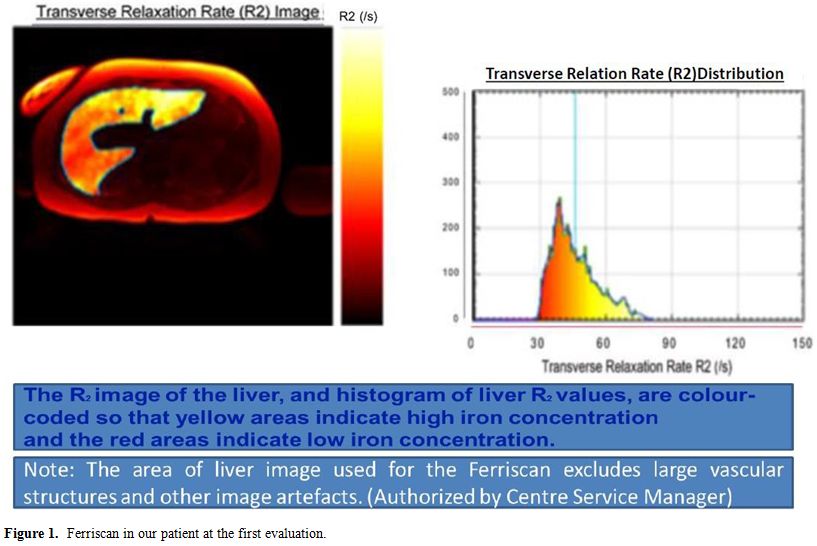 |
Figure 1. Ferriscan in our patient at the first evaluation. |
Discussion
The
2014 Annual Report of the American Association of Poison Control
Centers' (AAPCC) National Poison Data System reported 4024 single
exposures to iron or iron salts, with one major outcome and one death.
Overall, 75% of cases were in children younger than 6 years.[1]
Iron
is an essential element for normal cell metabolism, but in excess
quantities iron is highly cytotoxic and even lethal. Children may show
signs of toxicity with ingestions of 10-20 mg/kg of elemental iron.
Ingestions between 20 and 60 mg/kg of elemental iron can develop mild
to moderate clinical signs of iron intoxication, necessitating
treatment or monitoring. Serious toxicity is likely with ingestions of
more than 60 mg/kg and can result in serious poisoning or death.[4-7]
Manifestation
of acute iron poisoning, defined as a serum iron concentration >300
µg/dL (55 µmol/L) within 12 hours of ingestion. Early signs, presenting
within the first 6 hours, include abdominal pain, vomiting, diarrhoea
and gastrointestinal bleeding. In the stage of stabilisation (12 hours
post-ingestion), absorbed iron is rapidly cleared from the circulation
by cellular uptake, and then affects mitochondrial function.
Mitochondrial toxicity evokes signs of shock, acidosis, coagulopathy,
hyper- or hypoglycaemia, and acute tubular necrosis. Once a critical
amount of iron has reached the mitochondria, therapy has little effect
and outcome is poor. Subsequently, within 48 hours acute liver failure
predominates.[7,8]
Hepatotoxicity usually is
observed at serum iron levels higher than 500 µg/dL and patients
with serum iron levels higher than 1000 µg/dL may need intensive
care.[9] Two to 3 days post- ingestion, iron is
absorbed by Kupffer cells and hepatocytes, exceeding the storage
capacity of ferritin and causing oxidative damage. Pathologic changes
include cloudy swelling, peri-portal hepatic necrosis, and elevated
transaminase levels. This may result in hepatic failure. Intravenous
iron leads to preferential involvement of the reticuloendothelial
system of liver, spleen, bone marrow, and lymph nodes. After
saturation, the iron accumulates in hepatocytes and in parenchymal
cells of the pancreas, myocardium, and endocrine glands.[10]
Diagnosis
of acute iron poisoning is based on clinical symptoms, elevated serum
iron level and appearance of “vin rose" urine in deferoxamine test.[11]
For serum iron measurement, samples should be drawn at least 4 hours
post-ingestion, to allow levels to reach steady state; however, levels
drawn more than 6 hours after ingestion may underestimate toxicity
because of ferritin binding and redistribution of iron.
Our
patient came to our observation late (7 days after the last dose of
iron). Acute intoxication manifestations did not occur probably due to
the administration of iron doses over an extended period.[11]
However, it is possible that prompt iron chelation in our case had
prevented the cumulative pathological effects of acute and subacute
severe iron intoxication on the liver.
Liver iron concentration
provides the best measure of total body iron stores and is a validated
predictor of the risks a patient faces from the complications of iron
toxicity. Currents methods for quantifying hepatic iron include biopsy,
imaging, spectroscopy, and susceptometry. Biopsy is an invasive
procedure that carries a significant risk of complications. In
addition, biopsy specimen is subject to sampling error due to
non-uniformity in the distribution of liver iron.[14]
Several imaging non-invasive techniques are
available for measuring LIC. There are different validated MRI methods
for quantitating the liver iron burden, the most widely relaxometry
methods used include: the FerriScan® (T2/R2 based) and T2*/R2* based
methods: the FerriScan® and T2 methods. The noninvasive FerriScan® is
highly sensitive and specific for estimating LIC and is approved by the
Food and Drug Administration for routine clinical use. However, it is
usually not used to diagnose and monitor LIC in cases of acute iron
intoxication.[14-15]
In our case, significantly
increased LIC was documented despite moderately elevated serum iron and
ferritin and normal TIBC. This indicates a weak reliability of
biochemical parameters and the superiority of using this non-invasive
method if the patient presents late during iron toxicity. In support of
this finding, family studies on patients with idiopathic
haemochromatosis suggested that the serum ferritin concentration was
not a reliable index of hepatic iron overload and that a rise in serum
ferritin levels occurred only when liver damage was present. An
analysis of ferritin levels, estimated transfusion iron overload (TIL)
in patients who received simple transfusions prior to the start of
chelation therapy), and LIC (liver biopsy specimens) done on children
enrolled in two trials for stroke prevention showed serum ferritin
changes that were non-linear compared to TIL or LIC. Of note, serum
ferritin rose rapidly with transfusion initially, then slowed after
reaching 1,500–2,500 ng/ml, despite evidence of increasing iron load.
Serum ferritin levels greater than 3,000 ng/ml were associated with
both increased LIC and liver injury, as estimated by ALT levels.
However, because of the non-linear association between serum ferritin
level and LIC, authors recommended using more accurate methods for
assessing iron levels in those with levels between 1,500 and 3,000
ng/ml.[16-18]
The goals of pharmacotherapy are
to reduce iron levels, prevent complications, and reduce morbidity. DFO
is used for chelation of iron in both acute and chronic toxicity.
Approximately 8 mg of iron is bound by 100 mg of deferoxamine. DFO is a
chelating agent that, in acute iron intoxication, binds with ferric
iron (Fe3+) in the blood to form
water-soluble ferrioxamine that is then excreted by the kidneys.
Ferrioxamine gives urine the classical orange to reddish brown color.
DFO must be administered early in the treatment of iron overdose
because iron moves rapidly from the circulation into cells, where, in
acute intoxication, it is not readily accessible for chelation.[11,19]
Patients
who are symptomatic should receive DFO regardless of their iron level.
Indications of potential serious toxicity include the following:
ingestion of greater than 60 mg/kg of elemental iron; peak of serum
iron concentration greater than 500 µg/dL (90 µmol/L); persistent
serious symptoms such as vomiting, diarrhea, and/or altered
mental status; presence of systemic symptoms, including subtle symptoms
that can be seen in the latent phase of iron intoxication, such as
tachycardia, hypotension, poor peripheral tissue perfusion, and/or
tachypnea.[18,20] In acute iron
poisoning, intramuscular (IM) administration of deferoxamine is
indicated for patients who are not in shock; intravenous (IV)
administration should be reserved for patients in a state of
cardiovascular collapse or shock. Nevertheless, there is a lack of
knowledge about its optimal use.
Other chelator therapies are
still experimental. In 2005, the Food and Drug Administration approved
deferasirox as an oral iron chelating agent for chronic iron overload
due to blood transfusions in patients 2 year of age and older; it is
also approved for treatment of chronic iron overload resulting from
non–transfusion- dependent thalassemia. Efficacy of orally administered
iron chelator deferiprone in acute iron poisoning is still under
investigation.
Berkovitch M et al.[19] reported
that co- administration of 800 mg/kg deferiprone with LD50 dose of iron
decreased morbidity and mortality caused by acute iron overdose.
Histologically, there was a dose-dependent decrease in iron
accumulation in the gastrointestinal tract. These findings in animals
hold promise for its use in humans.
To determine the usefulness in management of acute iron ingestion, Griffith et al.[20]
studied the effect of orally administered deferasirox in 8 healthy
human adults. Subjects ingested 5 mg/kg of elemental iron in the form
of ferrous sulfate. One hour after iron ingestion, subjects were
randomized to receive 20 mg/kg of deferasirox or placebo. Deferasirox
significantly reduced serum iron levels when administered 1 hour after
iron ingestion during the 12- and 24-hour periods after acute ingestion.
Uncommon
serious side effects that may occur with deferasirox use include acute
renal failure and hepatic injury. Measuring and monitoring serum
creatinine and creatinine clearance and serum transaminases and
bilirubin in all patients prior to initiating treatment, and at least
monthly thereafter is recommended.
Conclusions
This
case demonstrates the efficacy of oral iron chelation therapy in
patients with mild to moderate clinical signs of iron intoxication with
normal renal and hepatic functions and the
usefulness of Ferriscan® to assess liver iron
overload and to monitor the effect of chelation therapy. It appears
that LIC of patients with acute iron intoxication can be accurately
diagnosed and monitored by Ferriscan.
References
- Mowry JB, Spyker DA, Brooks DE,
McMillan N, Schauben JL. 2014 Annual Report of the American Association
of Poison Control Centers' National Poison Data System (NPDS): 32nd
Annual Report. Clin Toxicol (Phila). 2015;53:962-1147. https://doi.org/10.3109/15563650.2015.1102927 PMid:26624241

- Erickson
TB, Thompson TM, Lu JJ. The approach to the patient with an unknown
overdose. Emerg Med Clin North Am. 2007;25:249-81. https://doi.org/10.1016/j.emc.2007.02.004 PMid:17482020

- Velez,
L, Delaney, K. Heavy metals. In: Emergency Medicine: Concepts and
Clinical Practice, 5th edition, Marx, J, Hockberger, R, Walls, R (Eds),
Mosby, St. Louis 2006. p.2418.
- Tenenbein M. Toxicokinetics and toxicodynamics of iron poisoning. Toxicol Lett. 1998;28:102-3

- Mills KC, Curry SC. Acute iron poisoning. Emerg Med Clin North Am.1994;12: 397- 413. PMid:8187690

- Link
G, Saada A, Pinson A, Konijn AM, Hershko C. Mitochondrial respiratory
enzymes are a major target of iron toxicity in rat heart cells. J Lab
Clin Med. 1998; 131: 466-74. https://doi.org/10.1016/S0022-2143(98)90148-2

- Manoguerra
AS, Erdman AR, Booze LL, Christianson G, Wax PM, Scharman EJ, Woolf AD,
Chyka PA, Keyes DC, Olson KR, Caravati EM, Troutman WG. Iron ingestion:
an evidenced-based consensus guideline for out-of-hospital management.
Clin Toxicol. 2005; 43:553-70. https://doi.org/10.1081/CLT-200068842

- Lawrence
DT, Bechtel L, Walsh JP, Holstege CD. The evaluation and management of
acute poisoning emergencies. Minerva Med.2007; 98:543-68. PMid:18043563

- Robertson A, Tenenbein M. Hepatotoxicity in acute iron poisoning. Hum Exp Toxicol. 2005;24:559-62. https://doi.org/10.1191/0960327105ht564oa PMid:16323571

- Siegelman
ES, Mitchell DG, Semelka RC. Abdominal iron deposition: metabolism, MR
findings, and clinical importance. Radiology.1996;199:13-22 https://doi.org/10.1148/radiology.199.1.8633135 PMid:8633135

- Baranwal AK, Singhi SC. Acute iron poisoning: management guidelines. Indian Pediatr. 2003; 40: 534-40. PMid:12824662

- Kontoghiorghe
CN, Kontoghiorghes GJ. Efficacy and safety of iron-chelation therapy
with deferoxamine, deferiprone, and deferasirox for the treatment of
iron-loaded patients with non- transfusion-dependent thalassemia
syndromes. Drug Des Devel Ther. 2016;10:465-81. https://doi.org/10.2147/DDDT.S79458 PMid:26893541 PMCid:PMC4745840

- Sirlin
CB, Reeder SB. Magnetic Resonance Imaging Quantification of Liver Iron.
Magn Reson Imaging Clin N Am. 2010;18:359-81. https://doi.org/10.1016/j.mric.2010.08.014 PMid:21094445 PMCid:PMC3430384

- St
Pierre TG, El-Beshlawy A, Elalfy M, Al Jefri A, Al Zir K, Daar S, Habr
D, Kriemler-Krahn U, Taher A. Multicenter validation of spin-density
projection-assisted R2-MRI for the noninvasive measurement of liver
iron concentration. Magn Reson Med. 2014;71:2215-2223. https://doi.org/10.1002/mrm.24854 PMid:23821350 PMCid:PMC4238736

- Garbowski
MW, Carpenter JP, Smith G, Roughton M, Alam MH, He T, Pennell DJ,
Porter JB. Biopsy-based calibration of T2* magnetic resonance for
estimation of liver iron concentration and comparison with R2
Ferriscan. J Cardiovasc Magn Reson. 2014 Jun 10;16:40.
doi:10.1186/1532-429X-16-40. https://doi.org/10.1186/1532-429X-16-40

- Feller
ER, Pont A, Wands JR, Carter EA, Foster G, Kourides IA, Isselbacher KJ.
Familial hemochromatosis. Physiologic studies in the
precirrhotic stage of the disease. N Engl J Med.
1977;296:1422-6. https://doi.org/10.1056/NEJM197706232962501 PMid:194151

- Adamkiewicz
TV1, Abboud MR, Paley C, Olivieri N, Kirby- Allen M, Vichinsky E,
Casella JF, Alvarez OA, Barredo JC, Lee MT, Iyer RV, Kutlar A, McKie
KM, McKie V, Odo N, Gee B, Kwiatkowski JL, Woods GM, Coates T, Wang W,
Adams RJ. Serum ferritin level changes in children with sickle cell
disease on chronic blood transfusion are nonlinear and are associated
with iron load and liver injury. Blood. 2009;114:4632-8. https://doi.org/10.1182/blood-2009-02-203323 PMid:19721013 PMCid:PMC2780299

- Tenenbein M. Benefits of parenteral deferoxamine for acute iron poisoning. J Toxicol Clin Toxicol.1996;34:485-489. https://doi.org/10.3109/15563659609028005 PMid:8800185

- Berkovitch
M, Livne A, Lushkov G, Segal M, Talmor C, Bentur Y, Klein J, Koren G.
The efficacy of oral deferiprone in acute iron poisoning. Am J Emerg
Med. 2000;18:36-40. https://doi.org/10.1016/S0735-6757(00)90045-7

- Griffith
EA, Fallgatter KC, Tantama SS, Tanen DA, Matteucci MJ. Effect of
deferasirox on iron absorption in a randomized, placebo-controlled,
crossover study in a human model of acute supratherapeutic iron
ingestion. Ann Emerg Med. 2011; 58:69-73. https://doi.org/10.1016/j.annemergmed.2010.11.020 PMid:21288598

[TOP]






















