Giuseppe Gritti1, Chiara Pavoni1 and Alessandro Rambaldi1,2
1 Hematology and Bone Marrow Transplant Unit, Ospedale Papa Giovanni XXIII, Bergamo, Italy
2 Department of Oncology and Oncohematology, Università degli Studi di Milano, Milano, Italy
Corresponding
author: Prof. Alessandro Rambaldi, MD. Università degli Studi di
Milano, Hematology and Bone Marrow Transplant Unit, Ospedale Papa
Giovanni XXIII, Piazza OMS, 1, 24127 Bergamo, Italy. Tel.:
+39-0352673684, Fax: +39-0352674968. E-mail:
arambaldi@asst-pg23.it
Published: January 1, 2017
Received: November 11, 2016
Accepted: November 24, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017010 DOI
10.4084/MJHID.2017.010
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
After
25 years, evaluation of minimal residual disease (MRD) in follicular
lymphoma (FL) has become a standardized technique frequently integrated
into clinical trials for its consistent and independent prognostic
significance. Achievement of a sustained MRD negativity is a marker of
treatment sensibility that has been associated with excellent clinical
outcome in terms of clinical response and progression-free survival,
independently from the employed therapy. However, no survival
advantages have been reported for MRD negative patients and despite the
compelling results of clinical trials, MRD evaluation has currently no
role in clinical practice. Ongoing clinical trials will help in
clarifying the potential setting in which MRD monitoring may have a
routine clinical application i.e. allowing de-escalation of standard
maintenance therapy in very low risk patients. In this review the
clinical implications of MRD monitoring in Rituximab-era are discussed
in light of the current treatment paradigms most aimed at reducing
toxicities, and the response definition that now routinely integrates
PET scan.
|
Introduction
Follicular
lymphoma (FL) is the most common indolent non-Hodgkin lymphoma (NHL),
accounting for 20-30% of all NHL in Western countries.[1]
It is characterized by a chronic course, with a projected survival of
more than 18 years in the modern chemo-immunotherapy era.[2]
While some patients with limited stage disease may be cured, those
presenting with advance stage or relapsing after local radiotherapy are
generally considered not curable with standard treatments.[1]
Early studies have shown that deferring treatment in asymptomatic
patients with low tumor burden is not associated to a worse survival
and, in many cases, the disease can remain stable for several years.[3,4] The usefulness of watchful waiting has been later on confirmed in the Rituximab era.[5]
Thus only patients bearing a high tumor burden disease and/or
symptomatic are currently treated with chemo-immunotherapy. Standard,
first line treatment includes the use of Rituximab plus chemotherapy
with an expected overall response rates of more than 90% and complete
remissions in the range of 25–70% with median progression-free survival
(PFS) exceeding 4 years.[6] A two years maintenance
with Rituximab in responders results in significant prolongation of
PFS, but not overall survival (OS).[7,8] Therefore,
despite the excellent improvement gained by chemo-immunotherapy, the
majority of the patients eventually progress or relapse.
Several
factors have been identified as of key importance in predicting PFS and
OS, among those the quality of first line response have been shown to
be remarkably associated to survival outcomes.[9]
Traditionally, response evaluation in FL has been made with the use of
contrast enhanced computed tomography (CT)-scan and bone marrow biopsy
(BMB) along with standard laboratory tests and clinical parameters.[10,11]
Immunohistochemical staining of BMB is the standard technique to assess
lymphoma infiltration, but more sensitive assays have been developed to
detect subclinical involvement. The presence of a hybrid BCL2/IGH gene
in 80-90% of FL has spurred the interest in applying polymerase chain
reaction (PCR) techniques to test the bone marrow (BM) and peripheral
blood (PB) of patients before and after treatment.[12,13]
In the current review we will discuss the methodological aspects of
molecular monitoring and its clinical significance in the modern
chemo-immunotherapy era.
Technical Aspects
The
genetic hallmark of FL is the t(14;18)(q32;q21) translocation that
leads to deregulated expression of the anti-apoptotic gene BCL2 in
tumor cells, thus allowing for the acquisition of secondary chromosomal
alterations in the germinal center environment, where the most
non-neoplastic B cells undergo apoptosis.[14] The
resulting hybrid gene BCL2/IGH is highly attractive for PCR based
assays as it is a disease specific clonal sequence directly linked to
FL pathogenesis and thus represents a highly stable marker. Five
different clusters of BCL2/IGH rearrangements occur: the major
breakpoint region (MBR), the minor clustering region (mcr), the
intermediate cluster region (ICR), the 3′-BCL-2 region and the 5′-mcr
region (Figure 1).[15]
To date, the molecular detection of minimal residual disease (MRD) has
been almost entirely based only on the study of MBR and mcr which
account for about 50 and 10% of all BCL2 rearrangements, respectively.[15]
Qualitative (nested) PCR (nPCR) has been widely used in molecular
testing FL patients and proved as a highly reproducible method with an
excellent sensitivity level able to detect 1 neoplastic cell in about a
hundred thousand normal cells (1x10-5).[16]
The advent of TaqMan-based approaches allowed the introduction of
quantitative PCR methods i.e. real-time quantitative PCR (RQ-PCR), a
significant step forward from the mere presence or absence of a
BCL2/IGH rearrangement.[17,18] This latter technique
made possible the quantification of BCL2/IGH tumor burden at diagnosis
and the dynamic of its reduction with treatment with lower risk of
contamination and higher inter-laboratory reproducibility. Conversely,
RQ-PCR has lower sensitivity than nPCR, probably as less amount of DNA
is tested, and it is more expensive and technically complex needing the
construction of standard reference curves.[19]
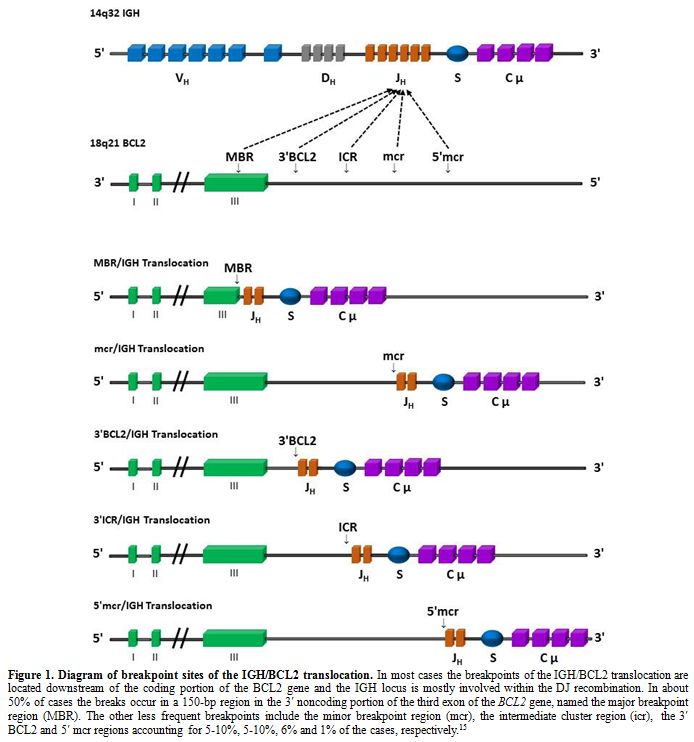 |
Figure 1. Diagram of
breakpoint sites of the IGH/BCL2 translocation. In most cases the
breakpoints of the IGH/BCL2 translocation are located downstream of the
coding portion of the BCL2 gene and the IGH locus is mostly involved
within the DJ recombination. In about 50% of cases the breaks occur in
a 150-bp region in the 3′ noncoding portion of the third exon of the
BCL2 gene, named the major breakpoint region (MBR). The other less
frequent breakpoints include the minor breakpoint region (mcr), the
intermediate cluster region (icr), the 3′ BCL2 and 5′ mcr regions
accounting for 5-10%, 5-10%, 6% and 1% of the cases, respectively.[15] |
The occurrence of
non-neoplastic BCL2/IGH rearrangements in the peripheral blood of
healthy donors or patients without lymphoma was regarded as a possible
confounder factor for MRD studies.[20,21] The low
chimeric gene levels found in non-FL patients and its clearance after
chemotherapy, however, confirmed the feasibility of MRD testing in this
setting.[22] Another key point for the diffusion of
MRD assessment is standardization of methodologies and definitions of
common MRD terms. Standardization of RQ-PCR, including data
interpretation and reporting, has been made by the efforts of the
European network project EURO-MRD and has been applied in clinical
trials.[19,23-26] New technical
approaches could in the near future improve the frequency and the
feasibility of BCL2/IGH rearrangements identification, as next
generation sequencing (NGS) or droplet digital PCR.[27,28] Clinical Implication of Minimal Residual Disease Monitoring
Twenty-five
years have passed since the first observations that MRD negativity
plays a role in predicting the outcome of patients with FL.[13]
Early studies showed that standard chemotherapy programs can achieve a
molecular remission (MR) in a minority of patients. First line
anthracycline containing protocols could attain a MR in about 30-50% of
the patients,[29,30] while intensification with autologous stem-cell transplant (SCT) can lead up to 60-70% of MRD negativity.[31] Conversely, the proportion was negligible in those with relapsed disease.[32]
In all of these studies, patients achieving a MR were characterized by
a significantly prolonged disease control. Notably, long term results
of two trials aiming at reducing neoplastic cell contamination before
ASCT with ex vivo purging, confirmed that persistence of residual
marrow involvement both at microscopic or molecular assessment were the
only significant factors for long term remission, but not for survival.[33]
The
association of Rituximab with chemotherapy dramatically improved
response rates, PFS and, most notably, OS of advanced FL patients
requiring treatment.[34,35] The ability of Rituximab
to deplete FL neoplastic cells from peripheral blood and bone marrow,
increased the rates of MR, accordingly. A clear demonstration of the
Rituximab activity on MRD was shown in a study in which only responsive
patients after CHOP therapy not achieving a MR were treated with 4
weekly infusion of Rituximab.[30] Overall, sequential
administration of CHOP followed by Rituximab resulted in complete BM
and PB molecular response in more than 70% of patients. Freedom from
recurrence at 3 years was 52-57% for those patients obtaining a durable
MR after CHOP or CHOP plus Rituximab, while it was significantly lower
(20%) for those failing to obtain or lost a MR. Residual disease
kinetic showed that most of the patients in MR after CHOP were negative
at the first interim evaluation after 3 cycles. Conversely, a delayed
maximum effect was noted after Rituximab, with 59%, 74% and 63% of the
patients in MR at week +12, +28 and +44 after treatment. This late
effect of Rituximab was observed in other trials, as well as the more
difficult clearance of MRD in the BM compared with the PB.[23,24]
Rituximab have been used as consolidation therapy after autologous SCT
in small series of patients, and proved to be safe and effective both
in increasing the quality of clinical response i.e. converting PR into
CR, as well as in achieving MR.[36,37]
The
efficacy of front line Rituximab plus chemotherapy in inducing MR have
been included as secondary end point in several large prospective
trials (Table 1).
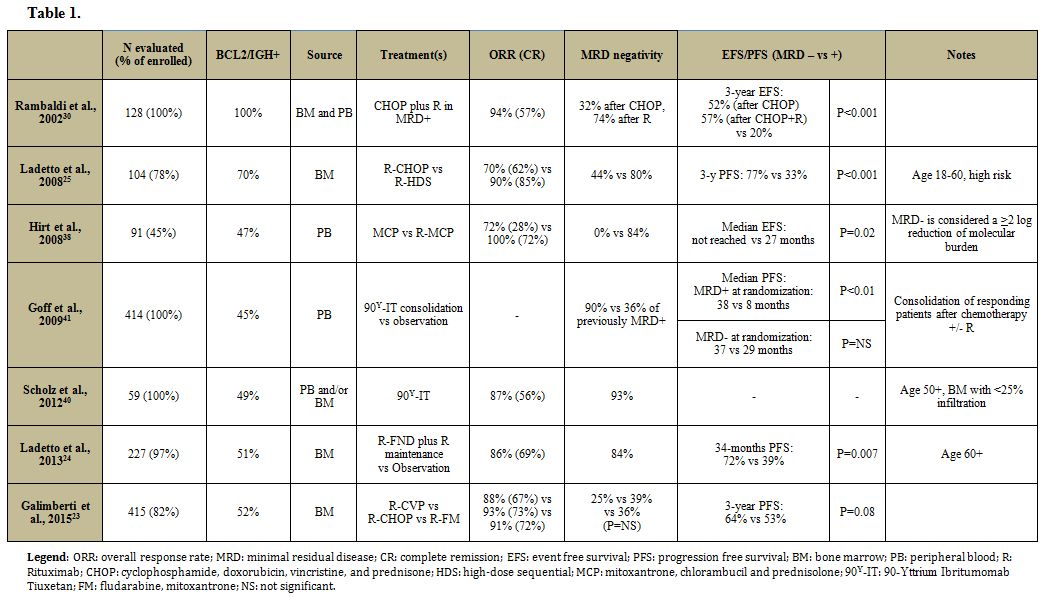 |
Table 1 |
In two different controlled studies, R-CHOP resulted in MR of 39-44%.[23,25]
Similar results were obtained with fludarabine and antracycline-based
induction regimens (R-FM, R-FND) and with mitoxantrone, chlorambucil,
and prednisolone (R-MCP).[23,24,38]
Indirect evidence suggests that the less intensive regimen R-CVP could
lead to inferior results both in term of clinical response and MR
rates, explaining the shorter PFS observed compared to R-CHOP/R-FM.[23]
Conversely, intensive regimens including upfront autologous SCT (R-HDS)
increase the molecular response rates up to 80% of patients.[25]
No data are currently available for the schema R-Bendamustine in the
front line setting, but Bendamustine alone or combined with the novel
anti-CD20 monoclonal antibody Obinutuzumab in relapsed/refractory
patients can induce MR.[39] Induction therapy with 90Y-Ibritumomab-Tiuxetan was associated with achievement of a MR in nearly all of the CR patients.[40]
Most importantly, all these trials confirmed the significant
improvement in disease control in terms of PFS or relapse/event free
survival in those patients achieving and maintaining the MR. The result
was independent from other prognostic factors, most importantly the
quality of response (CR vs PR), the chemotherapy induction regimen
chosen and clinical risk factors as the FL prognostic index (FLIPI).
However, the good results achieved in terms of disease control, did not
translate in survival improvement. The importance of MR was shown also
for a non-chemotherapy based consolidation. In a phase III trial
90Y-Ibritumomab-Tiuxetan was randomly given as consolidation therapy
after standard first line therapy.[41] Interestingly,
when compared to controls, consolidation with 90Y-Ibritumomab-Tiuxetan
did not improve the PFS of patients who already were in MR while a
significant prolongation of PFS was obtained in MRD positive patients
(38.4 vs 8.2 months of the control group, P<0.01). In the
relapsed/refractory setting, a randomized phase III study comparing
CHOP vs R-CHOP therapy with subsequent Rituximab maintenance vs
observation further confirmed the predictive value of MR, as almost all
of the few patients who were still BCL2/IgH PCR positive at the end of
the 2 years of maintenance treatment relapsed rapidly.[42]
Quantitative
PCR methods have been used to measure the BCL2/IGH chimeric gene burden
and early studies suggested that RQ-PCR evaluation before and after
autologous transplantation may predict the clinical course of these
patients.[43,44] In a clinical trial evaluating the
sequential administration of CHOP and Rituximab in MRD positive
patients, a high lymphoma cell burden at diagnosis was associated with
lower probability to achieve a clinical and molecular CR.[45]
The kinetic of BCL2/IGH positive cells during treatment showed that
CHOP and Rituximab were both able to remove approximately 2 logs of
tumor infiltration, thus explaining why patients with a limited
lymphoma infiltration can achieve a molecular remission after CHOP
chemotherapy alone, while the others necessitate the addition of
Rituximab. Quantification of BCL2/IGH chimeric gene burden in the BM,
but not in PB, was associated to a better event free survival. The
result of the MRD analysis of a large phase III study confirmed the
prognostic value of the molecular tumor burden both in term of
likelihood to achieve a CR, and PFS.[23] Of note,
high lymphoma cell burden at diagnosis was independent from FLIPI and
clinical response in determining PFS. The importance of RQ-PCR was
additionally shown in a study in which significant reduction (>2
logs) of circulating lymphoma cells rather than the mere MRD negativity
was associated with a favorable clinical response and prolonged
event-free survival.[38] However, not all the studies confirm these findings, probably due to the different induction regimen and Rituximab schedule.[24]
Current Issues and Future Perspective
Compelling
evidence indicates that MRD is a post treatment independent prognostic
factor that can be consistently used to guide subsequent consolidation
therapy in clinical trials. However a series of issues should be taken
into consideration. Firstly, in large randomized prospective trials
where molecular evaluation has been performed routinely, a molecular
marker could be detected in only 50 to 60% of the patients.[23-25]
In a large study including samples from 415 patients, a molecular
marker was present in 53% of the cases; in particular, 67.5% of
patients without BM infiltration were MRD positive, conversely 17.6% of
patients with microscopic marrow involvement at BMB were lacking the
molecular marker.[23] Several reasons could explain
this finding, mainly the presence of uncommon rearrangements and the
lack of significant marrow involvement in patients with nodal disease.[15]
Despite the availability of primers and probes for detecting rare
BCL2/IGH breakpoints will increase the cases with molecular marker, a
significant proportion of patients are eventually excluded from this
strategy. Persistence over time of MR is a major indication of
sustained remission. As MRD detection is more informative on BM,
especially in the Rituximab era, the need of multiple invasive
procedures additionally limits the feasibility of MRD monitoring over
time. Moreover, all the clinical trials reporting a prognostic
implication of MR included the evaluation of response according to the
1999 or 2007 International Working Group (IWG) criteria with the use of
the sole contrast enhanced CT-scan.[10,11] The
introduction of FDG-PET scan improved the accuracy of staging and
response assessment in FDG-avid lymphomas and is currently recommended
for the definition of response in FL.[46] Several
trials showed that concordance in CT-based and PET-based response
designation is critical especially for patients in PR or CR
unconfirmed, as PET scan is able to identify those patients with
metabolically active disease and thus can improve the predictive value
of response assessment.[47-49] To date, no data is
available regarding the integration of PET based response and MRD
evaluation. The only report in this setting is a retrospective
evaluation of a very limited proportion of patient (8%) enrolled in a
prospective trial.[50] This study suggests that PET
and MRD are not strongly correlated with each other, and thus could be
used as complementary techniques at the end of therapy to optimally
explore the nodal and bone marrow compartments, but further studies are
necessary to confirm the independent role of the two techniques.
The
current clinical significance of MRD evaluation should also be
evaluated when considering the evolving scenario of FL treatment. To
date, given the satisfactory median results of chemo-immunotherapy and
the lack of a survival impact of the chemotherapies available, the
routine selection of induction treatment is guided more from the
avoidance of unnecessary toxicity rather than the mere activity of the
regimen.[1] However, while most patients achieve
a prolonged disease control, a sizeable subset of cases remains
substantially refractory to front line treatment with a poor prognosis.[9]
Clinical scores currently available as FLIPI or FLIPI2 fail in
identifying such cases, and a growing numbers of prognostic factors
before or after treatment have been developed with this aim.[9,51-53]
Thus, definition of high risk patient and, accordingly, end points for
clinical trials are changing. Treatment results are satisfactory in low
risk patients and integration of new molecules should be made with
great caution in this group.[54] In this regard,
achievement of sustained MR could allow the de-escalation of standard
therapy in very low risk patients i.e. maintenance with Rituximab.
Conversely, high risk patients are a group for which standard treatment
need to be implemented and PFS should not represent per se the primary
end point. Efforts to consistently characterize this latter group are
ongoing and surrogate end points for survival as 2-year PFS have been
proposed.[9,51-53]
Conclusion
Although not yet integrated in clinical practice as compared to other setting such acute lymphoblastic leukemia,[55]
MRD evaluation is commonly integrated in clinical trials testing the
efficacy of new treatment protocols in FL patients. In this setting MRD
maintains its consistent and independent prognostic significance.
Achievement of MR is a marker of treatment sensibility that has been
associated with good clinical outcome in term of PFS, but not OS,
independently from the specific therapy. Some technical limitations
such as the limited coverage of the different breakpoints present in
the BCL2/IgH rearrangements will be likely overcome in the near future
by more appropriate molecular approaches.[27,28]
These laboratory improvements, most likely in combination with the new
imaging technologies currently tested by an ongoing clinical trial
(NCT02063685), will probably lead to a reappraisal of MRD evaluation in
FL patients
References
- Kahl BS, Yang DT. Follicular lymphoma: evolving therapeutic strategies. Blood. 2016;127:2055-2063. https://doi.org/10.1182/blood-2015-11-624288 PMid:26989204

- Tan D, Horning SJ, Hoppe RT, et al.
Improvements in observed and relative survival in follicular grade 1-2
lymphoma during 4 decades: the Stanford University experience. Blood.
2013;122:981-987. https://doi.org/10.1182/blood-2013-03-491514 PMid:23777769 PMCid:PMC3739040

- Brice
P, Bastion Y, Lepage E, et al. Comparison in low-tumor-burden
follicular lymphomas between an initial no-treatment policy,
prednimustine, or interferon alfa: a randomized study from the Groupe
d'Etude des Lymphomes Folliculaires. Groupe d'Etude des Lymphomes de
l'Adulte. J Clin Oncol. 1997;15:1110-1117. PMid:9060552

- Ardeshna
KM, Smith P, Norton A, et al. Long-term effect of a watch and wait
policy versus immediate systemic treatment for asymptomatic
advanced-stage non-Hodgkin lymphoma: a randomised controlled trial.
Lancet. 2003;362:516-522. https://doi.org/10.1016/S0140-6736(03)14110-4

- Solal-Celigny
P, Bellei M, Marcheselli L, et al. Watchful waiting in low-tumor burden
follicular lymphoma in the rituximab era: results of an F2-study
database. J Clin Oncol. 2012;30:3848-3853. https://doi.org/10.1200/JCO.2010.33.4474 PMid:23008294

- Hiddemann W, Cheson BD. How we manage follicular lymphoma. Leukemia. 2014;28:1388-1395. https://doi.org/10.1038/leu.2014.91 PMid:24577532

- Salles
G, Seymour JF, Offner F, et al. Rituximab maintenance for 2 years in
patients with high tumour burden follicular lymphoma responding to
rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled
trial. Lancet. 2011;377:42-51. https://doi.org/10.1016/S0140-6736(10)62175-7

- Barta
SK, Li H, Hochster HS, et al. Randomized phase 3 study in low-grade
lymphoma comparing maintenance anti-CD20 antibody with observation
after induction therapy: A trial of the ECOG-ACRIN Cancer Research
Group (E1496). Cancer. 2016;122:2996-3004. https://doi.org/10.1002/cncr.30137 PMid:27351685

- Tarella
C, Gueli A, Delaini F, et al. Rate of primary refractory disease in B
and T-cell non-Hodgkin's lymphoma: correlation with long-term survival.
PLoS One. 2014;9:e106745. https://doi.org/10.1371/journal.pone.0106745 PMid:25255081 PMCid:PMC4177839

- Cheson
BD, Horning SJ, Coiffier B, et al. Report of an international workshop
to standardize response criteria for non-Hodgkin's lymphomas. NCI
Sponsored International Working Group. J Clin Oncol. 1999;17:1244.
PMid:10561185

- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579-586. https://doi.org/10.1200/JCO.2006.09.2403 PMid:17242396

- Vaandrager
JW, Schuuring E, Raap T, Philippo K, Kleiverda K, Kluin P. Interphase
FISH detection of BCL2 rearrangement in follicular lymphoma using
breakpoint-flanking probes. Genes Chromosomes Cancer. 2000;27:85-94. https://doi.org/10.1002/(SICI)1098-2264(200001)27:1<85::AID-GCC11>3.0.CO;2-9

- Gribben
JG, Freedman AS, Neuberg D, et al. Immunologic purging of marrow
assessed by PCR before autologous bone marrow transplantation for
B-cell lymphoma. N Engl J Med. 1991;325:1525-1533. https://doi.org/10.1056/NEJM199111283252201 PMid:1944436

- Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol. 2015;15:172-184. https://doi.org/10.1038/nri3814 PMid:25712152

- Weinberg
OK, Ai WZ, Mariappan MR, Shum C, Levy R, Arber DA. ''Minor'' BCL2
breakpoints in follicular lymphoma: frequency and correlation with
grade and disease presentation in 236 cases. J Mol Diagn.
2007;9:530-537. https://doi.org/10.2353/jmoldx.2007.070038 PMid:17652637 PMCid:PMC1975105

- Weiss
LM, Warnke RA, Sklar J, Cleary ML. Molecular analysis of the t(14;18)
chromosomal translocation in malignant lymphomas. N Engl J Med.
1987;317:1185-1189. https://doi.org/10.1056/NEJM198711053171904 PMid:3657890

- Holland
PM, Abramson RD, Watson R, Gelfand DH. Detection of specific polymerase
chain reaction product by utilizing the 5'----3' exonuclease activity
of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci U S A.
1991;88:7276-7280. https://doi.org/10.1073/pnas.88.16.7276 PMid:1871133 PMCid:PMC52277

- Donovan
JW, Ladetto M, Zou G, et al. Immunoglobulin heavy-chain consensus
probes for real-time PCR quantification of residual disease in acute
lymphoblastic leukemia. Blood. 2000;95:2651-2658. PMid:10753847

- Bruggemann
M, Schrauder A, Raff T, et al. Standardized MRD quantification in
European ALL trials: proceedings of the Second International Symposium
on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia.
2010;24:521-535. https://doi.org/10.1038/leu.2009.268 PMid:20033054

- Summers
KE, Goff LK, Wilson AG, Gupta RK, Lister TA, Fitzgibbon J. Frequency of
the Bcl-2/IgH rearrangement in normal individuals: implications for the
monitoring of disease in patients with follicular lymphoma. J Clin
Oncol. 2001;19:420-424. PMid:11208834

- Dolken
G, Dolken L, Hirt C, Fusch C, Rabkin CS, Schuler F. Age-dependent
prevalence and frequency of circulating t(14;18)-positive cells in the
peripheral blood of healthy individuals. J Natl Cancer Inst Monogr.
2008:44-47. https://doi.org/10.1093/jncimonographs/lgn005 PMid:18648002

- Ladetto
M, Drandi D, Compagno M, et al. PCR-detectable nonneoplastic Bcl-2/IgH
rearrangements are common in normal subjects and cancer patients at
diagnosis but rare in subjects treated with chemotherapy. J Clin Oncol.
2003;21:1398-1403. https://doi.org/10.1200/JCO.2003.07.070 PMid:12663733

- Galimberti
S, Luminari S, Ciabatti E, et al. Minimal residual disease after
conventional treatment significantly impacts on progression-free
survival of patients with follicular lymphoma: the FIL FOLL05 trial.
Clin Cancer Res. 2014;20:6398-6405. https://doi.org/10.1158/1078-0432.CCR-14-0407 PMid:25316810

- Ladetto
M, Lobetti-Bodoni C, Mantoan B, et al. Persistence of minimal residual
disease in bone marrow predicts outcome in follicular lymphomas treated
with a rituximab-intensive program. Blood. 2013;122:3759-3766. https://doi.org/10.1182/blood-2013-06-507319 PMid:24085766

- Ladetto
M, De Marco F, Benedetti F, et al. Prospective, multicenter randomized
GITMO/IIL trial comparing intensive (R-HDS) versus conventional
(CHOP-R) chemoimmunotherapy in high-risk follicular lymphoma at
diagnosis: the superior disease control of R-HDS does not translate
into an overall survival advantage. Blood. 2008;111:4004-4013. https://doi.org/10.1182/blood-2007-10-116749 PMid:18239086

- van
der Velden VH, Cazzaniga G, Schrauder A, et al. Analysis of minimal
residual disease by Ig/TCR gene rearrangements: guidelines for
interpretation of real-time quantitative PCR data. Leukemia.
2007;21:604-611. https://doi.org/10.1038/sj.leu.2404586

- Drandi
D, Kubiczkova-Besse L, Ferrero S, et al. Minimal Residual Disease
Detection by Droplet Digital PCR in Multiple Myeloma, Mantle Cell
Lymphoma, and Follicular Lymphoma: A Comparison with Real-Time PCR. J
Mol Diagn. 2015;17:652-660. https://doi.org/10.1016/j.jmoldx.2015.05.007 PMid:26319783

- Kotrova
M, Muzikova K, Mejstrikova E, et al. The predictive strength of
next-generation sequencing MRD detection for relapse compared with
current methods in childhood ALL. Blood. 2015;126:1045-1047. https://doi.org/10.1182/blood-2015-07-655159 PMid:26294720 PMCid:PMC4551355

- Lopez-Guillermo
A, Cabanillas F, McLaughlin P, et al. The clinical significance of
molecular response in indolent follicular lymphomas. Blood.
1998;91:2955-2960. PMid:9531606

- Rambaldi
A, Lazzari M, Manzoni C, et al. Monitoring of minimal residual disease
after CHOP and rituximab in previously untreated patients with
follicular lymphoma. Blood. 2002;99:856-862. https://doi.org/10.1182/blood.V99.3.856 PMid:11806987

- Ladetto
M, Corradini P, Vallet S, et al. High rate of clinical and molecular
remissions in follicular lymphoma patients receiving high-dose
sequential chemotherapy and autografting at diagnosis: a multicenter,
prospective study by the Gruppo Italiano Trapianto Midollo Osseo
(GITMO). Blood. 2002;100:1559-1565. https://doi.org/10.1182/blood-2002-02-0621 PMid:12176870

- Gribben
JG, Freedman A, Woo SD, et al. All advanced stage non-Hodgkin's
lymphomas with a polymerase chain reaction amplifiable breakpoint of
bcl-2 have residual cells containing the bcl-2 rearrangement at
evaluation and after treatment. Blood. 1991;78:3275-3280.
PMid:1742487

- Brown
JR, Feng Y, Gribben JG, et al. Long-term survival after autologous bone
marrow transplantation for follicular lymphoma in first remission. Biol
Blood Marrow Transplant. 2007;13:1057-1065. https://doi.org/10.1016/j.bbmt.2007.05.012 PMid:17697968 PMCid:PMC4147857

- Hiddemann
W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added
to the combination of cyclophosphamide, doxorubicin, vincristine, and
prednisone (CHOP) significantly improves the outcome for patients with
advanced-stage follicular lymphoma compared with therapy with CHOP
alone: results of a prospective randomized study of the German
Low-Grade Lymphoma Study Group. Blood. 2005;106:3725-3732. https://doi.org/10.1182/blood-2005-01-0016 PMid:16123223

- Marcus
R, Imrie K, Belch A, et al. CVP chemotherapy plus rituximab compared
with CVP as first-line treatment for advanced follicular lymphoma.
Blood. 2005;105:1417-1423. https://doi.org/10.1182/blood-2004-08-3175 PMid:15494430

- Brugger
W, Hirsch J, Grunebach F, et al. Rituximab consolidation after
high-dose chemotherapy and autologous blood stem cell transplantation
in follicular and mantle cell lymphoma: a prospective, multicenter
phase II study. Ann Oncol. 2004;15:1691-1698. https://doi.org/10.1093/annonc/mdh425 PMid:15520073

- Morschhauser
F, Recher C, Milpied N, et al. A 4-weekly course of rituximab is safe
and improves tumor control for patients with minimal residual disease
persisting 3 months after autologous hematopoietic stem-cell
transplantation: results of a prospective multicenter phase II study in
patients with follicular lymphoma. Ann Oncol. 2012;23:2687-2695. https://doi.org/10.1093/annonc/mds202 PMid:22767588

- Hirt
C, Schuler F, Kiefer T, et al. Rapid and sustained clearance of
circulating lymphoma cells after chemotherapy plus rituximab: clinical
significance of quantitative t(14;18) PCR monitoring in advanced stage
follicular lymphoma patients. Br J Haematol. 2008;141:631-640. https://doi.org/10.1111/j.1365-2141.2008.07101.x PMid:18422779

- Pott
C, Belada D, Danesi N, et al. Analysis of Minimal Residual Disease in
Follicular Lymphoma Patients in Gadolin, a Phase III Study of
Obinutuzumab Plus Bendamustine Versus Bendamustine in
Relapsed/Refractory Indolent Non-Hodgkin Lymphoma. Blood.
2015;126:2015-2012-2003 2016:2028:2015
 .
.
- Scholz
CW, Pinto A, Linkesch W, et al. (90)Yttrium-ibritumomab-tiuxetan as
first-line treatment for follicular lymphoma: 30 months of follow-up
data from an international multicenter phase II clinical trial. J Clin
Oncol. 2012;31:308-313. https://doi.org/10.1200/JCO.2011.41.1553 PMid:23233718

- Goff
L, Summers K, Iqbal S, et al. Quantitative PCR analysis for Bcl-2/IgH
in a phase III study of Yttrium-90 Ibritumomab Tiuxetan as
consolidation of first remission in patients with follicular lymphoma.
J Clin Oncol. 2009;27:6094-6100. https://doi.org/10.1200/JCO.2009.22.6258 PMid:19858392

- van
Oers MH, Tonnissen E, Van Glabbeke M, et al. BCL-2/IgH polymerase chain
reaction status at the end of induction treatment is not predictive for
progression-free survival in relapsed/resistant follicular lymphoma:
results of a prospective randomized EORTC 20981 phase III intergroup
study. J Clin Oncol. 2010;28:2246-2252. https://doi.org/10.1200/JCO.2009.25.0852 PMid:20368567

- Ladetto
M, Sametti S, Donovan JW, et al. A validated real-time quantitative PCR
approach shows a correlation between tumor burden and successful ex
vivo purging in follicular lymphoma patients. Exp Hematol.
2001;29:183-193. https://doi.org/10.1016/S0301-472X(00)00651-2

- Galimberti
S, Guerrini F, Morabito F, et al. Quantitative molecular evaluation in
autotransplant programs for follicular lymphoma: efficacy of in vivo
purging by Rituximab. Bone Marrow Transplant. 2003;32:57-63. https://doi.org/10.1038/sj.bmt.1704102 PMid:12815479

- Rambaldi
A, Carlotti E, Oldani E, et al. Quantitative PCR of bone marrow
BCL2/IgH+ cells at diagnosis predicts treatment response and long-term
outcome in follicular non-Hodgkin lymphoma. Blood. 2005;105:3428-3433. https://doi.org/10.1182/blood-2004-06-2490 PMid:15637137

- Cheson
BD, Fisher RI, Barrington SF, et al. Recommendations for initial
evaluation, staging, and response assessment of Hodgkin and non-Hodgkin
lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. https://doi.org/10.1200/JCO.2013.54.8800 PMid:25113753 PMCid:PMC4979083

- Le
Dortz L, De Guibert S, Bayat S, et al. Diagnostic and prognostic impact
of 18F-FDG PET/CT in follicular lymphoma. Eur J Nucl Med Mol Imaging.
2010;37:2307-2314. https://doi.org/10.1007/s00259-010-1539-5 PMid:20717826

- Dupuis
J, Berriolo-Riedinger A, Julian A, et al. Impact of
[(18)F]fluorodeoxyglucose positron emission tomography response
evaluation in patients with high-tumor burden follicular lymphoma
treated with immunochemotherapy: a prospective study from the Groupe
d'Etudes des Lymphomes de l'Adulte and GOELAMS. J Clin Oncol.
2012;30:4317-4322. https://doi.org/10.1200/JCO.2012.43.0934 PMid:23109699

- Trotman
J, Fournier M, Lamy T, et al. Positron emission tomography-computed
tomography (PET-CT) after induction therapy is highly predictive of
patient outcome in follicular lymphoma: analysis of PET-CT in a subset
of PRIMA trial participants. J Clin Oncol. 2011;29:3194-3200. https://doi.org/10.1200/JCO.2011.35.0736 PMid:21747087

- Luminari
S, Galimberti S, Versari A, et al. Positron emission tomography
response and minimal residual disease impact on progression-free
survival in patients with follicular lymphoma. A subset analysis from
the FOLL05 trial of the Fondazione Italiana Linfomi. Haematologica.
2016;101:e66-68. https://doi.org/10.3324/haematol.2015.132811 PMid:26471485 PMCid:PMC4938338

- Casulo
C, Byrtek M, Dawson KL, et al. Early Relapse of Follicular Lymphoma
After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and
Prednisone Defines Patients at High Risk for Death: An Analysis From
the National LymphoCare Study. J Clin Oncol. 2015;33:2516-2522. https://doi.org/10.1200/JCO.2014.59.7534 PMid:26124482 PMCid:PMC4879714

- Pastore
A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk
prognostication for patients receiving first-line immunochemotherapy
for follicular lymphoma: a retrospective analysis of a prospective
clinical trial and validation in a population-based registry. Lancet
Oncol. 2015;16:1111-1122. https://doi.org/10.1016/S1470-2045(15)00169-2

- Jurinovic
V, Kridel R, Staiger AM, et al. Clinicogenetic risk models predict
early progression of follicular lymphoma after first-line
immunochemotherapy. Blood. 2016;128:1112-1120. https://doi.org/10.1182/blood-2016-05-717355 PMid:27418643

- Cheson BD. Speed bumps on the road to a chemotherapy-free world for lymphoma patients. Blood. 2016;128:325-330. https://doi.org/10.1182/blood-2016-04-709477 PMid:27222479

- Spinelli
O, Tosi M, Peruta B, et al. Prognostic significance and treatment
implications of minimal residual disease studies in
Philadelphia-negative adult acute lymphoblastic leukemia. Mediterr J
Hematol Infect Dis. 2014;6:e2014062. https://doi.org/10.4084/mjhid.2014.062 PMid:25237475 PMCid:PMC4165493

[TOP]










































 .
.














