Giovanni Caocci, Marianna Greco and Giorgio La Nasa
Hematology Unit, Bone
Marrow Transplant Center, R. Binaghi Hospital, Department of Medical
Sciences and Public Health, University of Cagliari, Cagliari, Italy.
Corresponding
author: Giovanni Caocci. Centro
Trapianti Midollo Osseo, Ematologia, Dipartimento di Scienze Mediche.
Ospedale “R. Binaghi”. Via Is Guadazzonis, 3, 09126 Cagliari, Italy.
Tel. ++390-70-6092800, Fax. ++390-70-6092936. E-mail:
giovanni.caocci@unica.it
Published: April 19, 2017
Received: January 14, 2017
Accepted: March 18, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017032 DOI
10.4084/MJHID.2017.032
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Homing
of hematopoietic stem cells (HSC) to their microenvironment niches in
the bone marrow is a complex process with a critical role in
repopulation of the bone marrow after transplantation. This active
process allows for migration of HSC from peripheral blood and their
successful anchoring in bone marrow before proliferation. The process
of engraftment starts with the onset of proliferation and must,
therefore, be functionally dissociated from the former process. In this
overview, we analyze the characteristics of stem cells (SCs) with
particular emphasis on their plasticity and ability to find their way
home to the bone marrow. We also address the problem of graft failure
which remains a significant contributor to morbidity and mortality
after allogeneic hematopoietic stem cell transplantation (HSCT). Within
this context, we discuss non-malignant and malignant hematological
disorders treated with reduced-intensity conditioning regimens or
grafts from human leukocyte antigen (HLA)-mismatched donors.
|
Introduction
Allogeneic
hematopoietic stem cell transplantation (HSCT) currently represents one
of the best standard treatment options for a variety of malignant and
non-malignant hematological diseases. This approach is based on the
ability of donor hematopoietic stem cells (HSC) to localize to
recipient bone marrow (BM) niches. Notably, only a small percentage of
infused HSCs (10%) engraft within the marrow microenvironment. This
process, known as “Homing” is
not fully elucidated and our ability to modulate it remains incomplete.
Engraftment failure is a rare but serious complication of HSCT. In
order to gather the most robust evidence in this area, we performed a
search of the literature available in Pubmed from January 2005 to
January 2017 on "Hemopoietic stem cell homing and engraftment,"
"Hemopoietic stem cell homing and engraftment defects" and "Hemopoietic
stem cell homing and chimerism." The present review covers the most
important aspects of recent insights into the mechanisms of engraftment
and defective engrafting activity of HSCs.
Biological Properties of Stem Cells
Stem
cells (SCs) are ancestral precursors common to all cell types. They are
responsible for the generation of the tissues that form organs during
embryogenesis and from there on maintaining the capacity of
self-renewal for the entire life of the organism. The concept of stem
cells dates back to the early 1960s when Till and McCulloch analyzed
bone marrow to find out which components were responsible for in vivo blood regeneration.[1]
Ten days after transplantation of syngeneic bone marrow (BM) cells in a
murine model, they observed the growth of nodules in the animal
spleens. These nodules, defined by the authors as “spleen colonies”,
appeared in proportion to the number of injected BM cells and were
therefore thought to derive from a single BM cell.[2]
These preliminary observations made it possible to establish two main
hallmarks of HSCs, namely, their ability to renew themselves (long-term self-renewal)
and to give rise to mature cell types with characteristic morphology
and specialized functions. Before reaching a fully differentiated
adult status, SCs generate intermediate cell types called precursors or progenitor cells. These cells are partially differentiated and committed to going through numerous cycles of cell division (committed precursors) to complete their developmental pathway in adult tissues.[3]
Experiments carried out on the Drosophila fruitfly suggest two
different mechanisms by which SCs can simultaneously generate identical
copies of themselves as well as more differentiated progeny.[4] These two modes of cell division are referred to as asymmetric cell division and symmetric cell division.
The first mode is characterized by an intrinsically asymmetric
mechanism whereby only one of the two daughter cells inherit the
regulating factors necessary for self-renewal and homeostatic control
of the stem cell pool. Hence each single SC produces a copy of itself
plus a differentiated cell (differentiative division).[5-7]
In
the second symmetric mode, homeostatic control is maintained at the
population level rather than at single cell level. Two types of
symmetric division have been distinguished: a proliferative division
which results in the generation of two new stem cells and a
differentiation division which generates two differentiated cells.[8]
Several mathematical algorithms have been developed and are currently
available for the simulation of stem cell proliferation kinetics.[9]
SCs
are classified as embryonic stem cells (ESCs), embryonic germ cells
(EGCs) or adult stem cells (ACSs), depending on their origin and
different properties. The cells that can virtually produce any kind of
tissue in the body, including extra-embryonic and placental tissues,
are known as totipotent cells.
These totipotent zygote cells appear about 5-7 days after fertilization
when the fertilized egg starts to divide and produces more totipotent
stem cells. After about 4 days of cell division, these cells begin to
specialize into pluripotent cells
that can generate all embryonic tissues but not an entire
organism. That is why totipotent stem cells are considered the
most versatile among the different types of SCs.
ESCs and induced
pluripotent stem cells (iPSCs) pertain to the category of pluripotent
stem cells. When pluripotent stem cells differentiate further, multipotent cells
are formed, these cells are less plastic and more specialized and can
develop into more than one cell type but never all types of cells of an
organism or tissue. Examples of multipotent cells are HSCs and
mesenchymal stem cells (MSCs). Oligopotent stem cells
are further specialized and are destined to become specific types of
cells. There are two kinds of hematopoietic oligolineage-restricted
cells: common lymphocyte progenitors (CLPs) which are programmed to
become either T or B lymphocytes or natural killer (NK) cells and
common myeloid progenitors (CMPs) which are progenitors for
myelo-erythroid lineages. CMPs give rise to cells that include
myelomonocytic progenitors (GMPs) and megakaryocytic/erythroid
progenitors (MEPs) (Figure 1).
More recently, an impressive study has proposed a new organization of
the hematopoiesis, suggesting a readjustment in the blood hierarchy
during in utero to adulthood time points.[10] Instead
of a three-tiers model, the authors propose a two-tiers scheme in adult
bone marrow: a top-tier which contains multipotent cells such as HSCs
and multipotent progenitors, and a bottom-tier composed of committed
unipotent progenitors (Figure 2).[10] Although often somewhat neglected by researchers in the past, unipotent stem cells are
unique in their ability to differentiate along only one cell lineage.
These cells are found in adult tissues and comparison to other stem
cells have the lowest differentiation potential.[11]
The potential difference between ESCs and ASCs can be summed up as
follows: the former are more versatile whereas the latter are
undifferentiated cells that are present in the differentiated tissue,
capable of replacing cells that have died or lost function. ASCs have
been identified in many different tissues including hematopoietic
(blood), epidermal, muscle, neural, mesenchymal, endothelial and
gastrointestinal tissues.
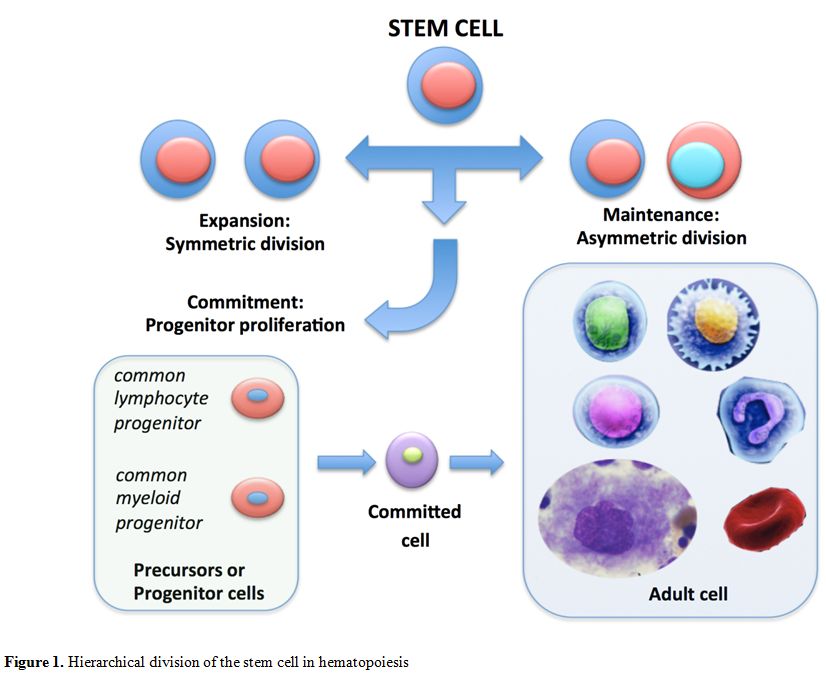 |
Figure 1. Hierarchical division of the stem cell in hematopoiesis |
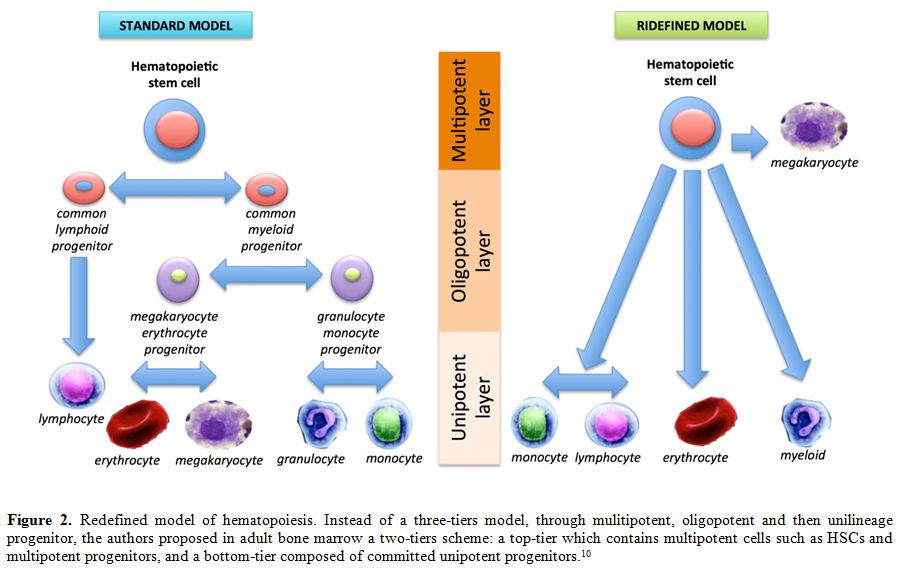 |
Figure 2. Redefined model of
hematopoiesis. Instead of a three-tiers model, through mulitipotent,
oligopotent and then unilineage progenitor, the authors proposed in
adult bone marrow a two-tiers scheme: a top-tier which contains
multipotent cells such as HSCs and multipotent progenitors, and a
bottom-tier composed of committed unipotent progenitors.[10] |
Most
of the tissue-specific ASCs persist for prolonged periods of time in G0
phase of cell cycle. This quiescent state of ASCs is also referred to
as homeostasis. Differences
in the expression of particular genes and transcription factors
determine the transaction from the quiescent state to an active phase
of the cell cycle, depending on the organism’s needs.[4] Thanks to the
presence of telomeres, the stem cell pool maintains longevity and
genomic stability and is protected against damage to DNA. Telomeres are
specialized repeat structures of TTAGGG and nucleoprotein complexes
localized at the ends of human chromosomes. These repetitive DNA
sequences at both ends of the chromosome protect cells from progressive
DNA shortening and degradation during each repeated cell division.[12,13]
The fate of HSCs is also strongly influenced by the BM microenvironment. This microenvironment is composed of specialized microanatomical areas called niches.
Numerous studies have shown that interactions between HSCs and their
non-stem cell neighbors in the niche are critical to the maintenance of
the stem cell pool in the quiescent state or promoting its self-renewal
and proliferation.[14] However, this complex network of signals that occurs in the niche is far from being fully elucidated.
Bone Marrow Homing
Regenerative
or gene HSC-based therapy is an interesting emerging field with a huge
potential for the cure of numerous congenital and acquired diseases.
There has been a rapid surge in clinical trials involving HSC therapies
over the last decade. These trials continue to demonstrate the
importance of stem cells both in replacing damaged tissue and in
providing extracellular factors capable of promoting endogenous
cellular salvage and replenishment.[15-18]
A
key feature of treatment with HSC is represented by their ability, once
introduced into the bloodstream to reach their final destination in a
distant target tissue. This intrinsic property is known as homing.
Homing is a crucial step toward successful engraftment after HSC
transplantation. It was first described several years ago as an active
process that allows for migration of HSCs through the blood and
vascular endothelium to different organs and BM niches. Nevertheless,
the full comprehension of this mechanism with its myriad of complex
molecular events remains a challenge. Homing is a process that relies
on intracellular signaling and interaction between chemokines,
chemokine receptors, adhesion molecules, and proteases, all of which
promote HSC adhesion to microvessels. E-endothelial and P-endothelial
selectin were found to be essential to cell movement (cell rolling) on BM microvessels (Figure 3).
The intimate contact with chemo-attractants promotes the expression of
HSC integrins, and through interactions with several members of the Ig
superfamily leads to the cell arrest on the endothelial surface.
Another important role in HSC homing has been assigned to intercellular
adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1
(VCAM-1). These two molecules have been shown to act as key
factors in cell trafficking between blood and BM.[19,20] Also α4β1 integrin and lectins would seem to have a primary function in HSC attachment to marrow stromal cells.[19]
Several studies have reported that α4β1/ligand interaction contributes
to cellular tethering and rolling. Additionally, it has been shown
that the homing ability of normal donor cells decreases after treatment
with anti-α4β1.[21-23] Further evidence suggesting the involvement of α4β1-integrin in the homing process is given in the points below.
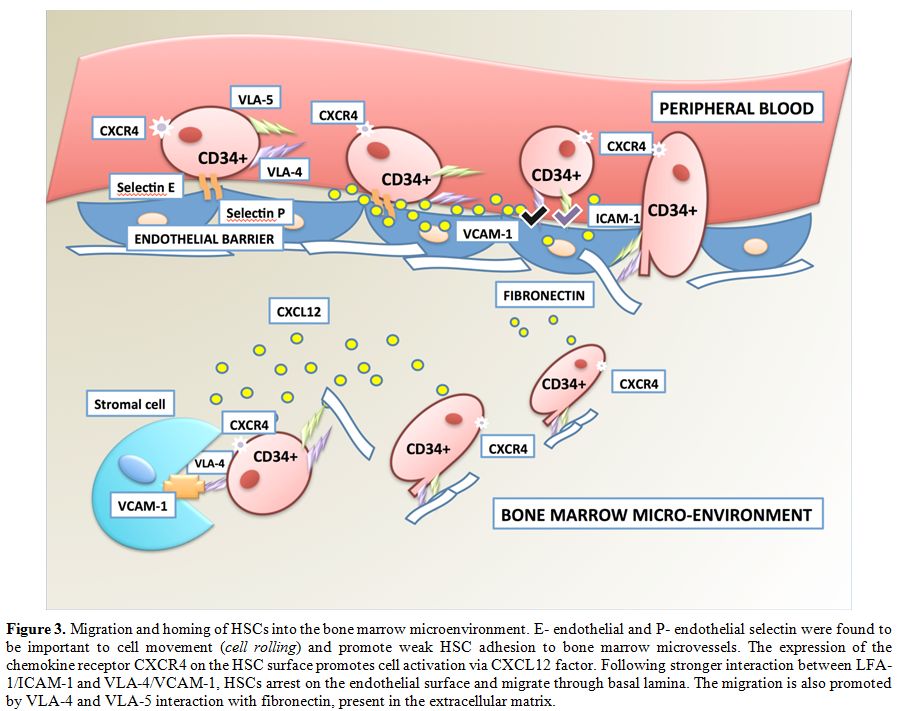 |
Figure 3. Migration and homing of HSCs
into the bone marrow microenvironment. E- endothelial and P-
endothelial selectin were found to be important to cell movement (cell
rolling) and promote weak HSC adhesion to bone marrow microvessels. The
expression of the chemokine receptor CXCR4 on the HSC surface promotes
cell activation via CXCL12 factor. Following stronger interaction
between LFA-1/ICAM-1 and VLA-4/VCAM-1, HSCs arrest on the endothelial
surface and migrate through basal lamina. The migration is also
promoted by VLA-4 and VLA-5 interaction with fibronectin, present in
the extracellular matrix. |
ì) α4β1 is widely expressed in both stem and progenitor cells, exceeding expression of both L-selectin and β2-integrin taken together;
ìì) α4β1 is constitutively active in HSC and progenitor cells;
ììì) α4β1 is usually inactive in committed cells. [24-26]
The
main ligand of α4β1 in committed cells is VCAM-1. It can, therefore, be
reasonably assumed that all functions are likely to be accomplished
through their interaction. However, homing mediated by VCAM-1 may rely
on other pathways.
Another important role in homing has been
assigned to concentration of stromal-cell-derived factor-1 (SDF-1)
ligand which increases in the BM microenvironment after conditioning
regimens for HSC transplantation (Figure 4).[27]
SDF-1 is a chemokine isolated from stromal fibroblasts, and it is
abundantly expressed by osteoblasts, endothelial cells and a subset of
reticular cells in the osteoblast and vascular niches of the bone
marrow.[28] SDF-1 is highly conserved among species
and constitutively produced in many tissues. At the basal homeostatic
concentration, SDF-1 interacts as a ligand with the G-protein coupled
receptor CXCR4, promoting HSC quiescence and survival. The expression
of the chemokine receptor CXCR4 on the HSC surface promotes migration
and homing into or from the BM.[29] Mouse embryos
knocked out for SDF-1 or CXCR4 show multiple lethal defects, as well as
the absence of BM homing by HSCs. Activation of the CXCR4 receptor by
SDF-1 is one of the transductional axes most studied in recent years
because of its fundamental importance in regulating trafficking of HSCs
to and from the BM. It has also been reported that CXCR4-depleted human
cells are insensitive to mobilization with agonists or antagonists of
the CXCR4 receptor.[30] Secretion of SDF-1 in the
bone marrow oscillates in a circadian manner. This process,
although not fully understood, also involves the activity of the
beta3-adrenergic (AdR) receptor.[31]
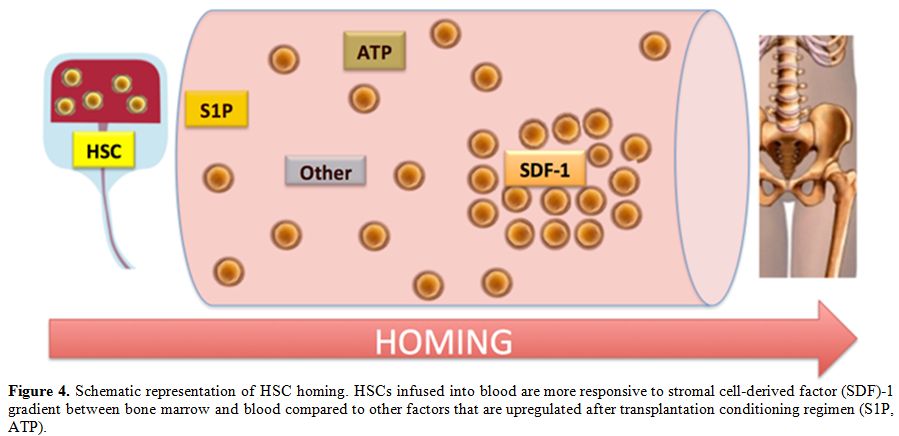 |
Figure 4. Schematic representation of HSC
homing. HSCs infused into blood are more responsive to stromal
cell-derived factor (SDF)-1 gradient between bone marrow and blood
compared to other factors that are upregulated after transplantation
conditioning regimen (S1P, ATP). |
SDF-1-CXCR4 interaction triggers chemotaxis via intracellular GTPase proteins (heterotrimeric G-proteins, typically Gαi subunits).[32]
After binding to SDF-1, CXCR4 undergoes down-modulation and
ubiquitination of the C-terminus (C-ter) by E3 ubiquitin ligase, in
this way promoting receptor degradation or its recycling via the
endosomal pathway.[33,34]
Other potential
factors involved in the homing process are the extracellular
nucleotides (eNTPs), such as adenosine triphosphate (ATP) and uridine
triphosphate (UTP), recently described as having a fundamental role in
the modulation of HSC migration in the presence of SDF-1. Since
extracellular UTP improves HSC migration toward SDF-1 gradients,
pretreatment with eUTP, it is likely to increase homing of HSCs to the
BM significantly as has been demonstrated in immunodeficient mice.[35]
The aforesaid eNTPs act through P2 nucleotide receptors (P2Rs);
particularly P2YRs. These seven transmembrane-spanning receptors, also
referred to as G-protein coupled receptors, activate their signal
transduction pathway via activation of phospholipase C or
activation/inhibition of adenylate cyclase.[36]
Although the influence of SDF-1 on HSC chemotactic responses has been well established,[37,38]
its role in the different molecular pathways underlying the early
stages of homing remains a highly discussed and contentious issue.[39,40]
Indeed, evidence has been produced of HSC homing to the BM independent
of the SDF-1–CXCR4 axis. Several observations support this
evidence. In 1999, Qing Ma and colleagues showed that
CXCR4-deficient HSCs could successfully seed BM and give rise to all
blood lineages in an SDF-1- independent manner.[41] A
study of HSC homing in a murine model made refractory to SDF-1 by
incubation and co-injection with AMD3100 (a CXCR4 receptor
antagonist) showed normal or only slightly reduced BM cellularity. In
yet another study, HSCs in which CXCR4 had been knocked down using an
SDF-1 intrakine strategy were competent to engraft. Myeloablative
conditioning for transplantation most likely induces a highly
proteolytic BM microenvironment that leads to SDF-1 proteolytic
degradation, thereby harshly sharpening its chemotactic homing
gradient.[42-44]
Adamiak and colleagues
recently confirmed the involvement of the bioactive phosphosphingolipid
sphingosine-1-phosphate (S1P) as a potent chemotactic factor for HSCs.
They performed hematopoietic transplantation in mice deficient in
BM-expressed sphingosine kinase 1 (Sphk1−/−), using HCs from normal
control mice as well as mice in which floxed CXCR4 (CXCR4fl/fl) had
been conditionally deleted. They found that homing and engraftment in
the Sphk1−/− mice was defective after transplantation of CXCR4−/− BM
cells, indicating that SIP expressed in the BM microenvironment was
involved in the homing process.
SIP levels in the BM are
regulated by a balance in activity between type 1 SP-1 kinase (Sphk1)
and S1P lyase, which has the role of degrading S1P.[45] Since 2010, it has been observed that S1P is a potent chemoattractant for HSCs, much stronger than SDF-1.[46]
It
has also been suggested that HSC homing could be improved by inhibiting
CD26 protein (DPPIV/dipeptidyl peptidase IV). Peptidase CD26 removes
dipeptides from the amino terminus of proteins, and it is has been
demonstrated that endogenous CD26 expression on donor cells
downregulates homing and engraftment. Therefore, it can be reasonably
assumed that by deleting or inhibiting CD26, it would be possible to
increase HSC transplantation efficiency.[42]
Besides
the BM microenvironment, other individual genetic factors can have an
impact on successful engraftment of HSCs. For example, HSC homing is
influenced by several molecules involved in inflammatory and other
signaling pathways of innate immune response.[47,48]
Ratajczak
and colleagues describe how innate immunity derived factors are
external modulators of the SDF-1–CXCR4 axis. Because SDF-1 is extremely
susceptible to degradation by proteolytic enzymes, its availability in
biological fluids may be somewhat limited. However, the authors
observed that at a minimum near threshold doses, SDF-1 was still able
to exert a robust chemotactic influence on engraftment. They showed
that chemotactic responsiveness of HSCs to several different types of
homing gradients could be modulated by ex vivo manipulations, using a
strategy that takes advantage of a hematopoietic stem and progenitor
cell (HSPC) -priming approach. Homing of HSPCs can be enhanced by ex
vivo cell exposure to C3a (cleavage fragments of the third protein
component of the complement cascade). A trial evaluating this procedure
is currently ongoing at the Masonic Cancer Center, University of
Minnesota.[49]
Another molecule that should be
tested in the clinical setting as a potential priming factor is
cathelicidin LL-37, a physiological factor secreted by BM stromal cells
with a more powerful priming potential than C3a.[50]
Despite
the many questions that still need to be answered, all these molecules
could support a rationale for the development of innovative strategies
aimed at improving HSC engraftment.
Hemopoietic Stem Cell Homing and Engraftment Defects
Graft
failure remains an important complication of allogeneic HSCT because of
the high morbidity and mortality associated with this event. Two
different clinical forms of defective engraftment have been
distinguished: graft failure (GF) and poor graft function (PGF), both
characterized by a primary or secondary form.[51]
Graft failure is defined as absolute neutrophil count of 0.5 x 109/L and/or platelet count of < 20 x 109/L. Primary graft failure is defined as failure to achieve absolute neutrophil count (ANC) ≥ 0.5 x 109/L for at least 3 consecutive days or ANC above 0.5 × 109/L,
without donor engraftment (autologous recovery). In secondary graft
failure, patients fail to sustain an absolute neutrophil count of ≥ 0.5
x 109/L after attainment of primary donor engraftment or fail to sustain a platelet count of ≥ 20 x 109/L,
despite neutrophil engraftment. Consequently, initial donor
engraftment with neutrophil recovery is followed by loss of the
functioning graft.
Both in primary and secondary graft failure,
chimerism may vary from a full recipient status to a mixed condition in
which donor and recipient cells coexist. Primary graft failure
following myeloablative conditioning regimens generally determines deep
and irreversible aplasia, often requiring re-transplantation. In
secondary graft failure, autologous recovery is common, particularly
after HSCT with reduced intensity conditioning (RIC); however, residual
pancytopenia and bone marrow hypocellularity may persist.[52]
From
a pathogenetic viewpoint, graft failure is determined by the
alloreactive immune responses of residual host immune effector cells
that survive the conditioning regimen.[51] Although the underlying mechanisms are not entirely known,[53]
it has been shown that residual host T cells with specific anti-donor
or suppressive activity play a fundamental role, both in HLA matched
and mismatched settings. Also, recipient natural killer (NK) cells are
involved in the pathogenetic pathways leading to graft failure. Their
cytotoxic activity against donor HSCs has been attributed to the
inability of inhibitory killer immunoglobulin-like receptors (KIRs) on
the NK cell surface to recognize HLA class I molecules expressed on
donor cells.[54] On the contrary, donor regulatory T
cells (Tregs and Tr1) and mesenchymal stem cells (MSC) seem to
facilitate engraftment and cco-transplantation of these cells with HSCs
appears to have the potential to reduce the risk of graft failure.[55-56] Donor-specific
HLA antibodies have also been found associated with an increased risk
of graft failure, mainly in HLA-mismatched and haploidentical
transplantation.[57-58]
Overall, the incidence
of graft failure has been reported to be between 3 and 15%, in relation
to the different sources of HSCs and transplant regimens.[51,52,59-62]
Several variables have been investigated as potential risk factors
associated with primary or secondary graft failure. In a large
retrospective study of 967 patients suffering from hematological
malignant and non-malignant disorders, the parameters increasing the
risk of graft failure were T-cell depletion, HLA-mismatched grafts,
non-malignant disorders and reduced-intensity conditioning. Conversely,
a total nucleated cell dose of ≥ 2.5 x 108/kg
conferred a reduced risk. Furthermore, primary or secondary graft
failure was associated with lower survival rates in malignant than in
non-malignant disorders.[61] Recent data,
retrospectively collected from 4684 consecutive patients who underwent
unrelated donor HSCT from 2006 to 2012, showed in univariate analysis
that only the type and status of disease at the time of transplantation
(complete remission versus no complete remission) were significant risk
factors for graft failure.[62]
Over the past
years, umbilical cord blood (UCB) has increasingly been used as a
source of HSCs for allogeneic transplantation. Compared to marrow
or mobilized peripheral blood stem cell grafts from adult donors,
significant delays in neutrophil and platelet engraftment have been
observed. Equally important limitations of this stem cell source are
poor immune reconstitution and an increased risk of graft failure, at
least partly due to defects in the homing capacity of these
cells. Poor homing of UCB cells has been associated with low
levels of fucosylation of cell surface molecules that are responsible
for binding to P- and E-selectins expressed in the BM microenvironment.[60]
Other factors linked to graft failure are low stem cell dose, major AB0
incompatibility, female donor grafts for male recipients and
myeloproliferative disease.[51]
Poor graft
function (PGF) is characterized by the presence of an initial full
donor engraftment. In the primary form, bone marrow cellularity
remains low, and patients present persistent cytopenias.[51]
In the secondary form, a prompt recovery is followed by a progressive
decrease in blood counts. This defect has an incidence after HSC
transplantation ranging between 5 to 25%.[63] Several
factors have been reported to be associated with PGF, but the most
relevant condition is represented by graft versus host disease (GVHD).[64]
A chronic inflammatory status, with overexpression of cytokines such as
tumor necrosis factor alfa (TNF-α) and interferon gamma (IFN-γ), may
lead to a decrease in HSC renewal and proliferation and thus determine
peripheral cytopenias.[65,66]
Mixed chimerism
(MC) after HSCT is an immunological condition characterized by the
simultaneous presence of different proportions of both donor- and
host-derived cells. This condition can be transient and evolve in the
direction of graft failure or complete chimerism (CC), or persist for
an extended period. Polymerase chain reaction (PCR) based on the
amplification of variable number tandem repeats (VNTRs) or short tandem
repeats (STRs) is currently the most common technique used to monitor
this condition.[67] In malignant hematological
disorders, MC anticipates secondary graft failure and
relapse. Therefore, early detection of this condition is essential
to ensure therapeutic interventions capable of reinforcing the graft,
such as donor lymphocyte infusion (DLI).[68]
Achievement
of persistent MC in patients transplanted for a chronic non-malignant
disease like thalassemia or sickle cell disease may lead to tolerance
of donor cells toward host tissues with no further need for
immunosuppressive therapy. Moreover, residual donor hematopoiesis may
be sufficient to eliminate transfusion dependency.[69-71] After
transplantation for thalassemia, MC occurs within the first 100 days
with an overall incidence ranging from 30% to 45%. This condition may
be stable or evolve to CC or rejection (secondary graft failure). Three
levels of MC have been established in thalassemia with different risk
categories for progression to rejection: 1) grade 1, residual host
cells <10%, rejection rates of 3-12%; 2) grade 2, residual host
cells ranging between 10 - 25%, rejection rates of 10-50%; 3) grade 3,
> 25% residual host cells, rejection rates of 50-90%.[69]
Variables reported to be associated with MC in thalassemia are
conditioning regimens, the dose of infused HSCs and the severity of
patient clinical conditions before transplantation.[70]
In recent years, it has been observed that induction of MC is an
effective way of inducing tolerance and sustained graft function.
Reprogramming of the immune system of the recipient to deliberately
establish MC has been investigated in the solid organ transplant
setting with the aim of improving the outcome and overall survival
rates.[71]
Conclusions
Homing
is a fascinating mechanism that allows HSCs to reach the BM
microenvironment, engraft and proliferate. This property has been
exploited both in auto and allo HSC transplant settings and is
currently attracting considerable attention in the field of gene and
regenerative therapy. Increasing advances in gene delivery techniques
have led to a surge of clinical trials over the past decade. The
possibility of using HSCs as possible carriers of modified genes using
viral vector delivery approaches is rapidly evolving. Gene therapy with
HSCs has an enormous potential, and different clinical trials have
resulted in functional cures for several inherited diseases.[72] New insights on how transplanted HSCs can reach the BM and which factors influence the homing process are thus critical.
Graft
failure continues to be a major contributor to morbidity and mortality
after allogeneic HSCT in patients with malignant and non-malignant
diseases, particularly when treated with reduced-intensity conditioning
regimens or grafts from HLA-mismatched donors. Such cases require close
surveillance and regular monitoring of chimerism. On the other hand,
deliberate induction of mixed chimerism by modulating the host immune
system could represent an attractive way to improve graft survival in
the future.
References
- Becker AJ, Mc CE, Till JE. Cytological
demonstration of the clonal nature of spleen colonies derived from
transplanted mouse marrow cells. Nature 1963;197:452-454. https://doi.org/10.1038/197452a0 PMid:13970094

- Till
JE, McCullough EA. A direct measurement of the radiation sensitivity of
normal mouse bone marrow cells. Radiat Res. 1961;14:213-222. https://doi.org/10.2307/3570892

- Robey PG. Stem cells near the century mark. J Clin Invest. 2000;105:1489-1491. https://doi.org/10.1172/JCI10256 PMid:10841501 PMCid:PMC300867

- Horvitz
HR, Herskowitz I. Mechanisms of asymmetric cell division: two Bs or not
two Bs, that is the question. Cell. 1992;68:237-255. https://doi.org/10.1016/0092-8674(92)90468-R

- Ho AD. Kinetics and symmetry of divisions of hematopoietic stem cells. Exp Hematol. 2005;33:1-8. https://doi.org/10.1016/j.exphem.2004.09.004 PMid:15661392

- Zhong W, Chia W. Neurogenesis and asymmetric cell division. Curr Opin Neurobiol. 2008;18:4-11. https://doi.org/10.1016/j.conb.2008.05.002 PMid:18513950

- Guilak
F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem
cell fate by physical interactions with the extracellular matrix. Cell
Stem Cell. 2009;5:17-26. https://doi.org/10.1016/j.stem.2009.06.016 PMid:19570510 PMCid:PMC2768283

- Zhang
YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal
and differentiation phases in the niche of infrequently dividing hair
follicle stem cells. Cell Stem Cell. 2009;5:267-278. https://doi.org/10.1016/j.stem.2009.06.004 PMid:19664980 PMCid:PMC2756832

- Mancuso
L, Liuzzo MI, Fadda S, Pisu M, Cincotti A, Arras M, et al. Experimental
analysis and modelling of in vitro proliferation of mesenchymal stem
cells. Cell Prolif. 2009;42:602-616. https://doi.org/10.1111/j.1365-2184.2009.00626.x PMid:19614674

- Notta
F, Zandi S, Takayama N, Dobson S, Gan OI, Wilson G, et al. Science.
Distinct routes of lineage development reshape the human blood
hierarchy across ontogeny. Science. 2016;351(6269) https://doi.org/10.1126/science.aab2116 PMid:26541609 PMCid:PMC4816201

- National
Institutes of Health (US) [NIH]. Stem Cells: Scientific Progress and
Future Research Directions. Bethesda (MD): NIH; 2001 Jun.

- Palm W, De Lasciencenge T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301-334. https://doi.org/10.1146/annurev.genet.41.110306.130350 PMid:18680434

- Flores I, Blasco MA. The role of telomeres and telomerase in stem cell aging. FEBS Lett. 2010;584:3826-30. https://doi.org/10.1016/j.febslet.2010.07.042 PMid:20674573

- He N, Zhang L, Cui J, Li Z. Bone marrow vascular niche: home for hematopoietic stem cells. Bone Marrow Res. 2014;2014:128436. https://doi.org/10.1155/2014/128436 PMid:24822129 PMCid:PMC4009113

- Nagree
MS, López-Vásquez L, Medin JA. Towards in vivo amplification:
Overcoming hurdles in the use of hematopoietic stem cells in
transplantation and gene therapy. World J Stem Cells. 2015;26:1233-1250.

- Vanhee
S, Vandekerckhove B. Pluripotent stem cell based gene therapy for
hematological diseases. Crit Rev Oncol Hematol. 2016;97:238-46. https://doi.org/10.1016/j.critrevonc.2015.08.022 PMid:26381313

- Nelson MH, Paulos CM. Novel immunotherapies for hematologic malignancies. Immunol Rev. 2015;263:90-105. https://doi.org/10.1111/imr.12245 PMid:25510273 PMCid:PMC4277117

- Powers
JM, Trobridge GD. Identification of Hematopoietic Stem Cell Engraftment
Genes in Gene Therapy Studies. J Stem Cell Res Ther. 2013. Suppl 3.

- Frenette
PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial
selectins and vascular cell adhesion molecule-1 promote hematopoietic
progenitor homing to bone marrow. Proc Natl Acad Sci U S A.
1998;95:14423-8. https://doi.org/10.1073/pnas.95.24.14423 PMid:9826716 PMCid:PMC24389

- Mazo
IB, Gutierrez-Ramos JC, Frenette PS, Hynes RO, Wagner DD, von Andrian
UH. Hematopoietic progenitor cell rolling in bone marrow microvessels:
parallel contributions by endothelial selectins and vascular cell
adhesion molecule 1. J Exp Med 1998;188:465-474. https://doi.org/10.1084/jem.188.3.465 PMid:9687524 PMCid:PMC2212463

- Alon
R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin
VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol.
1995;128:1243-1253. https://doi.org/10.1083/jcb.128.6.1243 PMid:7534768

- Ibbotson
GC, Doig C, Kaur J, Gill V, Ostrovsky L, Fairhead T, et al. Functional
alpha-4-integrin: a newly identified pathway of neutrophil recruitment
in critically ill septic patients. Nat Med 2001;7:465-470. https://doi.org/10.1038/86539 PMid:11283674

- Papayannopoulou
T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1
adhesion pathway defines contrasting mechanisms of lodgement of
transplanted murine hemopoietic progenitors between bone marrow and
spleen. Proc Natl Acad Sci USA. 1995;92:9647-9651. https://doi.org/10.1073/pnas.92.21.9647 PMid:7568190 PMCid:PMC40859

- Papayannopoulou
T, Brice M. Integrin expression profiles during erythroid
differentiation. Blood. 1992;79:1686-1694. PMid:1348432

- Ogawa
M, Kizumoto M, Nishikawa S, Fujimoto T, Kodama H, Nishikawa SI.
Expression of a4-integrin defines the earliest precursor of
hematopoietic cell lineage diverged from endothelial cells. Blood.
1999;93:1168-1177. PMid:9949159

- Wang
MWJ, Consoli U, Lane CM. Rescue from apoptosis in early (CD34-selected)
versus late (non-CD34-selected) human hematopoietic cells by very late
antigen 4- and vascular cell adhesion molecule (VCAM) 1-dependent
adhesion to bone marrow stromal cells. Cell Growth Differ.
1998;9:105-112. PMid:9486846

- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106: 1901-1910. https://doi.org/10.1182/blood-2005-04-1417 PMid:15890683

- Sugiyama
T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem
cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal
cell niches. Immunity. 2006;25:977-988. https://doi.org/10.1016/j.immuni.2006.10.016 PMid:17174120

- Asri
A, Sabour J, Atashi A, Soleimani M. Homing in hematopoietic stem cells:
focus on regulatory role of CXCR7 on SDF1a/CXCR4 axis. EXCLI J.
2016;15:134-4. PMid:27092040 PMCid:PMC4827072

- Liles
WC, Broxmeyer HE, Rodger E, Wood B, Hübel K, Cooper S, et al.
Mobilization of hematopoietic progenitor cells in healthy volunteers by
AMD3100, aCXCR4 antagonist. Blood. 2003;102:2728-2730. https://doi.org/10.1182/blood-2003-02-0663 PMid:12855591

- Spiegel
A, Kalinkovich A, Shivtiel S, Kollet O, Lapidot T. Stem cell regulation
via dynamic interactions of the nervous and immune systems with the
microenvironment. Cell Stem Cell. 2008;3:484-492. https://doi.org/10.1016/j.stem.2008.10.006 PMid:18983964

- Papayannopoulou
T, Priestley GV, Bonig H, Nakamoto B. The role of G-protein signaling
in hematopoietic stem/progenitor cell mobilization. Blood.
2003;101:4739-4747. https://doi.org/10.1182/blood-2002-09-2741 PMid:12595315

- Marchese
A, Benovic JL. Agonist-promoted ubiquitination of the G proteincoupled
receptor CXCR4 mediates lysosomal sorting. J Biol Chem.
2001;276:45509-45512. https://doi.org/10.1074/jbc.C100527200 PMid:11641392

- Marchese
A, Raiborg C, Santini F, Keen JH, Stenmark H, Benovic JL. The E3
ubiquitin ligase AIP4 mediates ubiquitination and sorting of the G
proteincoupled receptor CXCR4. Dev Cell. 2003;5:709722. https://doi.org/10.1016/S1534-5807(03)00321-6

- Rossi
L1, Manfredini R, Bertolini F, Ferrari D, Fogli M, Zini R, et al. The
extracellular nucleotide UTP is a potent inducer of hematopoietic stem
cell migration. Blood. 2007;109:533-542. https://doi.org/10.1182/blood-2006-01-035634 PMid:17008551

- Di
Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al.
Nucleotide receptors: an emerging family of regulatory mol- ecules in
blood cells. Blood 2001;97:587-600. https://doi.org/10.1182/blood.V97.3.587 PMid:11157473

- Imai
K, Kobayashi M, Wang J, Ohiro Y, Hamada J, Cho Y, et al. Selective
transendothelial migration of hematopoietic progenitor cells: a role in
homing of progenitor cells. Blood 1999;93:149-156. PMid:9864156

- Jo
DY, Rafii S, Hamada T, Moore MAS. Chemotaxis of primitive hematopoietic
cells in response to stromal cell-derived factor-1. J Clin Invest.
2000;105:101-111. https://doi.org/10.1172/JCI7954 PMid:10619866 PMCid:PMC382585

- Wiesmann
A, Spangrude GJ. Marrow engraftment of hematopoietic stem and
progenitor cells is independent of Gai-coupled chemokine receptors. Exp
Hematol. 1999;27:946-955. https://doi.org/10.1016/S0301-472X(99)00029-6

- Khaldoyanidi
S, Denzel A, Zoller M. Requirement for CD44 in proliferation and homing
of hematopoietic precursors. J Leuk Biol.
1996;60:579-592. PMid:8929548

- Ma
Q, Jones D, Springer TA. The chemokine receptor CXCR4 is required for
the retention of B lineage and granulocytic precursors within the bone
marrow microenvironment. Immunity. 1999;10:463-471. https://doi.org/10.1016/S1074-7613(00)80046-1

- Christopherson
KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem
cell homing and engraftment by CD26. Science. 2004;305:1000-1003. https://doi.org/10.1126/science.1097071 PMid:15310902

- Onai
N, Zhang YY, Yoneyama H, Kitamura T, Ishikawa S, Matsushima K.
Impairment of lymphopoiesis and myelopoiesis in mice reconstituted with
bone marrow-hematopoietic progenitor cells expressing SDF-1-intrakine.
Blood. 2000;96:2074-2080. PMid:10979950

- Kim
CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, et al.
Conditioning for hematopoietic transplantation activates the complement
cascade and induces a proteolytic environment in bone marrow: a novel
role for bioactive lipids and soluble C5b-C9 as homing factors.
Leukemia. 2012;26:106-116. https://doi.org/10.1038/leu.2011.185 PMid:21769103 PMCid:PMC3197954

- Adamiak
M, Borkowska S, Wysoczynski M. Evidence for the involvement of
sphingosine-1-phosphate in the homing and engraftment of hematopoietic
stem cells to bone marrow. Oncotarget. 2015;6:18819-18828. https://doi.org/10.18632/oncotarget.4710 PMid:26299919 PMCid:PMC4662458

- Ratajczak
MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel
insight into stem cell mobilization- Plasma sphingosine-1-phosphate is
a major chemoattractant that directs the egress of hematopoietic stem
progenitor cells from the bone marrow and its level in peripheral blood
increases during mobilization due to activation of complement
cascade/membrane attack complex. Leukemia. 2010;24:976-985. https://doi.org/10.1038/leu.2010.53 PMid:20357827 PMCid:PMC2946378

- Littera
R, Orrù N, Caocci G, Sanna M, Mulargia M, Piras E, et al. Interactions
between killer immunoglobulin-like receptors and their human leucocyte
antigen Class I ligands influence the outcome of unrelated
haematopoietic stem cell transplantation for thalassaemia: a novel
predictive algorithm. Br J Haematol. 2012;156:118-128. https://doi.org/10.1111/j.13652141.2011.08923. PMid:22077388

- Orrù
S, Orrù N, Manolakos E, Littera R, Caocci G, Giorgiani G, et al.
Recipient CTLA-4*CT60-AA genotype is a prognostic factor for acute
graft-versus-host disease in hematopoietic stem cell transplantation
for thalassemia. Hum Immunol. 2012;73:282-286.https://doi.org/10.1016/j.humimm.2011.12.014 PMid:22245568 PMCid:PMC3314940

- Ratajczak
MZ, Serwin K, and Schneider G. Innate Immunity Derived Factors as
External Modulators of the CXCL12 - CXCR4 Axis and Their Role in Stem
Cell Homing and Mobilization. Theranostics. 2013;3:3-10. https://doi.org/10.7150/thno.4621 PMid:23382780 PMCid:PMC3563075

- Wu
W, Kim CH, Liu R, Kucia M, Marlicz W, Greco N, et al. The bone
marrow-expressed antimicrobial cationic peptide LL-37 enhances the
responsiveness of hematopoietic stem progenitor cells to an SDF-1
gradient and accelerates their engraftment after transplantation.
Leukemia. 2012;26:736-745. https://doi.org/10.1038/leu.2011.252 PMid:21931324 PMCid:PMC3244577

- Masouridi-Levrat
S, Simonetta F, Chalandon Y. Immunological Basis of Bone Marrow Failure
after Allogeneic Hematopoietic Stem Cell Transplantation. Front
Immunol. 2016;7:362. https://doi.org/10.3389/fimmu.2016.00362 PMid:27695456 PMCid:PMC5025429

- Remberger
M, Mattsson J, Olsson R, Ringden O. Second allogeneic hematopoietic
stem cell transplantation (HSCT): a treatment for graft failure. Clin
Transplant 2011;25: E68-E76. https://doi.org/10.1111/j.1399-0012.2010.01324.x PMid:20946467

- Or-Geva
N, Reisner Y. The evolution of T-cell depletion in haploidentical
stem-cell transplantation. Br J Haematol. 2016;172:667-84 https://doi.org/10.1111/bjh.13868 PMid:26684279

- Ruggeri
L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al.
Effectiveness of donor natural killer cell alloreactivity in mismatched
hematopoietic transplants. Science. 2002;295:2097-100. https://doi.org/10.1126/science.1068440 PMid:11896281

- Hanash
AM, Levy RB. Donor CD4+ CD25+ T cells promote engraftment and tolerance
following MHC-mismatched hematopoietic cell transplantation. Blood.
2005;105:1828-36. https://doi.org/10.1182/blood-2004-08-3213 PMid:15494429

- Kallekleiv
M, Larun L, Bruserud Ø, Hatfield KJ. Co-transplantation of multipotent
mesenchymal stromal cells in allogeneic hematopoietic stem cell
transplantation: A systematic review and meta-analysis. Cytotherapy.
2016;18:172-85. https://doi.org/10.1016/j.jcyt.2015.11.010 PMid:26794711

- Spellman
S, Bray R, Rosen-Bronson S, Haagenson M, Klein J, Flesch S, et al. The
detection of donor-directed, HLA-specific alloantibodies in recipients
of unrelated hematopoietic cell transplantation is predictive of graft
failure. Blood. 2010;115:2704-8. https://doi.org/10.1182/blood-2009-09-244525 PMid:20089963 PMCid:PMC2852369

- Yoshihara
S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and
prevention of graft failure in patients with preexisting donor-specific
HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow
Transplant. 2012;47:508-15.https://doi.org/10.1038/bmt.2011.131 PMid:21691261

- Kallinikou
K, Anjos-Afonso F, Blundell MP, Ings SJ, Watts MJ, Thrasher AJ, et al.
Engraftment defect of cytokine-cultured adult human mobilized CD34(+)
cells is related to reduced adhesion to bone marrow niche elements. Br
J Haematol. 2012;158:778-87. https://doi.org/10.1111/j.1365-2141.2012.09219.x PMid:22816563

- Popat
U, Mehta RS, Rezvani K, Fox P, Kondo K, Marin D, et al. Enforced
fucosylation of cord blood hematopoietic cells accelerates neutrophil
and platelet engraftment after transplantation. Blood.
2015;125:2885-92. https://doi.org/10.1182/blood-2015-01-607366 PMid:25778529 PMCid:PMC4424412

- Olsson
R, Remberger M, Schaffer M, Berggren D, Svahn B, Mattsson J, et al.
Graft failure in the modern era of allogeneic hematopoietic SCT. Bone
Marrow Transplant. 2013;48:537-43. https://doi.org/10.1038/bmt.2012.239 PMid:23222384

- Cluzeau
T, Lambert J, Raus N, Dessaux K, Absi L, Delbos F, et al. Risk factors
and outcome of graft failure after HLA matched and mismatched unrelated
donor hematopoietic stem cell transplantation: a study on behalf of
SFGM-TC and SFHI. Bone Marrow Transplant. 2016; 51:687-91.https://doi.org/10.1038/bmt.2015.351 PMid:26855158

- Larocca
A, Piaggio G, Podestà M, Pitto A, Bruno B, Di Grazia C, et al. Boost of
CD34+-selected peripheral blood cells without further conditioning in
patients with poor graft function following allogeneic stem cell
transplantation. Haematologica. 2006;91:935-40. PMid:16818281

- Szyska M, Na I-K. Bone marrow GvHD after allogeneic hematopoietic stem cell transplantation. Front Immunol. 2016;7:11. https://doi.org/10.3389/fimmu.2016.00118 PMid:27066008 PMCid:PMC4811960

- Rezzoug
F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, et al.
TNF-a is critical to facilitate hemopoietic stem cell engraftment and
function. J Immunol. 2008;180:49-57. https://doi.org/10.4049/jimmunol.180.1.49 PMid:18097003

- Wang H, Yang YG. The complex and central role of interferon-γ in graft-versus-host disease and graft-versus-tumor activity. Immunol Rev. 2014;258:30-44. https://doi.org/10.1111/imr.12151 PMid:24517424 PMCid:PMC4040394

- Alizadeh
M, Bernard M, Danic B et al. Quantitative assessment of hematopoietic
chimerism after bone marrow transplantation by real-time quantitative
polymerase chain reaction. Blood. 2002;99:4618-25. https://doi.org/10.1182/blood.V99.12.4618 PMid:12036896

- Kumar
AJ, Hexner EO, Frey NV et al. Pilot study of prophylactic ex vivo
costimulated donor leukocyte infusion after reduced-intensity
conditioned allogeneic stem cell transplantation. Biol Blood Marrow
Transplant. 2013; 19:1094-101. https://doi.org/10.1016/j.bbmt.2013.04.021 PMid:23635453

- Andreani
M, Testi M, Lucarelli G. Mixed chimerism in haemoglobinopathies: from
risk of graft rejection to immune tolerance. Tissue Antigens.
2014;83:137-46. https://doi.org/10.1111/tan.12313 PMid:24571472

- La
Nasa G, Argiolu F, Giardini C, Pession A, Fagioli F, Caocci G, et al.
Unrelated bone marrow transplantation for beta-thalassemia patients:
The experience of the Italian Bone Marrow Transplant Group. Ann N Y
Acad Sci. 2005;1054:186-95. https://doi.org/10.1196/annals.1345.023 PMid:16339665

- La
Nasa G, Littera R, Locatelli F, Giardini C, Ventrella A, Mulargia M, et
al. Status of donor-recipient HLA class I ligands and not the KIR
genotype is predictive for the outcome of unrelated hematopoietic stem
cell transplantation in beta-thalassemia patients. Biol Blood Marrow
Transplant. 2007; 13:1358-68. https://doi.org/10.1016/j.bbmt.2007.07.011 PMid:17950922

- Reiser
J, Zhang XY, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential
of mesenchymal stem cells in gene therapy approaches for inherited and
acquired diseases. Expert Opin Biol Ther. 2005;5: 1571-1584. https://doi.org/10.1517/14712598.5.12.1571 PMid:16318421 PMCid:PMC1371057

[TOP]











































































