Alexandros Makis, Sophia Tsabouri, Paraskevi Karagouni, Maria Rogalidou, Irene Sionti and Nikolaos Chaliasos
Child Health Department, Faculty of Medicine, University of Ioannina, Ioannina, Greece.
Corresponding
author: Alexandros Makis, Assistant Professor of Pediatrics/Pediatric
Hematology, Child Health Department, Faculty of Medicine, University of
Ioannina, P.O. Box 1187, GR-45110 Ioannina, Greece. Tel: +30
2651099598, Fax: +30 2651007038. E-mail:
amakis@cc.uoi.gr
Published: January 1, 2017
Received: October 3, 2016
Accepted: November 18, 2016
Mediterr J Hematol Infect Dis 2017, 9(1): e2017011 DOI
10.4084/MJHID.2017.011
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
Visceral leishmaniasis (VL), caused by the protozoan parasite Leishmania donovani
infection, is characterized by a varied spectrum of clinical and
laboratory manifestations and by the potentiality of relapses (1,5-10%)
despite current therapy.[1] Following infection, many
cell adhesion interactions have been identified among
monocytes/macrophages, vascular endothelial cells and the parasite;[2,3]
and various changes in the expression of adhesion molecules VCAM-1
(vascular cell adhesion molecule-1), ICAM-1 (intracellular adhesion
molecule-1) and L-selectin have been found in experimental VL.[4,5]
We hypothesized that the monitoring of this cell to cell interaction
system through the course of VL could be useful in estimating the
disease progress.
We enrolled in the study 16 children (10 boys,
2-15 years) hospitalized for VL. Most of the children had presented
with fever, hepatosplenomegaly, anemia and thrombocytopenia. The
clinical diagnosis was confirmed by positive serology, PCR technology
or parasite presence in the bone marrow macrophages (Table 1).
The standard treatment regimen was liposomal amphotericin B (3 mg/kg)
on days 1 to 5, 14, and 21. The serum levels of VCAM-1, ICAM-1, and
L-selectin were determined in the patients at days 0, 15 and 30, as
well as in 20 gender and age-matched healthy children. Commercial ELISA
kits were used (Quantikine, R&D Systems, Inc., Minneapolis, USA).
Mann-Whitney U test, Wilcoxon matched pairs test and x2 test were appropriately used for the statistical analysis.
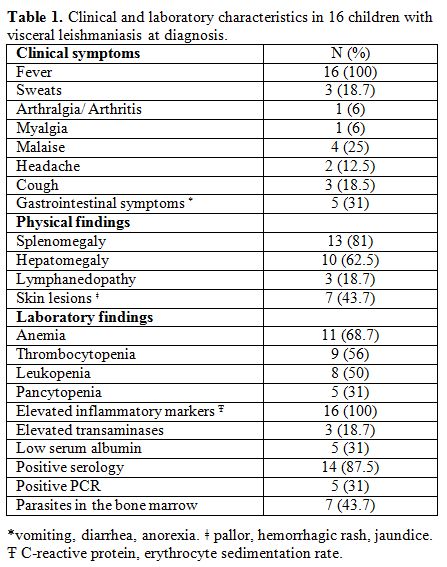 |
Table
1. Clinical and laboratory characteristics in 16 children with visceral leishmaniasis at diagnosis. |
All
children recovered completely, while three children relapsed 3, 5 and 6
months after treatment. At day 0, VCAM-1, ICAM-1, and L- selectin were
similar to controls (p>0.05). At day 15, VCAM-1 and ICAM-1 were
significantly increased (P=0.0012, P=0.0032) where L-selectin remained
stable (P=0.75). At day 30, VCAM-1 and ICAM-1 decreased at levels
comparable to pretreatment values in the 13 children who subsequently
had a good outcome without relapses (P=0.88), but not in the three
patients who relapsed (P=0.0007). No differences were noted in
L-selectin levels (P=0.19) (Table 2).
The adhesion molecules levels were further analyzed for both
non-relapsers and relapsers. Non-relapsers showed a significant decline
in VCAM-1 and ICAM-1 levels at day 30 (P=0.0006 and P=0.0008) compared
to day 15. By contrast, in relapsers day 30 serum VCAM-1 and ICAM-1 had
not significantly decreased as compared to day 15 (P=0.45, P=0,72). No
differences were demonstrated on day 0, 15 and 30 L-selectin values (Table 3).
No differences were noted regarding gender, age, symptoms and the
laboratory tests on admission, such as hemoglobin, white blood cell
counts, platelets, C-reactive protein, erythrocyte sedimentation rate,
total protein levels or albumin levels.
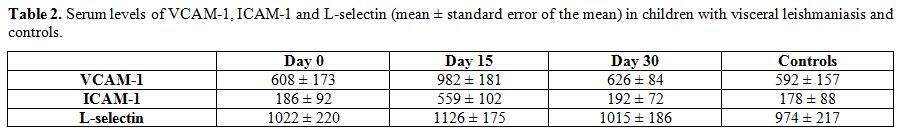 |
Table 2. Serum levels of VCAM-1, ICAM-1
and L-selectin (mean ± standard error of the mean) in children with
visceral leishmaniasis and controls. |
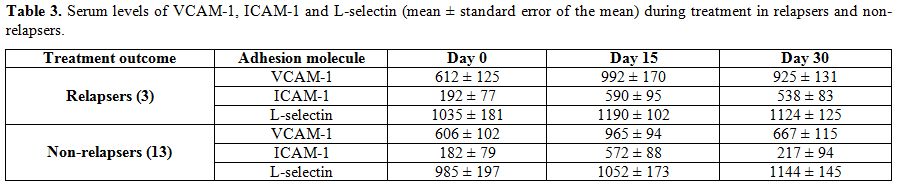 |
Table 3. Serum levels of VCAM-1, ICAM-1
and L-selectin (mean ± standard error of the mean) during treatment in
relapsers and non-relapsers. |
These
findings may be explained by the cell adhesion interactions during the
immune response and the effect of the anti-parasite treatment. The
suppressed VCAM-1 and ICAM-1 levels at diagnosis could reflect the
adverse effect of Leishmania
against the adhesive interactions to stop the leukocyte attraction to
the site of parasitic infection. The interaction of VCAM-1 with its
ligands is crucial for the efficient control of Leishmania donovani
infection, especially in the liver. Interestingly, the blockade of
VCAM-1 leads suppresses anti-leishmania immune responses and leads to
higher hepatic parasite accumulation.[6] A similar
mechanism of down-regulation of ICAM-1 has been found in human synovial
cells in vitro infected with Borrelia burgdorferi.[7]
The elevation of VCAM-1 and ICAM-1 in day 15 could be the beneficial
effect of liposomal amphotericin B which destroys the parasites and
allows the cell to cell interactions. After the end of treatment, the
number of tissue parasites dramatically diminishes, and this is
probably the reason why the VCAM-1 and ICAM-1 levels return to
pre-treatment levels in the children who had a good long-term outcome
without relapses. The persistence of high VCAM-1 and ICAM-1 values in
the children who relapsed despite they received the same treatment
possibly reflects the ongoing immune response to the remaining
parasites as well as the action of liposomal amphotericin
B. Maybe, these children had a tissue parasite burden larger at
front, and they could have taken advantage from the repetition of
second treatment schedule. L-selectin did not show any alterations
during the disease. One possible explanation is that L-selectin acts on
lymphocyte-endothelial cell interactions and activates Th2 (T helper 2)
immune response, which is not implicated in the host defense against Leishmania.[8]
In
conclusion, we found that serum levels of VCAM-1 and ICAM-1 at day 30
post-treatment demonstrated statistically significant correlation with
the possibility to relapse in this small group of patients, while
L-selectin showed no association. Despite the low number of the
patients of this study, our findings indicate that the measurement of
VCAM-1 and ICAM-1 during the course of VL may guide and predict disease
evolution and outcome in children. Although the mechanisms underlying
the association between serum VCAM-1 and ICAM-1 levels and the adverse
outcome has not yet been elucidated, further investigation with a
larger number of patients would clarify their role as factors of
disease severity and confirm their importance as prognostic markers. References
- Georgiadou SP, Stefos A, Spanakos G, Skrimpas S,
Makaritsis K, Sipsas NV, et al. Current clinical, laboratory, and
treatment outcome characteristics of visceral leishmaniasis: results
from a seven-year retrospective study in Greece. Int J Infect Dis.
2015;34:46-50. https://doi.org/10.1016/j.ijid.2015.02.021 PMid:25743761

- Rodrigues
V, Cordeiro-da-Silva A, Laforge M, Silvestre R, Estaquier J. Regulation
of immunity during visceral Leishmania infection. Parasit Vectors.
2016;9:118. https://doi.org/10.1186/s13071-016-1412-x PMid:26932389 PMCid:PMC4774109

- Figueira
CP, Carvalhal DG, Almeida RA, Hermida M, Touchard D, Robert P, et al.
Leishmania infection modulates beta-1 integrin activation and alters
the kinetics of monocyte spreading over fibronectin. Sci Rep.
2015;5:12862. https://doi.org/10.1038/srep12862 PMid:26249106 PMCid:PMC4528201

- Engwerda
CR, Ato M, Stager S, Alexander CE, Stanley AC, Kaye PM. Distinct roles
for lymphotoxin-alpha and tumor necrosis factor in the control of
Leishmania donovani infection. Am J Pathol. 2004;165(6):2123-33. https://doi.org/10.1016/S0002-9440(10)63262-2

- Colpitts
SL, Scott P. The early generation of a heterogeneous CD4+ T cell
response to Leishmania major. J Immunol. 2010;185(4):2416-23. https://doi.org/10.4049/jimmunol.1000483 PMid:20624946 PMCid:PMC2944829

- Stanley
AC, Dalton JE, Rossotti SH, MacDonald KP, Zhou Y, Rivera F, et al.
VCAM-1 and VLA-4 modulate dendritic cell IL-12p40 production in
experimental visceral leishmaniasis. PLoS Pathog. 2008;4(9):e1000158. https://doi.org/10.1371/journal.ppat.1000158 PMid:18802456 PMCid:PMC2528938

- Girschick
HJ, Meister S, Karch H, Huppertz HI. Borrelia burgdorferi downregulates
ICAM-1 on human synovial cells in vitro. Cell Adhes Commun.
1999;7(2):73-83. https://doi.org/10.3109/15419069909034398 PMid:10427961

- Seixas
Duarte MI, Tuon FF, Pagliari C, Kauffman MR, Brasil RA. Human visceral
leishmaniasis expresses Th1 pattern in situ liver lesions. J Infect.
2008;57(4):332-7. https://doi.org/10.1016/j.jinf.2008.07.005 PMid:18722018













