Alessia Castellino, Elisa Santambrogio, Maura Nicolosi, Barbara Botto, Carola Boccomini and Umberto Vitolo
Città della Salute e della Scienza University and Hospital, Hematology Unit, Turin, Italy
Corresponding
author: Alessia Castellino. Città della Salute e della Scienza
University and Hospital, Hematology Unit, Turin, Italy. E-mail:
acastellino@cittadellasalute.to.it
Published: Dacember 7, 2017
Received: November 2, 2016
Accepted: December, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017009 DOI
10.4084/MJHID.2017.009
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Follicular
lymphoma (FL) is the most common indolent non-Hodgkin lymphoma, which
typically affects mature adults and elderly, whose median age at
diagnosis is 65 years. The natural history of FL appears to have been
favorably impacted by the introduction of Rituximab. Randomized
clinical trials demonstrated that the addition of rituximab to standard
chemotherapy induction has improved the overall survival and new
strategies of chemo-immunotherapy, such as Bendamustine combined with
Rituximab, showed optimal results on response and reduced hematological
toxicity, becoming one of the standard treatments, particularly in
elderly patients. Moreover, maintenance therapy with Rituximab
demonstrated improvement of progression-free survival. Despite these
exciting results, FL is still an incurable disease. It remains a
critical unmet clinical need finding new prognostic factors to identify
poor outcome patients better, to reduce the risk of transformation and
to explore new treatment strategies, especially for patients not
candidate to intensive chemotherapy regimens, such as elderly patients.
Some progress was already reached with novel agents, but larger and
more validated studies are needed. Elderly patients are the largest
portion of patients with FL and represent a subgroup with higher
treatment difficulties, because of comorbidities and smaller spectrum
for treatment choice. Further studies, focused on elderly follicular
lymphoma patients, with their peculiar characteristics, are needed to
define the best-tailored treatment at diagnosis and at the time of
relapse in this setting.
|
Introduction
Follicular
lymphoma (FL) is the most common form of indolent lymphoma and accounts
for 20% to 30% of all newly diagnosed non-Hodgkin’s Lymphoma (NHL)[1]
and with an annual incidence of 1.6- 3.1/ 100000 cases in western
countries.[2,3] It typically occurs in mature and older adults, the
median age of 65 years and with frequently in patients older than 75
years. FL is considered as an indolent but incurable disease with a
median life expectancy of approximately ten years. Despite advances in
the treatment of FL, most of the patients remain incurable and, in 10
years, 15% to 28% of cases will transform into an aggressive phenotype,
typically diffuse large B-cell lymphoma (DLBCL).
FL arises from
malignant transformation of normal germinal center (GC) B cells and, in
approximately 85% of cases, harbours the translocation
(14;18)(q32;q21), resulting in an inability to down-regulate expression
of the anti-apoptotic protein B-cell lymphoma 2 (BCL2), which is absent
in normal GC B cells.[4] Most tumors are characterized by recurrent
secondary genetic alterations that may provide a growth advantage,
including genomic gains, losses, and mutations.
The histological
report should give the diagnosis according to the World Health
Organization (WHO) classification.[5] Grading of lymph node biopsies is
performed according to a number of blasts/high power field.
The
treatment depends on the stage of the disease, so initial staging
should be thorough, particularly in the small proportion of patients
with localized stages I and II (10%–15%). Staging should include a
computed tomography (CT) scan, Positron emission tomography(PET)-CT and
a bone marrow aspirate and biopsy.[6] Complete blood test, including
chemistry and screening for HIV, HCV, and HBV must be done at
baseline. The staging is performed according to the Ann Arbor
classification system.[7]
The prognosis of FL remains
heterogeneous. Thus, prognostic indices are necessary to guide the
physician’s decision-making process and to design clinical trials.
Several prognostic factors have been identified in patients with FL,
including age, stage, tumor burden, bone marrow (BM) involvement,
systemic symptoms, performance status (PS), serum lactate dehydrogenase
(LDH),
hemoglobin, erythrocyte sedimentation rate, and β2-microglobulin.[8-9]
As
result of international cooperation, the FL International Prognostic
Index (FLIPI) was established in 2004.[10] This model divided patients
affected by FL in three different classes of risk according to five
parameters, including age over 60 years, Ann Arbor stage III or IV,
hemoglobin value <12 mg/dL, more than four nodal sites involved,
increased value of serum LDH. However, the FLIPI was born before
rituximab era and was based on retrospective data, so a revised FLIPI 2
(incorporating beta2 microglobulin, the diameter of largest lymph node,
bone marrow involvement, and hemoglobin level) was introduced.[11]
Extended
knowledge of the biology of tumor lead to a clinic-genetic risk score
(m7-FLIPI) based on mutation status of 7 candidate genes,[12] but it is
not standardized yet.
Elderly Patient: the Impact of Age
Many
patients with FL are elderly and age by itself (>60 years) has been
shown to be one of the most powerful poor prognostic features into
Follicular Lymphoma International Prognostic Index (FLIPI).[10]
However, so far there are few clinical trials specifically designed for
these patients; in clinical practice elderly patients are often managed
in a palliative way or with the adoption of a “watchful waiting” policy
in low tumor burden or asymptomatic patients or, in most of the cases,
the planned whole treatment is stopped because of treatment-related
toxicity.
The clinical approach to elderly patients is a complex
issue and age alone could not be enough to guide the treatment
strategy. Older patients show alterations in tumor-host biology and
comorbidities which result in changes in pharmacokinetics and
pharmacodynamics, may be a possible reason for poorer outcome in this
setting.[13-14] Moreover, it is well known that immune system in older
adults displays a deterioration of DNA-damage repair mechanisms and a
decrease of both cellular mediated and humoral immune response.[15-16]
Older patients are also more likely to develop cardiotoxicity, neurotoxicity, kidney injury, and mucositis.
Indeed,
to explain the worst prognosis in elderly patients, some studies
suggested that lymphomas could be biologically more complex and
aggressive in older people.
Some evidence suggested for example
that CD69 expression on lymphoma cells was related to a poor outcome,
with a prognostic value independent from the treatment, evaluated in a
population of older adults.[12] A dense infiltrate of CD4-positive T
cells, especially when located interfollicular, was a good prognostic
sign irrespective of treatment. Dense infiltrate of FoxP3-positive T
cells and CD68 positive macrophage, especially with an interfollicular
component, was associated with better survival. However, contradictory
results regarding the
correlation between treatment heterogeneity
and clinical impact have been reported by a Finnish group:[17] they
showed that the addition of rituximab to chemotherapy is the cause of
reversing the negative prognostic impact of high macrophage content,
showed in previous series,[18] into favorable factor. In the rituximab
era, the high macrophage content showed a positive impact on prognosis
at both diagnosis and relapse, and it is likely to be associated with
antibody-dependent cytotoxicity. It was noted that the relative number
of lymphoma-associated macrophage is lower in younger patients.[18-19]
Also, the prognostic value of minimal residual disease (MRD) was
firstly evaluated in a cohort of elderly patients.[20]
Even if in
elderly patients there were biological differences compared to FL in
younger people, many trials showed that these patients, if treated with
a correct dose-intensity chemotherapy, could reach a response rate
similar to a younger population.[15]
According to the results of
these studies, an accurate, complete evaluation of elderly patients
affected by lymphoma remains a central issue for a good clinical
practice, in order to administer a tailored dose-intensity therapy to
obtain the best outcome for these patients.
The Comprehensive
Geriatric Assessment (CGA) is a score used to make a whole evaluation
of elderly people with cancer, based on age, comorbidities and
functional abilities of daily living and it represents an important
tool in older people, in order to personalize the treatment
discriminating among fit, unfit or frail patients.[21] It is based on
many different tests including: ADL scale, IADL scale, evaluation of
comorbidities (Charlson’s scale and CIRS-G scale), Mini Mental State
Examination (MMSE), evaluation of nutritional state (20% of patients
older than 70 years is underfed)[22] and socio-economic state. ADL
scale (or Katz’s scale)[23] is based on the possibility to perform
regular daily activities (such as eating, washing, dressing, etc..);
IADL scale (or Lawton’s scale)[24] evaluates the self-government in
social function, such as phoning, shopping, money management, etc. MMSE
shows alterations in more than 50% of people older than 85 years[25]
and Geriatric Depression Scale demonstrates a depression in 20% of
patients older than 70 years.[26]
On this basis, Tucci et al.[27]
conducted a pilot trial to analyze if a simplified CGA model could
identify elderly patients with aggressive lymphoma eligible for
anthracycline therapy on 84 patients aged more than 65 years. The
Italian Lymphoma Foundation (FIL) recently performed a prospective
multicenter trial to validate a simplified CGA evaluation model in a
cohort of 173 elderly patients with lymphoma. Based on this simplified
CGA elderly patients were classified into three categories: fit,
unfit and frail (Figure 1). The
results of this study showed that the 2y-OS was significantly better in
fit than in unfit or frail patients (84% vs. 47%, p <0.0001).
Survival in unfit and frail people was superimposable. CGA was
confirmed as very useful to guide clinical therapeutic decisions and to
identify elderly patients who can benefit from a curative approach,
while further efforts are needed to better tailor therapies in not fit
population.[28] However, it must be noted that this trial was conducted
in patients with aggressive diffuse large B-cell lymphoma and it was
not validated in a cohort of FL elderly patients.
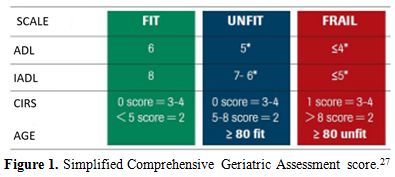 |
Figure 1. Simplified Comprehensive Geriatric Assessment score.[27] |
Recommendations
of the Authors: an accurate whole evaluation of elderly patient
affected by lymphoma is a central issue, and it represents the first
step for a tailored dose-intensity therapy, to obtain the best outcome
for these patients; CGA and comorbidity scale are useful instruments to
guide therapeutic decisions for a good clinical practice.
Treatment
An
ideal therapy for older adults should be brief, feasible in an
outpatient setting, effective and possibly with low related toxicity.
Despite
a variety of treatment approaches are currently available for the
initial treatment of follicular lymphoma, there are no universally
accepted first-line chemotherapy regimens for advanced stage disease.
The introduction of anti-CD20 monoclonal antibody (Rituximab) has
definitely improved the outcome of these patients as shown by many
studies. Rituximab and standard chemotherapy show no significant
overlapping toxicities. This evidence provides the rationale for
combining chemotherapy regimens with Rituximab, considered at
present the standard component of first-line treatment with a
complete remission rate ranging from 20 to 75%, a 4 years-progression
free survival (4y-PFS) improved at 61% (p=0.005) and a 4y-overall
survival (4y-OS) of 91% (p < 0.001).[29]
First-Line Therapy
In
the small proportion of limited non-bulky stages I–II, radiotherapy
alone is the preferred choice. Several centers reviewed the long-term
outcome of RT alone and demonstrated a freedom from relapse of 55%,
44%, 43% and 35% at 5, 10, 15 and 20 years of follow-up. Relapse
occurs in only 10% of high-risk patients at 10 years.[30-31]
The
most recent and largest retrospective study of 6,568 patients with
follicular lymphoma stage I or II diagnosed between 1973 and 2004 was
based on SEER data. Compared to the no RT group, patients who received
RT had higher rates of disease-specific survival (DSS) at 5 (81 % vs.
90%), 10 (66 % vs. 79%), 15 (57 % vs. 68%), and 20 (51 % vs. 63%)
years. Overall survival was also improved for patients who received
initial RT. Relapses usually occur distant from the RT site and are
rare after 10 years (1-11 %).[32] Data demonstrates that RT involved
filed 24 Gy is indicated to obtain a curative intent, whereas low dose
schedule (2x2 Gy) shows mainly a palliative effect.[33]
An initial
strategy of observation can also be considered. A Stanford report of
stage I and II patients who received no initial therapy showed that
more than half of the 43 patients did not require any therapy at a
median of 6 years, and 85% of patients were alive at 10 years.[34]
However this was performed in a small series of patients, and W&W
must be considered in selected case to avoid the usual side effects of
radiation (e.g. sicca syndrome, thyroid malfunction, mucositis,
myeloablative suppression, bladder disorders).
Asymptomatic,
low-tumor-burden patients may be candidates for a strategy of watch and
wait. The Groupe d’Etude des Lymphomes Folliculaires (GELF) criteria
are commonly used to assess tumor burden. For high-tumor-burden FL,
GELF criteria include at least 1 of the following: 3 distinct nodal
sites, each ≥3 cm; single nodal site ≥7 cm; symptomatic splenomegaly;
organ compression or compromise; pleural effusions, ascites. Therapy is
indicated in the presence of 1 criteria of high-tumor-burden; B
symptoms or any systemic symptoms; LDH or B2M above the upper limit of
normal. In the absence of high-tumor-burden criteria, there are no
benefits on overall survival by starting immediately specific
treatment.[35] (Table 1)
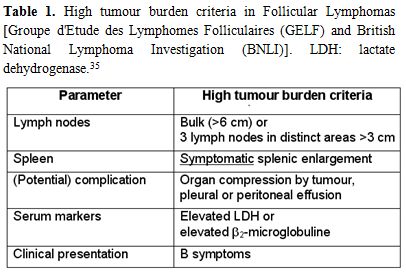 |
Table 1. High tumour burden criteria in
Follicular Lymphomas [Groupe d'Etude des Lymphomes Folliculaires (GELF)
and British National Lymphoma Investigation (BNLI)]. LDH: lactate
dehydrogenase.[35] |
The
F2-study, which compared the first-line treatment with Rituximab to the
Watch and Wait approach (W&W), did not show any differences on
freedom from treatment failure (FFTF) andoverall survival rates after
treatment in a selected prognostically favorable group. The median
studied population age was similar in two groups, 59 years (range 33-94
yrs) in W&W arm and 56 years (range 23-83 yrs) in Rituximab
receiving arm. Patients older than 60 years were respectively 46% and
39%.[36] Certainly, for elderly patients with a reduced life
expectancy, a W&W strategy is most appropriate in a
low-tumor-burden setting, as therapy is unlikely to alter the life
expectancy and could have detrimental effects on quality of life.
A
systemic more aggressive therapy is indicated for advanced stage FL
with high-tumor-burden or adverse prognostic features. At present,
advanced stage FL is still considered incurable, even if the discovery
and introduction of Rituximab as standard therapy in FL has
dramatically improved overall survival (OR) and progression-free
survival (PFS).[37-38] The optimal chemotherapy to associate with
Rituximab remains unsettled, and in clinical decisions, age,
comorbidities, and patients willingness have to be considered. The most
common associations were R-CHOP (rituximab, cyclophosphamide,
doxorubicin, vincristine, and prednisone), R-CVP (rituximab,
cyclophosphamide, vincristine, and prednisone), and R-fludarabine, even
if some of these options are not advisable in elderly patients for
their severe hematological toxicity. A randomized comparison of these
regimens indicated R-CHOP has the best risk-benefit profile, as it is
more active than R-CVP and less toxic than
Rituximab-fludarabine-mitoxantrone.[39]
In the last 20 years, the
re-discovery of Bendamustine has opened a new scenario in Indolent
Lymphoma treatment regimens. A phase 3 trial from the Study group
Indolent Lymphoma (StiL)[40] randomized 549 patients with
high-tumor-burden indolent NHL and mantle cell lymphoma (median age 64
years) to receive bendamustine 90 mg/m2 on days 1 and 2, with rituximab 375 mg/m2
on day 1, every 28 days (the BR group) or to receive standard R-CHOP
chemotherapy every 21 days. The overall response rates (ORRs) were
similar in the two groups (92.7% vs. 91.3%, respectively), but the
complete response (CR) was significantly higher in the BR group (39.8%)
compared with the R-CHOP group (30.0%). Evaluating just the FL
patients, with a median follow-up of 45 months, the median PFS was
significantly longer after BR compared with R-CHOP (not reached vs.
40.9 months). OS did not differ. There was less hematologic toxicity,
alopecia, infections, peripheral neuropathy, and stomatitis with BR.[40]
The
successful results of Bendamustine in FL were also confirmed in a
randomized, phase 3 trial (Bright) which enrolled 447 patients with
untreated indolent NHL and mantle cell lymphoma (MCL) to received
Rituximab-Bendamustine (BR) or standard therapy R-CHOP/R-CVP. 70% of
study’s population were FL with a median age of 60 years in BR group
and 58 years in R-CHOP/R-CVP group. The authors demonstrated the no
inferiority of BR to standard treatments, with ORR of 97% (CR in 31%)
vs. 91% (CR 25%) respectively. The toxicity pattern was different,
showing a higher incidence of nausea, vomiting and skin reactions in BR
arm, but rarely severe events (3%). Even if GCSF was used mainly in
R-CHOP, this group reported the higher number of cases of 3-4 grade
neutropenia.[41]
Another possible choice of treatment in FL is
Radioimmunotherapy, using an anti-CD20 antibody conjugated with a
radionuclide, 90Y-ibritumomab tiuxetan (Zevalin). It is recommended in
consolidation therapy, but it has also been evaluated in the first-line
treatment of advanced stage FL. In a phase II trial Zevalin was
administrated 8 days after a single dose of Rituximab (at 250 mg/mg).
50 patients were enrolled, and 25 of them had more than 60 years.
Objective response was in 94% of patients, with 86% of CR. Progression
or relapsed was reported in 34%, and 11% died for progression. At a
median follow-up of 38.8 months, median PFS and OS were not reached.
Three years PFS and OS were respectively 63% and 90%. Grade 3-4
myelosuppression was limited, with 30% of neutropenia and 26% of
thrombocytopenia. The study showed good efficacy and safety of single
dose of Zevalin in untreated patients, even in the elderly
population.[42]
Recommendations of the Authors: In limited stage,
FL radiotherapy alone is the preferred choice. In elderly patients with
advanced stage, low tumor burden FL the watch and wait approach is the
most appropriate strategy. Treatment is a need in high tumor burden
symptomatic FL. The introduction of Rituximab improved OS and PFS, but
the optimal chemotherapy to associate remains unsettled, above all in
elderly patients, for whom age, comorbidities, and frailty should be
considered for clinical decision. R-Bendamustine may be regarded as the
first choice, but also CHOP/CVP/FND are suitable alternatives, also in
elderly patients.
Maintenance/Consolidation Therapy
After first line
therapy, the majority of patients achieve complete remission of the
disease, however, most patients relapse. On this basis, many different
strategies were studied to delay the relapse and to ameliorate the
outcome of these patients, such as maintenance or consolidation
treatment.
Rituximab maintenance for 2 years improves PFS (75%
versus 58% after 3 years, p<0.0001), whereas a shorter maintenance
period results in an inferior benefit.[43-44]
As consolidation
strategy, radioimmunotherapy with Zevalin demonstrated to prolong PFS
after chemotherapy. However, the advantage after rituximab-containing
regimens has been not fully evaluated. This option would remain a valid
alternative in patients not eligible for high-dose chemotherapy and
autologous stem cell transplantation (ASCT) even if its benefit seemed
to be inferior in comparison to Rituximab maintenance for 2 years.[45]
Indeed a Spanish randomized phase II trial compared consolidation with
a single dose of Zevalin (arm A) versus maintenance with Rituximab (arm
B) for 2 years in newly diagnosed FL responding to R-CHOP. 146 patients
were enrolled (median age 55 yrs), 124 were randomized to induction
therapy and 22 patients were excluded for neutropenia or
thrombocytopenia, patient decision and unsatisfying response (< PR).
51% received Zevalin and 49% Rituximab. After a median follow-up of 37
months 32 patients relapsed/progressed with a 36-months PFS of 64% in
Zevalin arm and 86% with Rituximab. Number of PR which increased to CR
during maintenance were 50% and 46% in arm A, and B respectively. With
Zevalin 5 and 6 cases of >3-grade thrombocytopenia and
neutropenia were respectively described, whereas only one case
of >3-grade neutropenia was reported in Rituximab group. In
conclusion, maintenance with Rituximab was superior to Zevalin, in term
of PFS and toxicity. At present, no sufficient data are available on
long-term follow-up.[46]
Focus on the Phase III Trial ML17638[47]
The
goal of treatment in elderly patients with FL is to maintain clinical
efficacy while minimizing toxicity and preserving the patient’s quality
of life. The combination of rituximab and fludarabine-based
chemotherapy (fludarabine, mitoxantrone, dexamethasone; R-FND) has been
shown to be well-tolerated and efficient also in elderly patients.[48]
Regardless of induction therapy, rituximab maintenance has been shown
to prolong the duration of response in treatment-naive patients as well
as in those with relapsed/refractory disease.[49-52] However, none of
these trials were designed specifically for elderly patients, and there
is little data on maintenance therapy in the elderly. On
these basis the phase III trial ML17638 was designed by the Fondazione
Italiana Linfomi, with the aim to evaluate the efficacy and safety of a
short rituximab maintenance regimen compared to no further treatment in
elderly patients with advanced FL who had responded to a brief
first-line treatment regimen consisting of 4 courses of R-FND chemoimmunotherapy followed by 4 weekly doses of rituximab consolidation.[47]A
total of 234 elderly patients affected by treatment-naïve FL were
enrolled. It must be noted that median age was 66 years (range 60-75)
and patients aged more than 70 years were 23%; 41% of patients had no
comorbidities according to CGA score, while 23% of them presented more
than 2 concomitant comorbidities. All patients enrolled began a
chemoimmunotherapy with 4 monthly courses of R-FND followed by 4 weekly
cycles of rituximab consolidation. Of these, 202 responders were
randomized to rituximab maintenance (Arm A) once every 2 months for a
total of 4 doses or observation (Arm B). Median age in Arms A and B
were 66 and 65 years (range: 60-75). After induction and consolidation
therapy, the ORR was 86%, with 69% CR. After a 42 month median
follow-up from diagnosis, 3y-PFS and 3y-OS were 66% (95%CI:59-72%) and
89% (95%CI: 85-93%), respectively. After randomization, 2y- PFS was 81%
for rituximab maintenance versus 69% for observation with an HR of 0.63
(95%CI:0.38-1.05, p=0.079), although not statistically significant. Age
did not appear to have any significant effect on 3-year PFS. The
subgroup of patients below 70 years had a 3-year PFS of 67% (95%CI:
59-73%), compared to 63% (95%CI: 48-75%) for those ≥70 years. There
were no differences in 2y-PFS for patients with none, one or two or
more comorbidities. (Figure 2).
These data suggested that this therapy scheme could be safely
administered to older adults and also in those with comorbidities.
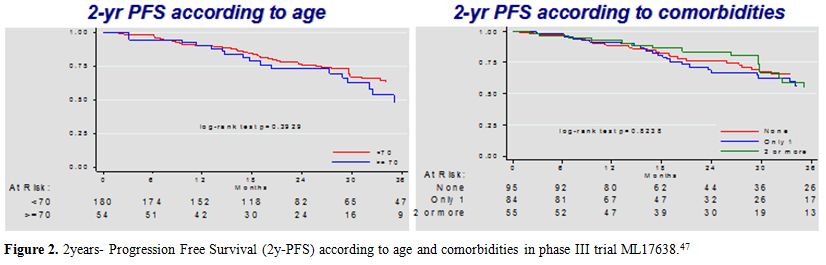 |
Figure 2. 2 years- Progression Free Survival (2y-PFS) according to age and comorbidities in phase III trial ML17638.[47] |
No differences between the two arms were detected by OS (9 deaths occurred, 5 in the maintenance and 4 in the observation arms).As
for safety profile of the treatment, the most frequent Grade 3-4
toxicity was neutropenia (25% of treatment courses), with 13
infections. Two toxic deaths (0.8%) occurred during treatment. Overall,
the regimen was well-tolerated. In the table (Table 2)
we reported the overall toxicity, treatment-related and other,
according to age and comorbidities reported as events in a total of
1119 treatment courses administered to 234 patients. The treatment was
well-tolerated, and there was presence of comorbidities, no significant
differences were found in the frequency of AEs.
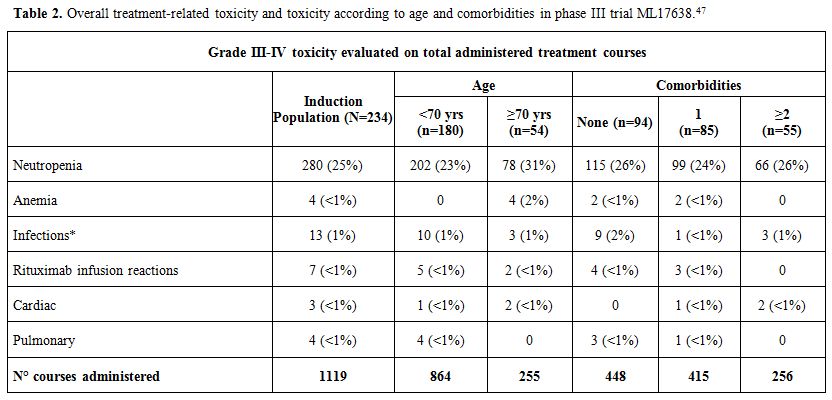 |
Table 2. Overall treatment-related toxicity and toxicity according to age and comorbidities in phase III trial ML17638.[47] |
Here
we present the results of a recent update of a prolonged follow-up of
the ML17638 trial, at 96 months from enrollment and 87 from
randomization. We collected data from 127 of 146 patients evaluable. Long-term
follow-up data confirmed the overall favorable outcome, with a 5y-PFS
of 57% and a 7y-PFS of 51%. Globally 5y-OS and 7y-OS were 85% and 80%
respectively (Figure 3).
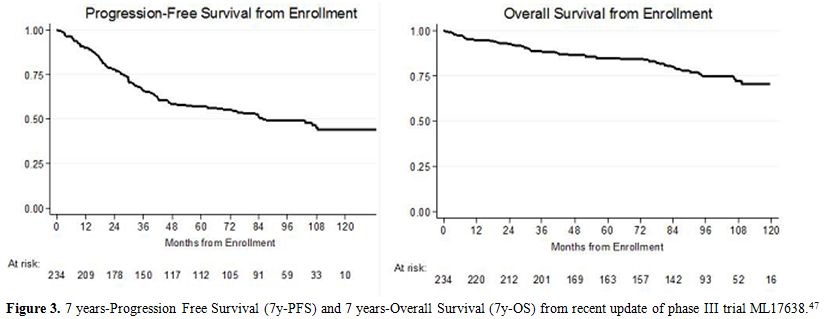 |
Figure 3. 7 years-Progression Free
Survival (7y-PFS) and 7 years-Overall Survival (7y-OS) from recent
update of phase III trial ML17638.[47] |
The
prognostic impact of FLIPI score was confirmed, with a benefit in both
PFS and OS in patients with a low-intermediate FLIPI score. The 7y-PFS
was 67% in patients with low-intermediate FLIPI vs. 38% in patients
with high FLIPI (p<0.001), moreover, 7y-OS was 86% vs. 75%
respectively in the two different prognostic groups (p=0.03). As
for maintenance treatment, no differences were shown between
maintenance and observation arms, with a 7y-PFS of 55% vs. 52%
respectively (p=0.331, HR 0.8).In
a multivariate analysis, male sex, the absence of molecular remission
and high-intermediate/high FLIPI score were confirmed as unfavorable
prognostic factors, with HR 1.91 (p=0.003), HR 1.7 (p=0.025) and HR
2.51 (p<0.0001) respectively.(Table 3)
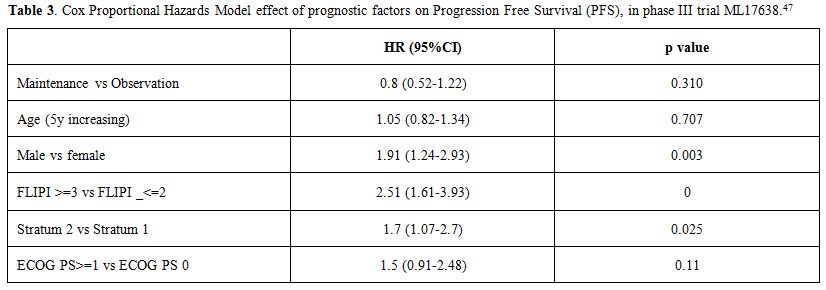 |
Table 3. Cox Proportional Hazards Model
effect of prognostic factors on Progression Free Survival (PFS), in
phase III trial ML17638.[47] |
No
differences were identified between the two arms maintenance vs.
observation in any subgroup neither in higher FLIPI score patients. Also
in this updated follow-up of the study, the achievement of a negative
PCR at the end of treatment (complete molecular remission) was
confirmed to be a favorable prognostic factor, predictive of a better
outcome, with a 7y-PFS of 58% vs 36% (p=0.084) respectively in patients
without or with minimal residual disease. (Figure 4)
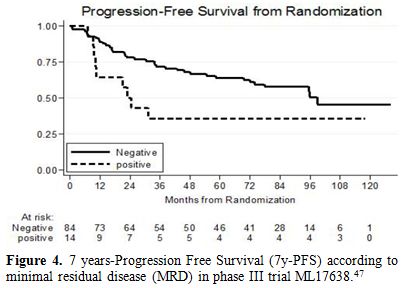 |
Figure 4. 7 years-Progression Free Survival (7y-PFS) according to minimal residual disease (MRD) in phase III trial ML17638.[47] |
No
differences between the two arms maintenance vs. observation were
observed in patients with minimal residual disease (MRD positive) at
the end of induction treatment. As
far as toxicities are concerned, 7y-follow up of ML17638 trial showed
similar toxicities in both maintenance and observation arm, for
infections, cardiac events, and secondary tumors. In particular, 13
secondary malignancies were observed in the maintenance group vs. 16 in
patients who underwent observation alone, with a cumulative incidence
of 13.9% (95% CI: 6.4 to 21.4) vs. 10.9% (95% CI: 4.4 to 17.4)
respectively.These
results underscore the importance of developing tailored therapies for
the elderly, exploring the use of brief chemoimmunotherapy regimens
beyond the age of 65.As
for maintenance treatment, the lack of statistical significance in our
findings may have different causes. First rituximab maintenance
may have a small clinical benefit, which could not be demonstrated with
the sample size of this study. However, the lack of statistically
significant difference is also confirmed at a longer follow-up.
Moreover, the maintenance strategy used in the present study was
relatively brief compared to “classical” 2-years maintenance, and this
may be the cause of the reduced efficacy. Furthermore, in our trial,
the results obtained in observation arm were better than expected, and
this may be the reason for a smaller absolute difference compared to
maintenance arm. Indeed, the lack of differences in PFS in this trial
suggests that the benefit of rituximab maintenance could be different
on the basis of induction chemotherapy administered. The PRIMA
study[43] allowed 3 different induction chemotherapy schemes
(R-CHOP, R-CVP and R-FCM (fludarabine, cyclophosphamide, mitoxantrone),
but the group of patients who received R-FCM was smaller (only 45
compared to 272 for R-CVP and 885 for R-CHOP) and was the only one
which did not seem to benefit from maintenance with rituximab. At
the same way, there are no clear data to support an advantage of
maintenance with rituximab after bendamustine-based treatment. The
MAINTAIN trial compared the results of observation only vs. 2 years vs.
4 years rituximab maintenance in patients with FL in remission after BR
induction therapy but failed to demonstrate any differences between the
different strategies.[53] In conclusion, the efficacy of rituximab
maintenance depends on the clinical contexts and induction therapy.[54]An
assessment of the prognostic value of minimal residual disease
(MRD)[20] in patients enrolled in ML17638 trial was done. MRD for the
bcl-2/IgH translocation was determined on bone marrow cells in a
centralized laboratory belonging to the Euro-MRD consortium, using
qualitative and quantitative polymerase chain reactions (PCRs). Of 234
enrolled patients, 227 (97%) were screened at diagnosis. A molecular
marker (MM) was found in 51%. Patients with an MM were monitored at 8
subsequent times. Conversion to PCR negativity predicted better
progression-free survival (PFS) at all post-treatment times (eg, end of
therapy: 3-year PFS, 72% vs 39%; P <.007). MRD was predictive in
both maintenance (83% vs 60%; P <.007) and observation (71% vs 50%;
P <.001) groups. PCR positivity at the end of induction was an
independent adverse predictor (hazard ratio, 3.1; 95% confidence
interval, 1.36-7.07). MRD is one of the most powerful independent
outcome predictor in FL patients who receive rituximab-intensive
programs, suggesting a need to investigate its value for
decision-making, also in an older population.On
the behalf of FIL, based on favorable safety and efficacy profile of
Bendamustine and on the results of the ML17638 trial, another study
(FLE09 trial) was designed to evaluate the efficacy and the safety
profile of a treatment with a combination scheme with rituximab plus
bendamustine and mitoxantrone for 4 courses, followed by a
consolidation with 4 additional doses of weekly rituximab, in elderly
FL patients, extending the upper limit of age to 80 years. Preliminary
data from this study are promising, and the publication of the final
results of the trial is ongoing. Recommendations
of the Authors: Since relapse is a common event in FL, even in patients
achieved complete remission after first-line therapy, maintenance or
consolidation therapy is needed. Maintenance
with rituximab for 2 years seems to be an effective strategy and should
also be administered in elderly patients. However, the efficacy of
rituximab maintenance depends on the clinical contexts and induction
therapy used. Second-Line Therapy and New Drugs
At
relapse of disease, it is strongly recommended to obtain a new biopsy
to exclude any transformation into an aggressive lymphoma. Targeting
the biopsy with a PET scanning may be useful.As at first presentation, observation is an accepted approach in asymptomatic patients with low tumor burden.Selection
of salvage treatment depends on the efficacy of prior regimens. In
early relapse occur (<12-24 months), a non-cross-resistant scheme
should be preferred (e.g. bendamustine after CHOP or vice versa). Other
options, including fludarabine-based, platinum salts-based or
alkylating agents-based regimens, could also be useful, but not
applicable in older or unfit patients. Rituximab
should be added if the previous anti-CD20 antibody-containing scheme
achieved >6-12-month duration of remission, while in
rituximab-refractory cases, the recently introduced new anti-CD20
antibodies of the second generation, such as obinutuzumab, demonstrated
to improve PFS in comparison to chemotherapy alone.[55]The
results of the randomized phase III GADOLIN trial that compared the
results of bendamustine alone vs obinutuzumab in association to
bendamustine in relapsed/refractory setting in indolent lymphomas have
recently been published.[55] 396 patients were enrolled: after a median
follow-up of 21.9 months, the PFS was significantly longer with
obinutuzumab plus bendamustine (median not reached [95% CI 22·5
months–not estimable]) than with bendamustine monotherapy (14·9 months
[12·8–16·6]; hazard ratio 0·55 [95% CI 0·40–0·74]; p=0·0001). Grade 3–5
adverse events occurred in 132 (68%) of 194 patients in the
obinutuzumab plus bendamustine group and in 123 (62%) of 198 patients
in the bendamustine monotherapy group. This treatment showed to be
manageable also in older patients, with acceptable safety profile.
Another study that investigated the role of obinutuzumab in association
to chemotherapy in relapsed and rituximab refractory FL is GAUDI’
trial.[56] Fifty-six patients were enrolled and were randomized to
receive obinutuzumab plus cyclophosphamide, doxorubicin, vincristine,
and prednisone (G-CHOP; every 3 weeks for 6 to 8 cycles) or
obinutuzumab plus fludarabine and cyclophosphamide (G-FC; every 4 weeks
for 4 to 6 cycles). Median age was 62.5 years (range 32-75) in G-CHOP
arm vs. 61 years (range 45-77) in G-FC group. Treatment responders were
eligible for obinutuzumab maintenance every 3 months for up to 2 years.
Grade 1/2 infusion-related reactions (IRRs) were the most common
treatment-related adverse event. Neutropenia was the most common
treatment-related hematologic toxicity. Obinutuzumab plus chemotherapy
resulted in 93% to 96% response rates, with manageable toxicity also in
older people, supporting the need for a phase-3 investigation.Also,
radioimmunotherapy may represent an effective therapeutic approach, in
particular in elderly patients with comorbidities not appropriate for
high dose chemotherapy. Pisani et al.[57] published the results of a
retrospective study that investigated the long-term efficacy and safety
of a fludarabine, cyclophosphamide and rituximab (FCR) regimen followed
by 90Y-ibritumomab tiuxetan consolidation for the treatment of nine
patients (median age 63 years, range 46–77), with grades 1 and 2
relapsed FL. After FCR, 7 patients obtained CR and 2 PR; after 90Y-RIT
2 patients in PR converted to CR 12 weeks later. With a median
follow-up of 88 months (range 13–104) since 90Y-RIT 3 deaths were not
related to lymphoma; all 3 deceased patients obtained CR before 90Y-RIT
and died still in CR. The median OS and PFS have not been reached. The
most common grade 3 or 4 adverse events were hematologic. The authors
concluded that these results confirm the long-term efficacy and safety
of 4 cycles of FCR followed by 90Y-RIT in relapsed grades 1 and 2 FL.
They suggest that this regimen could be a therapeutic option for this
setting of patients, especially at the age of 60–75, who cannot receive
high-dose chemotherapy and autologous stem cell transplant, with no
unexpected toxicities.In
further relapses, a lot of novel drugs may play a role in monotherapy
or in association to other chemotherapy. These new molecules represent
an available strategy also in older adults, who are not eligible for
high-dose chemotherapy and autologous stem cell transplant programs.
Idelalisib,
a phosphatydil-inositol-3 kinase (PI3K) inhibitor, has been registered
in double-refractory FL, based on a phase II study, showing on ORR of
54% in this setting of patients.[58] New trials with idelalisib in
association to rituximab are ongoing.Immunomodulatory
drugs, such as Lenalidomide, in monotherapy or in association to
chemotherapy or monoclonal antibody such as rituximab, demonstrated
additional inhibition of the B-cell signaling pathway and had proved
activity in phase II studies, but randomized phase III trial are needed
to confirm these data. Fowler
et al.[59] presented the results of a phase 2 trial to assess the
efficacy and safety of lenalidomide plus rituximab (R2) in patients
with untreated, advanced stage indolent non-Hodgkin lymphoma. A total
of 110 patients were enrolled, among that 50 FL (whose median age is
relatively young: 56 years, range 35-84). ORR for all patients was 90%
(95% CI 83–95), with 63% of CR (95% CI 53–72). Of 46 evaluable patients
with FL 87% achieved CR. The most common grade 3 or 4 adverse events
were neutropenia (35%).This study suggested that lenalidomide plus
rituximab is well tolerated and highly active as initial treatment for
indolent non-Hodgkin lymphoma, and it could be applied in elderly
patients not eligible for chemotherapy regimen. An international phase
3 study (RELEVANCE trial) comparing this regimen with chemotherapy in
patients with untreated follicular lymphoma is ongoing.In
relapsed/refractory setting, Leonard et al.[60] presenting the results
of a randomized phase II trial on 91 patients affected by previously
treated FL, whose median age was 63 years (range 34-89). Patients were
randomized to receive rituximab (375 mg/m2
weekly for 4 weeks), lenalidomide (15 mg per day on days 1 to 21,
followed by 7 days of rest, in cycle 1 and then 20 mg per day on days 1
to 21, followed by 7 days of rest, in cycles 2 to 12), or a combination
therapy rituximab plus lenalidomide (LR). In the lenalidomide and LR
arms, grade 3 to 4 adverse events occurred in 58% and 53% of patients.
Dose-intensity exceeded 80% in both arms. ORR was 53% (CR 20%) and 76%
(CR 39%) for lenalidomide alone and LR, respectively (p=0.029). At the
median follow-up of 2.5 years, median TTP was 1.1 year for lenalidomide
alone and 2 years for LR (p=0.0023). The combination scheme LR is more
active than lenalidomide alone in recurrent FL with similar toxicity,
manageable also in elderly patients, warranting further studies.On
behalf of FIL, a randomized phase III multicenter trial to compare a
combination of rituximab and lenalidomide vs. rituximab alone as
maintenance after R-Bendamustine in relapsed/refractory FL patients
(FIL-RENOIR12) is ongoing. There are no age limits for enrollment, and
this trial is dedicated mainly to patients over the age of 65 or with
comorbidities, who cannot be eligible for high-dose therapy and
transplant. Other combinations, such as bortezomib plus rituximab, have
shown only a minor benefit compared with antibody monotherapy.Nivolumab,
a monoclonal antibody antiPD1, showed an ORR of 40% in
relapsed/refractory FL,[61] supporting the hypothesis of the important
role of immunosurveillance in disease control. Recommendations
of the Authors: In early relapsed FL, a non-cross-resistant
chemoimmunotherapy scheme should be used. In elderly and frail
patients, novel agents (such as new monoclonal antibodies, idelalisib,
lenalidomide, and nivolumab), with a good safety profile, should be
considered. Conclusion
Follicular
lymphoma is the most common indolent non-Hodgkin lymphoma, typically
affects older adults, whose median age at diagnosis is 65 years. FL is
considered as an indolent but incurable disease with a median life
expectancy of approximately ten years. Randomized clinical trials have
demonstrated that the addition of rituximab to standard chemotherapy
induction has improved the overall survival. Moreover, maintenance
therapy with Rituximab showed improvement of progression-free survival.
Despite advances in the treatment of FL, most FL patients remain
incurable and, in 10 years, 15% to 28% of cases will transform to an
aggressive phenotype, typically diffuse large B-cell lymphoma. New
clinical and biological prognostic factors are needed, to tailor
therapy better, above all in elderly patients not eligible for
aggressive chemotherapy. Some progress were already made with novel
agents, but further studies, especially focused on elderly follicular
lymphoma patients, with their peculiar characteristics, are needed to
define the best-tailored treatment at diagnosis and at the time of
relapse in this challenging clinical setting. References
- Harris NL, Jaffe ES, Stein H, et al: A Revised
European-American Classification of Lymphoid Neoplasms: A proposal from
the International Lymphoma Study Group. Blood 1994; 84: 1361-1392.
PMid:8068936

- Shirley
MH, Sayeed S, Barnes I, Finlayson A, Ali R. Incidence of haematological
malignancies by ethnic group in England, 2001-7. Br J Haematol.
2013;163:465-77. https://doi.org/10.1111/bjh.12562 PMid:24033296

- Smith
A, Howell D, Patmore R, et al. Incidence of haematological malignancy
by sub-type: a report from the Haematological Malignancy Research
Network. Br J Cancer. 2011;105:1684-92. https://doi.org/10.1038/bjc.2011.450 PMid:22045184 PMCid:PMC3242607

- Roulland
S, Faroudi M, Mamessier E, Sungalee S, Salles G, et al.: Early steps of
follicular lymphoma pathogenesis. Adv Immunol 111:1-46, 2011. https://doi.org/10.1016/B978-0-12-385991-4.00001-5 PMid:21970951

- Swerdlow
SH, Campo E, Pileri SA, et al. The updated who classification of
hematological malignancies. The 2016 revision of the World Health
Organization classification of lymphoid neoplasms. Blood 2016, Vol 127,
n 20. https://doi.org/10.1182/blood-2016-01-643569 PMid:26980727

- Cheson
BD, Fisher RI, Barrington SF, et al. Recommendations for initial
evaluation, staging and response assessment of Hodgkin and non-Hodgkin
lymphoma - the Lugano Classification. J Clin Oncol 2014;32:3059-3068. https://doi.org/10.1200/JCO.2013.54.8800 PMid:25113753 PMCid:PMC4979083

- Cheson
BD. New staging and response criteria for non-Hodgkin lymphoma and
Hodgkin lymphoma. Radiol Clin North Am. 2008; 46(2):213-23. https://doi.org/10.1016/j.rcl.2008.03.003 PMid:18619377

- Decaudin
D, Lepage E, Brousse N, Brice P, Harousseau JL, et al.: Low-grade stage
III-IV follicular lymphoma: ultivariate analysis of prognostic factors
in 484 patients - a study of the groupe dEtude des lymphomes de
lAdulte. J Clin Oncol 17:2499-2505, 1999.
PMid:10561315

- Federico M, Vitolo U,
Zinzani PL, Chisesi T, Clò V, et al.: Prognosis of follicular lymphoma:
A predictive model based on a retrospective analysis of 987 cases.
Intergruppo Italiano Linfomi. Blood 95:783-789, 2000.
PMid:10648386

- Solal-Céligny P, Roy P,
Colombat P, White J, Armitage JO, et al.: Follicular lymphoma
international prognostic index. Blood. 104:1258-1265, 2004. https://doi.org/10.1182/blood-2003-12-4434 PMid:15126323

- Federico
M, Bellei M, Marcheselli L, Luminari S, Lopez-Guillermo A, et al.:
Follicular lymphoma international prognostic index 2: a new prognostic
index for follicular lymphoma developed by the international follicular
lymphoma prognostic factor project. J Clin Oncol 27:4555-4562, 2009. https://doi.org/10.1200/JCO.2008.21.3991 PMid:19652063

- Pastore
A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk
prognostication for patients receiving first-line immunochemotherapy
for follicular lymphoma: a retrospective analysis of a prospective
clinical trial and validation in a population-based registry. Lancet
Oncol 2015 16:1111-1122. https://doi.org/10.1016/S1470-2045(15)00169-2

- Goss PE: Non-Hodgkin's lymphomas in elderly patients. Leuk Lymphoma132, 993;(10):147-156.

- Ballester
OF, Moscinski L, Spiers A, et al: Non-Hodgkin's lymphoma in the older
person: A review. J Am Geriatr Soc 1993; (41): 1245-1254. https://doi.org/10.1111/j.1532-5415.1993.tb07310.x PMid:7693787

- Vose
JM, Armitage JO, Weisenburger DD, et al: The importance of age in
survival of patients treated with chemotherapy for aggressive
non-Hodgkins lymphoma. J Clin Oncol 1988; (6): 1838-1844.
PMid:2462026

- Extermann M1, Overcash
J, Lyman GH, et al. Comorbidity and functional status are independent
in older cancer patients. J Clin Oncol 1998(16): 1582-1587.
PMid:9552069

- Taskinen M,
Karjalainen-Lindsberg M-L, Nyman H, Eerola L-M, Leppä S: A high
tumor-associated macrophage content predicts favorable outcome in
follicular lymphoma patients treated with rituximab and
cyclophosphamide-doxorubicin-vincristine- prednisone. Clin Cancer Res
13:5784-5789, 2007. https://doi.org/10.1158/1078-0432.CCR-07-0778 PMid:17908969

- Farinha
P, Masoudi H, Skinnider BF, Shumansky K, Spinelli JJ, et al.: Analysis
of multiple biomarkers shows that lymphomaassociated macrophage (LAM)
content is an independent predictor of survival in follicular lymphoma
(FL). Blood 106:2169- 2174, 2005. https://doi.org/10.1182/blood-2005-04-1565 PMid:15933054

- Takumi
Sugimoto* and Takashi Watanabe. Follicular Lymphoma: The Role of the
Tumor microenvironment in Prognosis. J Clin Exp Hematop Vol. 56, No. 1,
June 2016.

- Ladetto M, Lobetti-Bodoni C, Mantoan B, et
al. Persistence of minimal residual disease in bone marrow predicts
outcome in follicular lymphomas treated with a rituximab-intensive
program. Blood 2013 (122)23: 3759-3766. https://doi.org/10.1182/blood-2013-06-507319 PMid:24085766

- Extermann M, et al. Studies of comprehensive geriatric assessment in patients with cancer. Cancer Control; 2003 (10): 465-468.

- Ferrucci L. et al: The frailty syndrome: a critical issue in geriatric oncology. Critb Rev Oncol Hematol. 2003; (46): 127-137.

- Katz
S. et al. Studies of illness in the age. The index of ADL: a
standardised measure of biological and psychological functions. JAMA
1963(185): 914-919. https://doi.org/10.1001/jama.1963.03060120024016 PMid:14044222

- Lawton
M.P, Brody E.M: Assessment of older people: self-maintaining and
instrumental activities of daily living. Gerontologist (1969)9:
179-186. https://doi.org/10.1093/geront/9.3_Part_1.179 PMid:5349366

- Folstein
M.F. et al: Mini Mental State: a practical method for grading the
cognitive state of patients for the clinician. J Psichiatr Res 1975;
(12): 189-198.

- Hickie C. e Snowdon J. Depression scales for the elderly: GDS. Clin gerontol 1987; (6): 51-53.

- Tucci
A , F errari S , B ottelli C , e t a l. A comprehensive geriatric
assessment is more eff ective than clinical judgment to identify
elderly diff use large cell lymphoma patients who benefi t from
aggressive therapy. Cancer 2009; 115: 4547- 4553. https://doi.org/10.1002/cncr.24490 PMid:19562776

- Tucci
A, Martelli M. Rigacci L, et al. Comprehensive geriatric assessment is
an essential tool to support treatment decisions in elderly patients
with diff use large B-cell lymphoma: a prospective multicenter
evaluation in 173 patients by the Lymphoma Italian Foundation
(FIL). Leuk Lymphoma. 2015 Apr;56(4):921-6. doi:
10.3109/10428194.2014.953142

- Fisher
RI, LeBlanc M, Press OW, et al.: New treatment options have changed the
survival of patients with follicular lymphoma. J Clin Oncol 2005;
(23):8447- 8452. https://doi.org/10.1200/JCO.2005.03.1674 PMid:16230674

- Mac
Manus MP, Hoppe RT. Is radiotherapy curative for stage I and II
low-grade follicular lymphoma? Results of a long-term follow-up study
of patients treated at Stanford University. Journal of Clinical
Oncology, 1996, 14.4: 1282-1290. PMid:8648385

- Pugh
TJ, Ballonoff A, Newman F, et al. Improved survival in patients with
early stage low-grade follicular lymphoma treated with radiation: a
Surveillance, Epidemiology, and End Results database analysis. Cancer.
2010;116:3843-3851. doi: 10.1002/cncr.25149. https://doi.org/10.1002/cncr.25149

- Hoskin
PJ, Kirkwood AA, Popova B et al. 4 Gy versus 24 Gy radiotherapy for
patients with indolent lymphoma (FORT): a randomised phase 3
non-inferiority trial. Lancet Oncol 2014; 15: 457-463. https://doi.org/10.1016/S1470-2045(14)70036-1

- Advani
R, Rosenberg SA, Horning SJ. Stage I and II follicular non-Hodgkins
lymphoma: long-term follow-up of no initial therapy. J Clin Oncol
2004;22(8):1454-1459 https://doi.org/10.1200/JCO.2004.10.086 PMid:15024027

- Brice
P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden
follicular lymphomas between an initial no-treatment policy,
prednimustine, or interferon alfa: a randomized study from the Groupe
dEtude des Lymphomes Folliculaires. Groupe dEtude des Lymphomes de
lAdulte. J Clin Oncol 1997;15(3):1110-1117
PMid:9060552

- Solal-Céligny P, Bellei
M, Marcheselli L et al. Watchful waiting in low-tumor burden follicular
lymphoma in the rituximab era: results of an F2-study database. J Clin
Oncol 2012; 30: 3848-3853. https://doi.org/10.1200/JCO.2010.33.4474 PMid:23008294

- Brad S. Kahl, David T. Yang. Follicular lymphoma: evolving therapeutic strategies. Blood 2016 127:2055-2063;. https://doi.org/10.1182/blood-2015-11-624288

- Hiddemann
W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added
to the combination of cyclophosphamide, doxorubicin, vincristine, and
prednisone (CHOP) significantly improves the outcome for patients with
advanced-stage follicular lymphoma compared with therapy with CHOP
alone: results of a prospective randomized study of the German
Low-Grade Lymphoma Study Group. Blood 2005;106(12):3725-3732. https://doi.org/10.1182/blood-2005-01-0016 PMid:16123223

- Bachy
E, Houot R, Morschhauser F, et al; Groupe dEtude des Lymphomes de
lAdulte (GELA). Long-term follow up of the FL2000 study comparing
CHVP-interferon to CHVP-interferon plus rituximab in follicular
lymphoma. Haematologica 2013;98(7):1107-111. https://doi.org/10.3324/haematol.2012.082412 PMid:23645690 PMCid:PMC3696615

- Federico
M, Luminari S, Dondi A, et al. R-CVP versus R-CHOP versus R-FM for the
initial treatment of patients with advanced-stage follicular lymphoma:
results of the FOLL05 trial conducted by the Fondazione Italiana
Linfomi [published correction appears in J Clin Oncol.
2014;32(10):1095]. J Clin Oncol 2013;31(12):1506-1513 https://doi.org/10.1200/JCO.2012.45.0866 PMid:23530110

- Rummel
MJ, Niederle N, Maschmeyer G, et al; Study group indolent Lymphomas
(StiL). Bendamustine plus rituximab versus CHOP plus rituximab as
first-line treatment for patients with indolent and mantle-cell
lymphomas: an open-label, multicentre, randomised, phase 3
non-inferiority trial. Lancet 2013;381(9873):1203-1210. https://doi.org/10.1016/S0140-6736(12)61763-2

- Flinn
IW et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in
first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood,
2014, 123.19: 2944-2952. https://doi.org/10.1182/blood-2013-11-531327 PMid:24591201 PMCid:PMC4260975

- Ibatici
A et al. Safety and efficacy of 90Yttrium-Ibritumomab-Tiuxetan for
untreated follicular lymphoma patients. An Italian cooperative study.
British journal of haematology, 2014, 164.5: 710-716. https://doi.org/10.1111/bjh.12695 PMid:24344981

- Seymour
JF, Feugier P, Offner F, et al. Updated 6 Year Follow-Up Of The PRIMA
Study Confirms The Benefit Of 2-Year Rituximab Maintenance In
Follicular Lymphoma Patients Responding To Frontline
Immunochemotherapy. Blood 2013;122: abstr. 509
- Taverna
CJ, Martinell G, Hitz F, et al. Rituximab maintenance treatment for a
maximum of 5 years in follicular lymphoma: results of the randomized
phase III trial SAKK 35/03. ASH 2013; 122: abstr. 508.
- Morschhauser
F, Radford J, Van Hoof A, et al. 90Yttrium-ibritumomab-tiuxetan
consolidation of first remission in advanced-stage follicular
non-Hodgkin lymphoma: updated results after a median follow up of 7.3
years from the international, randomized, phase III first-line indolent
trial. J Clin Oncol 2013, 31:1977-1983. https://doi.org/10.1200/JCO.2012.45.6400 PMid:23547079

- Lopez-Guillermo
A, Canales MA, Dlouhy I, et al. A randomized phase II study comparing
consolidation with a single dose of 90Y ibritumomab tiuxetan (Zevalin)
(Z) vs. maintenance with rituximab (R) for two years in patients with
newly diagnosed follicular lymphoma (FL) responding to R-CHOP.
Preliminary results at 36 months from randomization. ASH 2013; 122:
abstr. 369.
- Vitolo U, Ladetto M, Boccomini C et al:
Rituximab maintenance compared with observation after brief first-line
R-FND chemoimmunotherapy with rituximab consolidation in patients age
older than 60 years with advanced follicular lymphoma: a phase III
randomized study by the Fondazione Italiana Linfomi, J Clin Oncol 2013;
31(27):3351-9 https://doi.org/10.1200/JCO.2012.44.8290 PMid:23960180

- McLaughlin
P, Hagemeister FB, Rodriguez MA, et al.: Safety of fludarabine,
mitoxantrone, and dexamethasone combined with rituximab in the
treatment of stage IV indolent lymphoma. Semin Oncol 27:37-41, 2000.
PMid:11225999

- van Oers MH: Rituximab maintenance therapy: a step forward in follicular lymphoma. Haematologica 92:826-833, 2007. https://doi.org/10.3324/haematol.10894 PMid:17550856

- Forstpointner
R, Unterhalt M, Dreyling M, et al.: Maintenance therapy with rituximab
leads to a significant prolongation of response duration after salvage
therapy with a combination of rituximab, fludarabine, cyclophosphamide,
and mitoxantrone (R-FCM) in patients with recurring and refractory
follicular and mantle cell lymphomas: Results of a prospective
randomized study of the German Low Grade Lymphoma Study Group (GLSG).
Blood 108:4003-4008, 2006. https://doi.org/10.1182/blood-2006-04-016725 PMid:16946304

- van
Oers MH, Klasa R, Marcus RE, et al.: Rituximab maintenance improves
clinical outcome of relapsed/resistant follicular non-Hodgkin lymphoma
in patients both with and without rituximab during induction: results
of a prospective randomized phase 3 intergroup trial. Blood
108:3295-3301, 2006. https://doi.org/10.1182/blood-2006-05-021113 PMid:16873669

- Van
Oers MH, van GM, Giurgea L, et al.: Rituximab maintenance treatment of
relapsed/resistant follicular non-Hodgkin's lymphoma: long-term outcome
of the EORTC 20981 phase III randomized intergroup study. J Clin Oncol
28:2853-2858, 2010. https://doi.org/10.1200/JCO.2009.26.5827 PMid:20439641 PMCid:PMC2903319

- Rummel
MJ, Viardot A, Greil R, et al. Bendamustine Plus Rituximab Followed By
Rituximab Maintenance for Patients with Untreated Advanced Follicular
Lymphomas. Results from the StiL NHL 7-2008 Trial (MAINTAIN trial).
Blood 2014 124:3052.

- Jacobson CA and Freedman AS. One
Size Does Not Fit All in Follicular Lymphoma. Journal of Clinical
Oncology, Vol 31, No 27 (September 20), 2013: pp 3307-3308. https://doi.org/10.1200/JCO.2013.50.0454 PMid:23960176

- Sehn
LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus
bendamustine monotherapy in patients with rituximab-refractory indolent
non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label,
multicentre, phase 3 trial. Lancet Oncol. 2016 Jun 23.

- John
Radford, Andrew Davies, Guillaume Cartron, et al. Obinutuzumab
(GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma:
results of the GAUDI study BO21000). Blood. 2013 Aug 15;122(7):1137-43. doi: 10.1182/blood-2013-01-481341

- Francesco Pisani, Rosa Sciuto, Maria Laura
Dessanti. Long term efficacy and safety of Fludarabine,
Cyclophosphamide and Rituximab regimen followed by 90Y-ibritumomab
tiuxetan consolidation for the treatment of relapsed grades 1 and 2
follicular lymphoma. Experimental Hematology & Oncology (2015) 4:17
https://doi.org/10.1186/s40164-015-0012-3 PMid:26120498 PMCid:PMC4482187

- Gopal
AK, Kahl BS, de Vos S, et al. PI3Kd inhibition by idelalisib in
patients with relapsed indolent lymphoma. N Engl J Med 2014; 370:
1008-1018. https://doi.org/10.1056/NEJMoa1314583 PMid:24450858 PMCid:PMC4039496

- Nathan
H Fowler, R Eric Davis, Seema Rawal . Safety and activity of
lenalidomide and rituximab in untreated indolent lymphoma: an
open-label, phase 2 trial. Lancet Oncol 2014; 15: 1311-18. https://doi.org/10.1016/S1470-2045(14)70455-3

- John
P. Leonard, Sin-Ho Jung, Jeffrey Johnson, et al. Randomized Trial of
Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With
Recurrent Follicular Lymphoma: CALGB 50401 (Alliance). J Clin Oncol
2015; 33:3635-3640. https://doi.org/10.1200/JCO.2014.59.9258 PMid:26304886 PMCid:PMC4622102

- Lesokhin
AM, Ansell SM, Armand P, et al. Preliminary results of a phase I study
of nivolumab in patients with relapsed or refractory lymphoid
malignancies. ASH 2014, abs 291. PMid:26827906

[TOP]


































































