Farida H. El-Rashedy1, Mahmoud A. El-Hawy1, Sally M. El Hefnawy2 and Mona M. Mohammed3
1 Department of Pediatrics, Faculty of Medicine, Menoufia University, Shebin El-Kom, Egypt.
2 Department of Biochemistry, Faculty of Medicine, Menoufia University, Shebin El-Kom, Egypt.
3 Department of Pediatrics, Benha Educational Hospital, Benha, Egypt.
Corresponding
author: Mahmoud Ahmed El-Hawy. Department Pediatrics, Faculty of Medicine , Menoufia University , Shebin El-Kom , Egypt Email:
mahmodelhawy18@yahoo.com
Published: April 15, 2017
Received: September 9, 2016
Accepted: March 27, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017026 DOI
10.4084/MJHID.2017.026
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Childhood acute lymphoblastic leukemia (ALL) with current cure rates
reaching 80% emphasizes the necessity to determine treatment-related
long-term effects. The aim of this study is to estimate the prevalence
of overweight, obesity, and hepatic late adverse effects in a cohort of
ALL survivors treated at the Hematology and Oncology Unit, Pediatrics
Department, Menoufia University, Egypt.
Methods:
In this case-control study, height, weight, and body mass index (BMI)
were assessed for 35 pediatric ALL survivors and 35 healthy children.
These parameters were plotted on the growth and WHO standard deviation
charts for both males and females. Overweight and obesity were defined
by BMI > 85th and 95th percentile respectively. Laboratory
investigations were done in the form of iron profile, liver enzymes,
total and direct bilirubin levels, serum urea &creatinine and
detection of hepatitis C virus antibodies by ELISA.
Results:
The weight and BMI were significantly greater in the survivors than
controls (P value =0.002 and 0.039 respectively). ALT, total &
direct bilirubin, serum ferritin and transferrin saturation were
considerably higher in the survivors than the controls (P value = 0.03,
0.036, 0.044, 0.006 and 0.03 respectively). Ten (28.6%) of survivors
had hepatitis C antibodies with none (0%) of controls (P value =0.02)
Conclusions:
Pediatric ALL survivors are at increased risk of overweight/obesity,
hepatic dysfunction in the form of elevated liver enzymes, bilirubin
levels, and C viral hepatitis. Screening of those survivors for such
complications should be considered.
|
Introduction
In
Egypt, childhood acute lymphoblastic leukemia (ALL) is the most common
cancer in children, accounting for almost one-third of newly diagnosed
pediatric cancer cases. The annual incidence of pediatric ALL is
approximately four cases per 100,000 children per year in the National
Cancer Institute NCI, Cairo University, Egypt. Cases show male to
female ratio of 2.3:1. The 2-10 years age group constitutes 68.5%.[1,2]
Fortunately,
improvements in treatment, including multimodal therapy and hospital
care, have improved survival such that over 80% of children diagnosed
with ALL survive at least five years.[2,3]
However,
childhood cancer survivors are at increased risk of developing chronic
health conditions, some of which manifest during or soon after
treatment whereas others emerge years after therapy.[4]
Obesity is a particularly significant problem among ALL survivors,
which can intensify cardiovascular outcomes and place these individuals
at greater risk for other chronic health conditions.[5]
Evidence
from the Childhood Cancer Survivor Study (CCSS) suggests that survivors
of ALL (who lived 5ys after treatment) experience a higher rate of
obesity than their same-sex siblings, especially for female survivors
(ALL: 31.7% vs. siblings: 22.2%).[6] Obesity may
further compound the risk of other late effects, such as increased rate
of cardiovascular diseases observed in childhood cancer survivors.[7]
Previous
studies have attributed obesity to the cranial irradiation (CRT)
patients received to prevent central nervous system (CNS) relapse,
However, since the 1990s, CNS prophylaxis with CRT protocols has
gradually been replaced with intrathecal and systemic chemotherapy by
several consortia.[8] A study of the Children’s
Oncology Group (COG) found excessive weight gain also occurred in
children receiving chemotherapy alone.[9] Treatment
with glucocorticoids has been implicated in the physiology of
adiposity, and there is data that dexamethasone may act more potently
than prednisone.[10] Prolonged use of corticosteroids
has shown effects on body composition associated with increases in the
percentage of body fat in pediatric ALL survivors.[11]
Also,
Hepatic abnormalities are well documented in survivors of childhood
malignancies. A spectrum of liver diseases has been described including
hepatitis B and C, iron overload, hepatic fibrosis, cirrhosis and
hepatocellular carcinoma.[12] Less commonly reported
hepatobiliary complications include cholelithiasis, focal nodular
hyperplasia (FNH), nodular regenerative hyperplasia, hepatic
microvesicular fatty change and siderosis.[13] The
Childhood Cancer Survivor Study (CCSS) noted an almost two-fold excess
risk of gallbladder disease among childhood cancer survivors compared
to sibs (1.9 95 % 1.7–2.2).[13]
Acute or
sub-acute hepatobiliary injury is recognized with varying incidence
following radiation, multiple chemotherapies, or hematopoietic stem
cell transplantation (HSCT).[14] Additionally,
hepatobiliary toxicity is associated with supportive care measures,
such as transfusion-acquired hepatitis, transfusion-associated iron
overload or cholestatic disease from total parenteral nutrition (TPN).
These conditions may predispose to clinically significant liver disease
in aging childhood cancer survivors.[15] In this
study, we aimed to estimate the prevalence of overweight, obesity, and
hepatic late adverse effects in pediatric ALL survivors who lived 5
years after treatment.
Patients and Methods
A
comparative cross-sectional case-control study was performed on
thirty-five ALL survivors, treated at Hematology and Oncology Unit,
pediatric department, Menoufia University Hospital. All of them
completed treatment with St Jude Total XV Chemotherapy Protocol 5 years
before the time of examination. In that protocol, the remission
induction therapy began with prednisone, vincristine, daunorubicin, and
asparaginase. Subsequent remission induction therapy included
cyclophosphamide, mercaptopurine, and cytarabine. On hematopoietic
recovery, consolidation therapy with high-dose methotrexate,
mercaptopurine, triple intrathecal treatment began, and the dose of
methotrexate was based on risk classification. During initial
continuation therapy, patients with low-risk disease received daily
mercaptopurine and weekly methotrexate with pulses of mercaptopurine,
dexamethasone, and vincristine. Two re-induction treatments were given
between weeks 7 to 9 and weeks 17 to 19. Patients with standard-risk
disease received weekly asparaginase and daily mercaptopurine with
pulses of doxorubicin plus vincristine plus dexamethasone. They also
received two re-induction treatments between weeks 7 to 9 and weeks 17
to 20. For the remaining part of continuation therapy, patients with
low-risk disease received mercaptopurine and methotrexate, with pulses
of dexamethasone, vincristine, and mercaptopurine, and patients with
standard-risk disease received three rotating drug pairs mercaptopurine
plus methotrexate, cyclophosphamide plus cytarabine, and dexamethasone
plus vincristine. Continuation treatment lasted 120 weeks for girls and
146 weeks for boys.[16]
None of those survivors
received radiotherapy. The study was done in the period between March
2015 and December 2015. A control group of 35 healthy children with
matched age and sex was selected from volunteers from a local school.
They were apparently healthy with no history of chronic illnesses or
previous history of steroid intake. A written informed consent was
obtained from the parents of all children, and oral assent was obtained
from children of both groups. This study was approved by the ethical
committee of the Faculty of Medicine, Menoufia University.
The survivors and controls were subjected to anthropometric measurements and laboratory investigations:
Anthropometric
measurements (weight, height, and body mass index), BMI was assessed by
Z-score. Body Mass Index = Weight in Kilograms/ (Height in meters) ²
was plotted on age and gender-specific percentile charts (for 2 to
20-year-olds). BMI over the 95th percentile indicates obesity, between 85th and 95th, indicates risk of overweight.[17]
We evaluated longitudinal changes in obesity rate and BMI Z scores in
survivors of pediatric ALL as for survivors of childhood cancer aged
<20 y. The BMI Z-score or percentile is often used to evaluate
weight status, rather than the absolute BMI because an increased BMI is
part of the normative/adolescent development and also varies by sex.[18] The BMI Z-score or percentile can be calculated on the basis of age and sex-specific mean BMI of a reference population.[19]
7 ml of venous blood were withdrawn from every child then transferred
into a plain tube, centrifuged for 10 min at 4000 r.p.m. The serum
obtained was kept frozen at - 20 ºC till analysis (Liver, kidney
function tests, iron profile and HCV antibodies detection). Serum ALT
& AST were estimated by enzymatic colorimetric method using Randox
kit, United Kingdom.[20] Serum total and direct bilirubin were estimated by enzymatic colorimetric method using Diamond Diagnostics kit, Germany.[20] Serum urea was determined by Mod Berthelot enzymatic colorimetric method using Diamond Diagnostics Kit, Germany.[21] Serum creatinine was determined by the fixed rate kinetic chemical method, using Diamond Diagnostics Kit, Germany.[22]
Serum iron and total iron binding capacity (TIBC) were determined by a
colorimetric method using SPECTRUM diagnostics kit (Germany).[23,24]
Transferrin saturation index (TSI) was calculated by the following
formula: iron concentration divided by TIBC and multiplied by 100. The
TSI > 16% values were regarded as correct ones.[25]
Serum ferritin levels were measured to assess the iron status of our
patients by Enzyme Linked Immune Sorbent Assay (ELISA) technique using
(RAMCO LABORATORIES kit, INC., USA), and HCV antibodies were detected
by ELISA using (AUTOBIO DIAGNOSTICS, China) kit on microplate reader
(Bio-Rad 680 Hercules, California, USA).
Statistical Analysis
The
statistical presentation and analysis of the present study were
conducted using SPSS V.20 (SPSS Inc., Chicago, IL, USA). Data were
expressed in two phases: Continuous parametric variables were presented
as means± SD while for categorical variables numbers (%) were used.
Chi-square (χ2 test) and Fisher's
exact test were used for qualitative variables, student’s t-test for
parametric continuous variables and Man Whitney (U) test for
non-parametric variables
Results
The
mean age of the survivors at the time of the study was (11.01±4.6)
years. Fourteen of them (40%) are females, and 21 (60%) are males.
Sixty% were leukemia of low risk, and 40% were of standard risk The
mean age of them at diagnosis was (5.86±1.5) years. The mean age of
controls was (9.6±3.3). Nine (60%) are males, and 6(40%) are females.
The weight and BMI were significantly higher in the survivors’ controls
(P value =0.002 and 0.039 respectively) while no significant difference
was found between the two groups regarding the height (P-value =0.351) (Table 1).
Survivors had a significant positive correlation with younger age at
diagnosis and BMI (P-value =0.003) and highly significant correlation
with weight at diagnosis and after chemotherapy (P value =<0.001).
Also, a highly significant value was detected between obese survivors
and positive family history of obesity (P value =<0.001) (Table 2).
ALT, total & direct bilirubin, serum ferritin and transferrin
saturation were significantly higher in the survivors than controls (P
value =0.03, 0.036, 0.044, 0.006 and 0.03 respectively) (Table 3).
While no significant difference was found between the two groups
regarding AST, albumin, creatinine, BUN, serum iron and TIBC (Table 3). Ten (28.6%) of survivors had hepatitis C antibodies with none (0%) of controls (P value =0.02) (Table 4).
A correlation was calculated between cumulative doses of asparaginase
and ALT, AST, total bilirubin, direct bilirubin. A positive highly
significant correlation was found between cumulative dose of
asparaginase and liver enzymes (ALT, AST) (P value =<0.001) and with
total bilirubin (P-value = 0.003) but not with direct bilirubin
(P-value = 0.052) (Table 5). No
significant correlation was found between liver function (transaminases
and hyperbilirubinemia) and ferritin levels in survivors (Table 6).
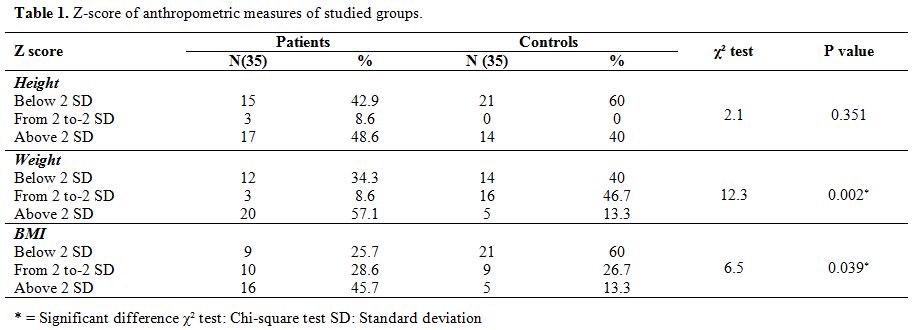 |
Table 1.
Z-score of anthropometric measures of studied groups |
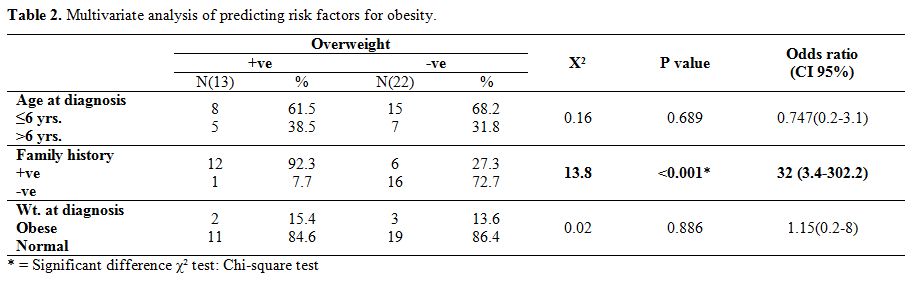 |
Table 2.
Multivariate analysis of predicting risk factors for obesity. |
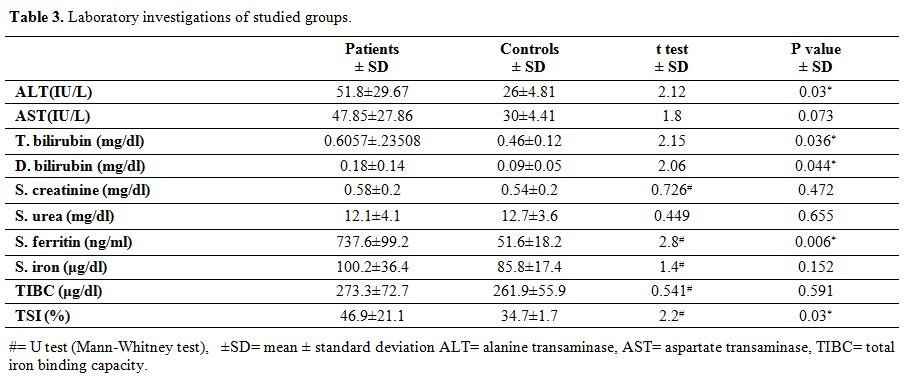 |
Table 3.
Laboratory investigations of studied groups. |
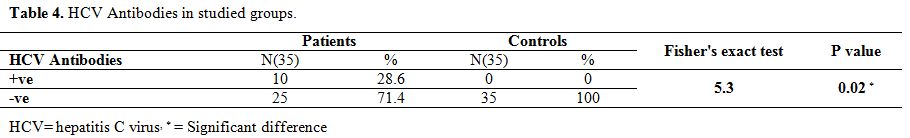 |
Table 4.
HCV Antibodies in studied groups. |
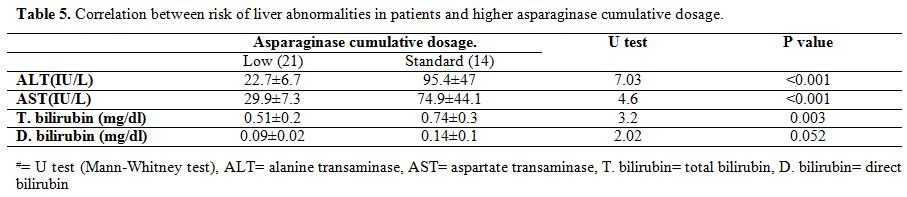 |
Table 5.
Correlation between risk of liver abnormalities in patients and higher asparaginase cumulative dosage. |
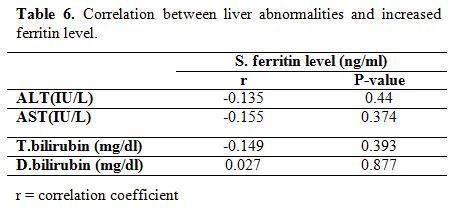 |
Table 6. Correlation between liver abnormalities and increased ferritin level. |
Discussion
The
first aim of this study was to assess the prevalence of overweight and
obesity in pediatric ALL survivors. Our results revealed that survivors
of pediatric ALL were at risk becoming overweight or obese with
long-term follow-up. Based on U.S. Centers for Disease Control and
Prevention (CDC) definition growth charts, Salazar-Martinez et al.[26]
said that the prevalence of overweight and obesity was 12.1% and 6.2%,
respectively, among the healthy Egyptian adolescents. This study
revealed that overweight and obesity were more prevalent in ALL
survivors compared to general Egyptian population, suggesting an impact
of chemotherapy on weight gain in ALL survivors. This datum is in
agreement with Asner et al.[27] who examined the
prevalence and the risk factors for overweight and obesity in a cohort
of ALL survivors treated and living in the French speaking part of
Switzerland reported that there is a significant prevalence of obesity
in young ALL survivors.
Fang et al.[28]
indicated a significantly higher BMI in pediatric ALL survivors than
the reference population. However, a study by Murphy et al.[29]
found that on-treatment and survivor groups had a significantly lower
body cell mass index than matched controls, and 53% of the survivors
were considered undernourished.
In a study done on 56 adolescent,
ALL survivors in Saudi Arabia, with a mean age of 13.4 years an average
of 9.1 years post-diagnosis who did not receive CRT, the prevalence of
BMI for age defined overweight, and obesity (combined 28.5%) were lower
than in the general population in Saudi Arabia. The authors supposed
that overweight and obesity observed were probably not an ALL specific
problem.[30]
Our results demonstrated that the
survivors who had high BMI z-score at diagnosis also had increased risk
of being overweight /obese after treatment completion. This result was
in line with Fang et al. study which is a retrospective cohort of 83
pediatric patients with ALL; they examined BMI status at several key
time points: diagnosis; end of induction; end of consolidation; every 6
months during maintenance; and yearly for up to 5 years post-treatment.
At diagnosis, 21% were overweight (BMI = 85–94.9th percentile) or obese (BMI ≥95th
percentile). At the end of treatment and 5 years post-treatment,
approximately 40% were overweight or obese. Weight gain during
treatment was associated with being overweight/obese 5 years
post-treatment (OR = 3.8, 95% CI: 1.1–12.5).[31]
All of the involved survivors had received dexamethasone with the mean cumulative dose of 927 ± 135 mg/m2
which may be the cause of weight gain. It is not entirely understood
why ALL survivors gain excess fat mass. One theory is that, during
glucocorticoid treatment, ALL patients have an increased energy intake[32] and reduced energy expenditure on habitual physical activity[33]
and that this effect continues after treatment ceases. Other theories
are that glucocorticoid treatment causes increased adiposity by
suppressing growth hormone secretion or that it causes resistance to
leptin.[34]
The second aim of this study was to
assess the hepatic late adverse effects in pediatric ALL survivors. At
our study, there was a significant increase in D. Bilirubin, T.
Bilirubin, ALT, serum ferritin and soluble transferrin saturation in
the survivors’ group more than the control group. Ten of our survivors
(28.6 %) have HCV positive antibodies detected by ELISA. These results
go with the previous findings of Mulder et al.[35] who concluded that abnormal high ALT level was detected in survivors of childhood cancer. Also, Schempp et al.[36]
found elevated levels of serum ferritin and soluble transferrin (iron
overload) in survivors of childhood cancer and attributed this to
Transfusion volume. This iron overload causes tissue damage through the
chronic formation of free radicals leading to liver dysfunction.[37]
In
a study of 118 children (with standard-risk leukemia) receiving native
E. coli asparaginase or PEG-asparaginase, abnormal liver function
(grade 3/4), including elevated transaminases and hyperbilirubinemia,
was found in 8% of patients receiving native E. coli asparaginase and
in 5% of patients receiving PEG-asparaginase.[38]
There
are no clear pediatric guidelines for the management of asparaginase in
patients with hepatic toxicity, and treatment recommendations vary
across protocols. In the DCOG ALL-11 pediatric protocol, patients are
required to display aspartate aminotransferase/alanine aminotransferase
< 10×ULN and no signs of jaundice with bilirubin < 3× ULN before
starting asparaginase treatment.[39]
Patients
with hematologic malignancies were at a very high risk of HCV infection
due to the large transfusional support they often needed.[40]
The previously immunocompromised status of the leukemia survivors may
have promoted more rapid viral replication or impaired host viral
clearance and led to rapidly progressive liver disease.[41]
Moreover,
it is known that chemotherapeutic drugs (methotrexate and
6-mercaptopurine) increase the risk of liver toxicity during or soon
after cancer treatment.[13]
Study Limitations
This study had some limitations as small sample size and short duration.
Conclusions
Pediatric
ALL survivors are at increased risk of overweight/obesity, iron
overload, HCV infection and delayed hepatic complications.
References
- Shalaby R, Ashaat N, El-Wahab N, El-Hamid M,
El-Wakeel S. Bcl-2 expression and chromosomal abnormalities in
childhood acute lymphoblastic leukemia. Academic Journal of Cancer
Research 2010; 3(2):34-43.

- Ibrahim
AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer Incidence in
Egypt: Results of the National Population-Based Cancer Registry
Program. J Cancer Epidemiol. 2014; 2014: 437971.
doi:10.1155/2014/437971. https://doi.org/10.1155/2014/437971

- Tantawy
AA, El-Rashidy FH, Ragab IA, Ramadan OA, El-Gaafary MM. Outcome of
childhood acute Lymphoblastic leukemia in Egyptian children: a
challenge for limited health resource countries. Hematology. 2013;
18(4):204-210. https://doi.org/10.1179/1607845412Y.0000000061 PMid:23394310

- Hudson
MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al.
Clinical ascertainment of health outcomes among adults treated for
childhood cancer. JAMA 2013; 309, 2371-81. https://doi.org/10.1001/jama.2013.6296 PMid:23757085 PMCid:PMC3771083

- Zhang
F, Liu S, Chung M, Kelly M. Growth patterns during and after treatment
in patients with pediatric ALL: A meta-analysis. Pediatr Blood Cancer.
2015;62(8):1452-1460 https://doi.org/10.1002/pbc.25519 PMid:25808413 PMCid:PMC4482769

- Garmey
EG, Liu Q, Sklar CA, Meacham LR, Mertens AC, Stovall MA, et al.
Longitudinal changes in obesity and body mass index among adult
survivors of childhood acute lymphoblastic leukemia: A report from the
Childhood Cancer Survivor Study. J Clin Oncol. 2008; 26:4639-45. https://doi.org/10.1200/JCO.2008.16.3527 PMid:18824710 PMCid:PMC2653124

- Oeffinger
KC. Are survivors of acute lymphoblastic leukemia (ALL) at increased
risk of cardiovascular disease? Pediatr Blood Cancer. 2008; 50(2
Suppl):462-7; discussion 468. [PubMed: 18064658]. https://doi.org/10.1002/pbc.21410 PMid:18064658

- Oeffinger
KC, Mertens AC, Sklar CA, Yutaka Yasui, Thomas Fears, Marilyn Stovall,
et al. Obesity in adult survivors of childhood acute lymphoblastic
leukemia: A report from the Childhood Cancer Survivor Study. J Clin
Oncol 2003; 21(7):1359-65. https://doi.org/10.1200/JCO.2003.06.131 PMid:12663727

- Withycombe
JS, Post-White JE, Meza JL, Hawks RG, Smith LM, Nancy Sacks et al.
Weight patterns in children with higher risk ALL: a report from the
Children's Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer
2009; 53(7):1249- 54. https://doi.org/10.1002/pbc.22237 PMid:19688832 PMCid:PMC3044478

- Tonorezos
ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, Qianxing Mo, et al.
Adipokines, body fatness, and insulin resistance among survivors of
childhood leukemia. Pediatr. Blood Cancer 2012; 58: 31-6. https://doi.org/10.1002/pbc.22964 PMid:21254377 PMCid:PMC3520427

- Chow
EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and
hypertension among children after treatment for acute lymphoblastic
leukemia. Cancer 2007; 110: 2313-20. https://doi.org/10.1002/cncr.23050 PMid:17896787

- Bano
G, Chong H, Vlahos I. A new long term hepatic complication in survivors
of childhood haematological malignancy. Med Hypotheses 2012;
79(5):663-6. https://doi.org/10.1016/j.mehy.2012.08.004 PMid:22951417

- Goldsby
R, Chen Y, Raber S, Linda Li, Diefenbach K, Shnorhavorian M, et al.
Survivors of childhood cancer have increased risk of gastrointestinal
complications later in life. Gastroenterology 2011; 140: 1464-71. https://doi.org/10.1053/j.gastro.2011.01.049 PMid:21315721 PMCid:PMC3081911

- Rodriguez-Frias EA, Lee WM. Cancer chemotherapy II: atypical hepatic injuries. Clin Liver Dis. 2007; 11(3):663-76. https://doi.org/10.1016/j.cld.2007.06.012 PMid:17723925

- Castellino
S, Muir A, Shah A, Shope S, McMullen K, Ruble K, et al. Hepato-Biliary
Late Effects in Survivors of Childhood and Adolescent Cancer: A Report
from the Children's Oncology Group. Pediatr Blood Cancer 2010;
54(5):663-9. PMid:19890896 PMCid:PMC2838980

- Pui
CH, Sandlund JT, Pei D. Improved outcome for children with acute
lymphoblastic leukemia: results of Total Therapy Study XIIIB at St Jude
Children's Research Hospital. Blood 2004; 104:2690-6. https://doi.org/10.1182/blood-2004-04-1616 PMid:15251979

- Zhang
FF, Rodday AM, Kelly MJ, Must A, Macpherson C, Roberts SB, et al.
Predictors of being overweight or obese in survivors of pediatric acute
lymphoblastic leukemia (ALL). Pediatr Blood Cancer 2014; 61:1263-9. https://doi.org/10.1002/pbc.24960 PMid:24482072 PMCid:PMC4435552

- Barlow
SE. Expert committee recommendations regarding the prevention,
assessment, and treatment of child and adolescent overweight and
obesity: summary report. Pediatrics 2007; 120(Suppl 4):S164-92. https://doi.org/10.1542/peds.2007-2329C PMid:18055651

- Centers
for Disease Control and Prevention. CDC Growth Chart Training Modules:
Overweight Children and Adolescents: Recommendations to Screen, Assess
and Manage. Available at: http://www.cdc.gov/nccdphp/dnpa/growthcharts/training/modules/module3 /text/page4a.htm. Accessed: 26 February 2006.
- Tietz NW, 1995. Clinical Guide to Laboratory Tests. 3rd Edn., W.B. Saunders Co, Philadelphia, PA. PMCid:PMC228439

- Tobacco
A; Meiathini F; Moda E and Tarli P (1979): Simplified
enzymic/colorimetric serum urea nitogen determination. Clin Chem
25:336-337.

- Bowers
L and Wong E (1980): kinetic serum creatinine assays. A critical
evaluation and review. Clin Chem; 26:555-561. PMid:7020989

- Stookey L. (1970): Ferrozine – a new spectrophotometric reagent for iron. Anal chemistry 42: 779-781. https://doi.org/10.1021/ac60289a016

- Fairbanks
V and Klee G. (1987): Biochemical aspects of hematology In: Tietz NW,
ed. Fundamentals of clinical chemistry 3rd ed. Philadelphia WB
saunders. 789-824.

- Kamer
B et al. (2012): The usefulness of soluble transferrin receptor (sTfR)
in differentiating anemia occurring in young children. FOLIA
HISTOCHEMICA ET CYTOBIOLOGICA,Vol. 50: 473–479. https://doi.org/10.5603/FHC.2012.0066

- Salazar-Martinez
E, Allen B, Fernandez-Ortega C,Torres-Mejia G, Galal O, Lazcano-Ponce
E. Overweight and obesity status among adolescents from Mexico and
Egypt. Arch Med Res, 2006; 37 (4):535-42. https://doi.org/10.1016/j.arcmed.2005.10.014 PMid:16624655

- Asner
S, Ammann RA, Ozsahin H, Beck-Popovic M, von der Weid N.X. Obesity in
Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr
Blood Cancer 2008; 51:118-22. https://doi.org/10.1002/pbc.21496 PMid:18338394

- Fang
F. Zhang, Michael J. Kelly, Edward Saltzman, Aviva Must Susan B.
Roberts, Susan K. Parsons. Obesity in Pediatric ALL Survivors A
Meta-Analysis. Pediatrics 2014;133 :e704-e15.

- Murphy
AJ, White M, Elliott SA, Lockwood L, Hallahan A, Davies PSW. Body
composition of children with cancer during treatment and in
survivorship. Am J Clin Nutr. 2015 Oct; 102(4):891-6. https://doi.org/10.3945/ajcn.114.099697 PMid:26269368

- Aldhafiri
F, Al-Nasser A, Al-Sugair A, Al-Mutairi H, Young D, Reilly JJ. Obesity
and metabolic syndrome in adolescent survivors of standard risk
childhood acute lymphoblastic leukemia in Saudi Arabia Pediatr Blood
Cancer. 2012; 59 (1):133-7

- Fang
Fang Zhang, Angie Mae Rodday, Michael J. Kelly, Aviva Must, Cathy
MacPherson, Susan B. Roberts, Edward Saltzman, and Susan K. Parsons
(2014): Predictors of Being Overweight or Obese in Survivors of
Pediatric Acute Lymphoblastic Leukemia (ALL). Pediatr Blood Cancer.
2014 July ; 61(7): 1263–1269. doi:10.1002/pbc.24960. https://doi.org/10.1002/pbc.24960

- Reilly
JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect
of glucocorticoid therapy on energy intake in children treated for
acute lymphoblastic leukemia. J Clin Endocrinol Metab 2001; 86:3742-5. https://doi.org/10.1210/jcem.86.8.7764 PMid:11502805

- Warner
JT, Bell W, Webb DK, Gregory JW. Daily energy expenditure and physical
activity in survivors of childhood malignancy. Pediatr Res
1998;43:607-13 https://doi.org/10.1203/00006450-199805000-00008 PMid:9585006

- Davies
JH, Evans BAJ, Jones E, Evans WD, Jenney MEM, Gregory JW. Osteopenia,
excess adiposty and hyperleptinaemia during 2 years of treatment for
childhood acute lymphoblastic leukemia without cranial irradiation.
Clin Endocrinol 2004;60:358-65 https://doi.org/10.1111/j.1365-2265.2003.01986.x

- Mulder
RL, Kremer LC, Koot BG, Benninga MA, Knijnenburg SL, van der Pal HJ, et
al. Surveillance of hepatic late adverse effects in a large cohort of
long-term survivors of childhood cancer: prevalence and risk factors.
Eur J Cancer. 2013 Jan; 49(1):185-93. Epub 2012 Aug 15. https://doi.org/10.1016/j.ejca.2012.07.009 PMid:22901831

- Schempp
A, Lee J, Kearney S, Mulrooney DA, Smith AR. Iron Overload in Survivors
of Childhood Cancer. J Pediatr Hematol Oncol. 2016 Jan; 38(1):27-31. https://doi.org/10.1097/MPH.0000000000000444 PMid:26422286

- Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009 May; 23(3):95-104. https://doi.org/10.1016/j.blre.2008.08.001 PMid:18835072 PMCid:PMC2717717

- Dinndorf
PA, Gootenberg J, Cohen MH, et al. FDA drug approval summary:
pegaspargase (Oncaspar_) for the first-line treatment of children with
acute lymphoblastic leukemia (ALL). Oncologist 2007; 12: 991–998. https://doi.org/10.1634/theoncologist.12-8-991 PMid:17766659

- Dutch
Childhood Oncology Group. Treatment study protocol of the Dutch
Childhood Oncology Group for children and adolescents (1–19 year) with
newly diagnosed acute lymphoblastic leukemia; April 10, 2013. Accessed
December 1, 2014 from: https:// www.skion.nl/workspace/uploads/Onderzoeksprotocol-ALL11-version-4-1-april-2013.pdf

- Kebudi
R, Ayan I, Y´ilmaz G, Akící F, Görgün O, Badur S. Seroprevalence of
hepatitis B, hepatitis C and immunodeficiency virus infections in
children with cancer at diagnosis and following therapy in Turkey. Med
Pediatr Oncol.2000;34: 102-5. https://doi.org/10.1002/(sici)1096-911x(200002)34:2<102::aid-mpo5>3.0.co;2-#

- Paul
IM, Sanders J, Ruggiero F, Andrews T, Ungar D, Eyster ME. Chronic
hepatitis C virus infections in leukemia survivors: prevalence, viral
load, and severity of liver disease. Blood. 1999 Jun 1;93(11):3672-7
PMid:10339473

[TOP]














































