Paola Magro1, Ilaria Izzo1, Barbara Saccani1, Salvatore Casari1, Silvio Caligaris1, Lina Rachele Tomasoni1, Alberto Matteelli1, Annamaria Lombardi2, Antonella Meini2 and Francesco Castelli1
1 University
Department of Infectious and Tropical Diseases, University of Brescia
and Spedali Civili General Hospital, Brescia, Italy.
2 University Department of Pediatrics, University of Brescia and Spedali Civili General Hospital, Brescia, Italy.
Corresponding
author: Ilaria Izzo, MD. University Department of Infectious and
Tropical Diseases, University of Brescia and Spedali Civili General
Hospital, Brescia, Italy. Tel: +39303995677. E-mail:
izzo.ilaria@hotmail.it
Published: March 1, 2017
Received: December 12, 2016
Accepted: February 14, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017023 DOI
10.4084/MJHID.2017.023
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The
protective role of Sickle Cell Trait (SCT) in malaria endemic areas has
been proved, and prevalence of HbS gene in malaria endemic areas is
high. Splenic infarction is a well-known complication of SCT, while the
association with malaria is considered rare. A Nigerian boy was
admitted to our ward after returning from his country of origin, for P. falciparum malaria.
He underwent abdominal ultrasound for upper right abdominal pain,
showing cholecystitis and multiple splenic lesions suggestive of
abscesses. Empiric antibiotic therapy was undertaken. Bartonella, Echinococcus, Entamoeba
serologies, blood cultures, Quantiferon test, copro-parasitologic exam
were negative; endocarditis was excluded. He underwent further blood
exams and abdomen MRI, confirming the presence of signal alterations
areas, with radiographic appearance of recent post-infarction outcomes.
Hemoglobin electrophoresis showed a percentage of HbS of 40.6% and a
diagnosis of SCT was then made.
Splenic infarction should be taken
into account in patients with malaria and localized abdominal
pain. Moreover, diagnosis of SCT should be considered.
|
Case Report
In
1948 Haldane firstly hypothesized the existence of a protective
relationship between an otherwise harmful genetic mutation and a
population with a high frequency of parasite infection. Since then, the
protective role of Sickle Cell Trait (SCT) in malaria endemic areas has
been proved in several studies.[1,2] In fact, if red cells are abnormal, the chance of success of the parasite is affected, reducing death rate due to Plasmodium spp.
Splenic
infarction is a well-known complication of SCT, in particular during
exercise at high altitude, but it has also been described at rest in
aircrafts or with exercise at sea level. Splenic vaso-occlusion is
related to hemoglobin S polymerization and red cell deformation.
Clinical presentation consists of severe upper quadrants abdominal
pain, vomiting, and nausea. Fever, leukocytosis and elevation of LDH
usually occur in the first 3 days.[3]
In
contrast, malaria-associated splenic infarctions are considered rare.
Anyway, reports of single or small series of cases have appeared almost
annually.[4] Is therefore really unusual to find these three conditions co-existing?
We report the case of an 11-year-old Nigerian boy, living in Italy since 2010. On August, 26th he flew back from a one-month stay in Nigeria, and two days later he started presenting fever. On August, 31st
he complained vomiting and abdominal pain and was then conducted to the
Emergency Room of our Hospital. Here, after an initial suspect of
appendicitis, a diagnosis of Plasmodium falciparum
malaria was made, and the patient was admitted to the Infectious
Diseases ward. Body temperature was 38°C, while other vital signs were
normal. His complete blood count showed leukopenia (WBC 4060, nv
4500-10800) and thrombocytopenia (PLT 59,000, nv 100,000-400,000),
while hemoglobin was 13 g/dL (nv 13-16.5 g/dL). His chemistry profile
showed hyperbilirubinemia (2.93 mg/dL, nv 0.2-0.5 mg/dL), AST 136 UI/L
and ALT 122 UI/L (nv 13-51 UI/L and 15-47 UI/L, respectively),
creatinine was 1.06 mg/dL (nv 0.3-0.9 mg/dL) and an increase of LDH
values (657 U/L) was present (nv 120-330 U/L); C-reactive protein was
85.6 mg/L (nv <5 mg/L). He started therapy with
piperachin/diidroartemisin 320/40 mg, 3 tablets qd, and as long as he
kept presenting well-localized right upper quadrant abdominal pain, an
ultrasound (US) was performed on September, 1st.
The exam showed a starred sky appearance of the liver associated with a
slightly enlarged gallbladder, with transonic content and thickened
walls, suggestive of cholecystitis. A slight increase of the spleen’s
dimensions (pole-to-pole diameter 11 cm) was present, and in its
context a hypo-anechoic, inhomogeneous ovalar lesion (diameter 1.5 cm)
was found, raising a diagnostic doubt (Abscess? Hematoma? Cyst?). The
pediatric surgeon confirmed a non-surgical approach for cholecystitis
and antibiotic therapy with piperacilline/tazobactam 4.5 g tid (body
weight of 42 Kg) was undertaken. As long as he kept presenting fever (Figure 1) although therapy for malaria was considered over (piperachin/diidroartemisin 320/40 mg, 3 tablets qd, from August, 31st to September, 2nd)
and hemoscopy resulted negative, as suggested by the radiologist, the
next day the US was repeated, showing multiple splenic abscesses.
Therefore, serologies for Bartonella, Echinococcus and Entamoeba and blood cultures were sent.
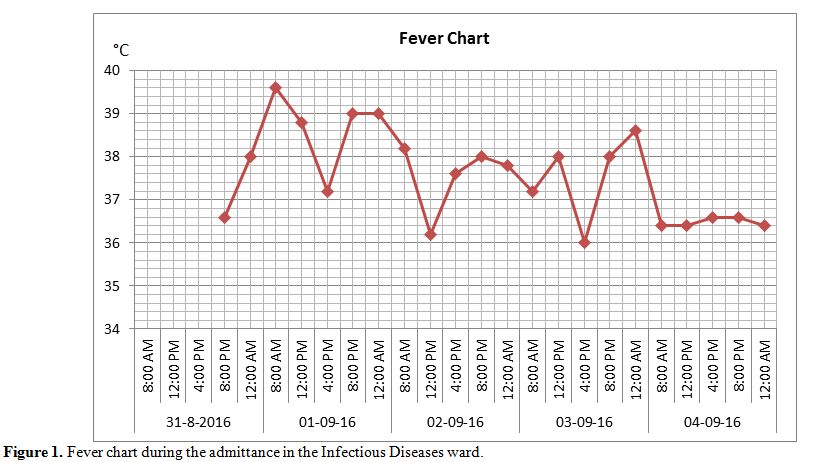 |
Figure 1. Fever chart during the admittance in the Infectious Diseases ward. |
Quantiferon
test and coproparasitologic exam were executed, resulting both
negative. An echocardiogram showed no endocarditic vegetations. On
September, 5th
the boy was transferred to the Pediatrics ward for cholecystitis and
splenic abscesses. Antimicrobial spectrum was broadened with
Claritromicine 250 mg bid per os. Blood tests were performed, including
peripheral blood smear, quantitative Ig, total IgE, lymphocyte typing,
hemoglobin electrophoresis and dihydrorhodamine 123 test, in order to
evaluate any underlying hematologic diseases. On September, 8th,
magnetic resonance imaging (MRI) of the abdomen was performed,
confirming the presence of seven focal areas of signal alteration, with
prevalent peripheral subcapsular distribution (maximum size 1.7 mm).
The
morphology of the formations, especially of the most voluminous, was a
pyramidal wedge with the apex pointing towards the hilum, and the base
of the splenic capsule, while profiles looked finely irregular (Figure 2 and 3).The
radiographic appearance then made it less likely the possibility of
splenic abscesses, in favour of a diagnosis of recent post-infarction
outcomes or hematomas in a subacute phase. While all other requested
exams resulted negative, on September, 13th,
hemoglobin electrophoresis showed a percentage of HbS of 40.6%, HbA was
55.2% while HbA2 and HbF were respectively 3.5 and 0.7%. A diagnosis of
SCT was then made.
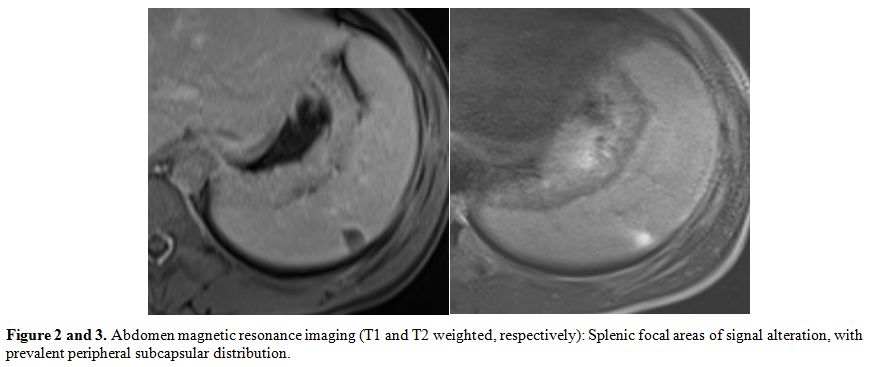 |
Figure 2 and 3. Abdomen magnetic resonance
imaging (T1 and T2 weighted, respectively): Splenic focal areas of
signal alteration, with prevalent peripheral subcapsular distribution. |
On September, 15th
the boy was discharged, with recommendations to radiologic follow-up
and preconception counseling for him and his parents and without
further antibiotic therapy.
On October, 13th
the boy repeated the US, which showed a normal spleen. The timing of
regression is compatible with the diagnosis of splenic infarction.
Discussion and Conclusions
SCT has a protective role in the pathogenesis of severe malaria, whereas HbS is not an absolute impediment to the infection by Plasmodium spp.
Probably an early phagocytose of the parasited sickled cells by the
reticulo-endothelial system is the mechanism for the increased fitness
of patients with SCT.[1] Prevalence of HbS gene in
malaria endemic areas can reach a percentage >20%; therefore this
diagnosis must be taken into account in a patient hailing from one of
these countries,[2] especially when anemia is present. Splenic infarction is a complication of SCT,[3]
and it has been reported at rest in aircrafts, moreover it has been
described as a rare complication of malaria, it is primarily caused by P. falciparum, occurring mostly during the acute phase of the infection,[4]
even if the exact frequency of malaria-associated splenic infarction
remains unclear because of under-diagnosis and under-reporting.[5]
In
our case, as long as fever kept on after the conclusion of an adequate
cycle of antimalarial therapy, in association with abdominal pain in
the right upper quadrant and evidence of splenic lesions, further
analysis have been undertaken in the suspect of a concomitant
condition. Trauma was excluded. Therefore we considered as differential
diagnosis splenic abscesses, both primary or secundary (e.g.
endocarditis, patent foramen ovale), splenic infarction, primary
hematologic diseases and granulomatous diseases (e.g. tuberculosis and
chronic granulomatous disorder). Contrary to our case, abdominal pain
is generally reported as localized to the left upper quadrant, when
splenic infarction is present.[4] In the reported
case, atypical clinical presentation, concomitant cholecystitis and
radiological diagnosis of splenic abscesses were misleading.
Splenic
infarction should be taken into account in a patient with malaria and
abdominal pain, particularly when localized to upper quadrants, and US
and CT scan should be performed to confirm the diagnosis. Computed
tomography scan with contrast is the gold standard for diagnosis of
splenic infarction. In our case, however, US has been primarly
performed in the suspect of cholecystitis. MRI was then performed,
taking into account the young age of the patient and the good
sensitivity of this technique for spleen lesions.[5] Conservative therapy must be attempted, and the prognosis is good.[6]
Splenectomy should be reserved for those patients with severe damage to
the spleen, also taking into account possible future complications,[7] including severe malaria.
Finally,
as long as the number of international migrants worldwide kept on
growing over the past decades, all of us clinicians should be aware of
pathologies we haven’t been familiar with in our daily activity.
References
- Luzzatto L. Sickle Cell Anaemia and Malaria. Mediter J Hematol Infect Dis 2012, 4(1): e2012065. https://doi.org/10.4084/mjhid.2012.065 PMid:23170194 PMCid:PMC3499995

- Russo G et al. Italian Guidelines for the Sickle Cell Disease in Pediatrics. AIEOP. Article available at http://www.aieop.org/files/files_htmlarea/tutto%20giu12.pdf last accessed on November 12th, 2016.

- Kark J. Sickle cell trait. Article available at http://sickle.bwh.harvard.edu/sickle_trait.html, last accessed on November 12th, 2016

- Hwang JH, Lee CS. Malaria-Induced Splenic Infarction. Am. J. Trop. Med. Hyg., 2014;91(6):1094–1100 https://doi.org/10.4269/ajtmh.14-0190 PMid:25294615 PMCid:PMC4257629

- Parikh M, Pachter L, Stewart GD. Splenic Infarct Workup. Article available at http://emedicine.medscape.com/article/193718-workup#c5, last accessed on January 30th, 2017

- Cinquetti
G, Banal F, Rondel C, et al. Splenic infarction during Plasmodium ovale
acute malaria: first case reported. Malar J 2010;9: 288. https://doi.org/10.1186/1475-2875-9-288 PMid:20955610 PMCid:PMC2984568

- Leone
G, Pizzigallo E. Bacterial infections following splenectomy for
malignant and nonmalignant hematologic diseases. Mediterr J Hematol
Infect Dis 2015, 7(1): e2015057 https://doi.org/10.4084/mjhid.2015.057 PMid:26543526 PMCid:PMC4621170

[TOP]










