Ashraf Siddig Yousif1,2* and Atif Abdelrahman Elagib3
1 Ragon
Institute of Massachusetts General Hospital, Massachusetts Institute of
Technology and Harvard University, 400 Technology Square, Cambridge
02139 USA
2 Department of Immunology and Biotechnology, Tropical Medicine Research Institute. National Centre for Research, Khartoum, Sudan
3 National Centre for Research, Ministry of Higher Education and Scientific Research, Khartoum, Sudan
Corresponding
author: Dr. Ashraf Siddig Yousif. Ragon
Institute of Massachusetts General Hospital (MGH), Massachusetts
Institute of Technology (MIT) and Harvard University, 400 Technology
Square, Cambridge 02139 USA, Tel: (617) 735-5205; FAX: (857) 268-7142;
E-mail:
ahamadelneel@mgh.harvard.edu and
ashtmri@gmail.com
Published: July 1, 2017
Received: March 26, 2017
Accepted: June 8, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017042 DOI
10.4084/MJHID.2017.042
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Haptoglobin
(Hp) is an acute phase protein that binds the free hemoglobin (Hb),
thus preventing iron loss and renal damage. Hp also has antioxidative
and immunomodulatory properties. Three Hp phenotypes have been
identified in human: Hp1-1, Hp2-1, and Hp2-2. Hp polymorphisms have
been related to susceptibility of various diseases. In this study, we
aimed to assess the possible association of Hp phenotypes polymorphism
to Schistosoma parasites infection in central Sudan. We have investigated the Hp phenotypes polymorphism distribution in the serum of 125 (93 S. mansoni, 13 S. haematobium
and 19 infected with both ‘’co-infection’’) parasitologically confirmed
infected individuals and 208 healthy individuals served as control. Hp
phenotypes have been determined by polyacrylamide gel electrophoresis
followed by benzidine staining. Our study revealed that Hp1-1
percentage frequency was significantly higher in infected individuals
than healthy control individuals 51% and 26% respectively. Our data
suggest that Hp1-1 phenotype may upsurge the susceptibility to Schistosoma parasites infection in central Sudan.
|
Introduction
Haptoglobin
(Hp) is an acute phase plasma protein with a high affinity-binding to
free hemoglobin (Hb) and subsequently responsible for its removal from
the circulation.[1,2] The Hp gene
has been identified in all mammals and in humans it has been
characterized by a genetic polymorphism leading to three phenotypes: Hp
1-1, Hp 2-1 and Hp 2-2.[3] Early studies demonstrated
that the distribution of the three phenotypes varies worldwide
depending on racial origin in particular among races and tribes in
Africa suggesting such critical point must be taken into consideration
in all Hp association studies with infection and diseases progression.[4,5]
Hp
phenotypes revealed different anti-inflammatory, immunomodulatory and
anti-oxidative properties that have clinical consequences in different
pathologies including cancer, infections and also the lifespan
expectancy.[6-9] As an immunomodulatory, Hp1-1 and
Hp2-1 have been reported to affect the T-lymphocyte functions by direct
binding to the resting and activated CD4+ and CD8+ T lymphocytes resulting in a strong suppression of induced T-cell proliferation.[10] Furthermore, Hp1-1 and Hp2-1 display strong in vitro inhibitory effect on Th2 cytokine release and subsequently promote Th1 activation over Th2 activation in vivo.[11]
Haptoglobin also acts as a potent antioxidant, oxidative damage to DNA
induced by hydrogen peroxide have been investigated among the three Hp
phenotypes and finding revealed that Hp1-1 has least DNA damage
compared to Hp2-1 and Hp2-2.[12]
Schistosomiasis
or bilharzia is an infection caused by trematodes (blood flukes) and is
widespread in Sudan, especially in the major irrigation systems of the
Gezira agriculture scheme between the Blue and White Nile Rivers.[13] Infection in Sudan mainly belongs to two species of the genus Schistosoma: S. mansoni which cause intestinal schistosomiasis and S. haematobium that causes urinary schistosomiasis.[13,14]
The proportion of the population infected with schistosomiasis is
growing in the endemic areas. The disease has very serious
socioeconomic consequence e.g. decreasing work capacity, restricting
marriage and occupational mobility.[15] Some studies in Sudan revealed the association of Hp phenotypes polymorphism with infectious and non-infectious diseases.[16-18]
Therefore, in this study, we investigated the possible association of
Hp phenotypes and susceptibility to Schistosoma parasites infection
acquisition in central Sudan. Our finding suggests that the individuals
with Hp1-1 are at higher risk of attaining the Schistosoma infection
compared to individuals with other Hp phenotypes.
Materials and Methods
Study area, population and samples collection.
This study was conducted in two permanent agricultural camps in Gezira
irrigated scheme, central Sudan which is endemic with both S. mansoni and to less extent S. haematobium
parasitic infection. The camps are without water supply systems, and
their main source of water is the canal. The inhabitants of these camps
are originally from western Sudan. The majorities of the populations in
the camps are agricultural field laborers and were equally exposed to
schistosome infection. After obtaining a written informed consent
from all participants, the standard microscopic parasitological
examination was performed to detect the Schistosome eggs in stool and
urine samples which confirm the infection and the type of Schistosoma parasite infection.[19] Blood was collected from 125 infected individuals (93 S. mansoni, 13 S. haematobium and
19 infected with both ‘’co-infection’’) and 208 healthy individuals
served as control. Serum was separated and obtained from all blood
samples by centrifugation at 2000 rpm for 15 min and stored at -70°C.
Identification of Haptoglobin (Hp) phenotypes.
Haptoglobin (Hp) phenotypes were separated in discontinuous
polyacrylamide gel electrophoresis (non-reducing) according to Davis
and Orenstein[20] method and modified by Linke[21]
and was applied using the Mini-V 8.10 system (BRL, Life Technologies
Inc, Gaithersburg, USA). In brief, 10 μl of serum was mixed with 4 μl
of erythrocyte hemolysate which contains free hemoglobin (Hb) and 5 μl
loading buffer, then 10 μl
from each prepared mixture was added to each well of 4.7%
polyacrylamide gel. After completion of the run, the gel was stained
for 10-15 min with benzidine
stain.
Statistical analysis.
Statistical significance was assessed by Chi-square test to determine
the association of Hp phenotypes distribution among infected
individuals and healthy control individuals. Statistical analysis and
charts preparation were performed using Graphpad Prism version 5.0
(GraphPad Software Inc.).
Results and Discussion
Disparities
in infection acquisition among individuals to prevailing endemic
pathogens obviously demonstrate the significance of the host genetic
variability to pathogens vulnerability.[22,23] Since
the identification of haptoglobin (Hp) molecular heterogeneity in
humans, many reports have associated the Hp phenotypes polymorphism to
susceptibility and progression of various diseases such as cancer,
diabetes mellitus, liver disorders and infections including malaria,
Chagas disease, and HIV.[6,24-28] In this study, we have aimed to assess the possible association of Hp phenotypes polymorphism to the susceptibility to Schistosoma
parasites infection acquisition in central Sudan, which is an endemic
area of schistosomiasis. Serum was collected from 125 parasitologically
confirmed infected individuals either with S. mansoni (93), S. haematobium
(13) or with co-infection (19) and 208 healthy individuals served as
control. Hp phenotypes determined by separation by polyacrylamide gel
electrophoresis followed by benzidine staining (Figure 1). As described previously by Langlois and Delanghe,[3]
Hp phenotypes have very well distinctive patterns in complex with the
Hb. Hp1-1 appeared as one band with low molecular weight, Hp2-1 has
multiple bands with high molecular weight in addition to the Hp1-1 band
while Hp2-2 has only the multiple bands with high molecular weight but
no Hp1-1 band (Figure 1). Among
overall infected individuals, we found that the Hp1-1 phenotype was
higher and significantly distributed among infected individuals
compared to healthy control, 51% to 26% respectively (Figure 2). However Hp2-1 phenotype was less frequent in the infected individuals compared to healthy control, 37%, 59% respectively (Figure 2).
Overall, the Hp2-2 phenotype was the least distributed phenotype in
both infected and healthy individuals with no apparent difference among
infected individuals and healthy control (12%, 18% respectively) (Figure 2). Moreover, we have analyzed the Hp phenotypes distribution among individuals infected either with S. mansoni, S. haematobium or both “Co-infection” separately (Figure 3). Comparing Hp phenotypes patterns in the overall Schistosoma-infected
individuals and healthy controls we found that the Hp1-1 phenotype is
more frequent in each infected group irrespective to the type of
infection suggesting that individuals with Hp1-1 phenotype may at
higher risk of Schistosoma parasites infection acquisition compared to those carrying Hp2-1 or Hp2-2.
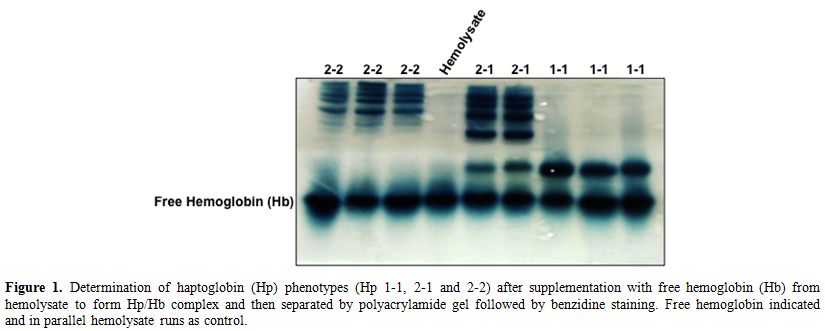 |
Figure 1.
Determination of haptoglobin (Hp) phenotypes (Hp 1-1, 2-1 and
2-2) after supplementation with free hemoglobin (Hb) from hemolysate to
form Hp/Hb complex and then separated by polyacrylamide gel followed by
benzidine staining. Free hemoglobin indicated and in parallel
hemolysate runs as control. |
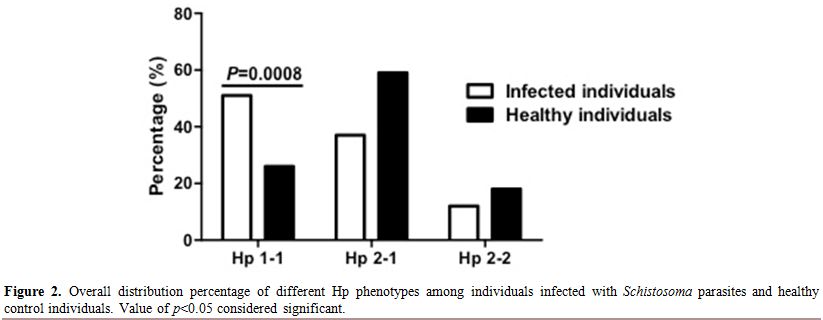 |
Figure 2. Overall distribution percentage of different Hp phenotypes among individuals infected with Schistosoma parasites and healthy control individuals. Value of p<0.05 considered significant. |
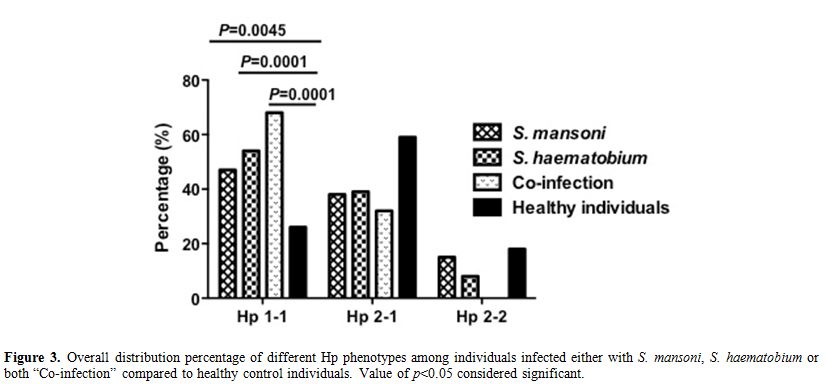 |
Figure 3. Overall distribution percentage of different Hp phenotypes among individuals infected either with S. mansoni, S. haematobium or both “Co-infection” compared to healthy control individuals. Value of p<0.05 considered significant. |
It
has been demonstrated that Hp 1-1 possess differentially and extremely
greater antioxidant activity than Hp 2-1 and 2-2 which may reflect in
clinical implications in various diseases.[29] Indeed
Hp1-1 have been associated with malaria infection in some African
countries such as Ghana, Cameroon, and Sudan as well.[18,28,30]
Some early studies in Sudan also reported that Hp1-1 phenotype is
associated with liver disorders including hepatitis B virus (HBV).[16,17] Interestingly a significant increase of Hp concentration following the Schistosoma mansoni cercariae infection in both mice and baboons have been observed and linked to schistosomiasis infection and pathogenesis.[31-33] Our current study findings attest the involvement of Hp in schistosoma infection in particular Hp1-1. Intriguingly Cook and his colleagues found that Schistosoma
adult worm expresses antioxidant enzymes to neutralize the effects of
reactive oxygen and nitrogen species as a mechanism in evading immune
killing.[34] Moreover, Hp1-1 and Hp2-1 playing a
critical role in modulating the Th1/Th2 balance and promoting a
dominant Th1 cellular response over Th2.[10,11]
Th1/Th2 balancing is crucial to fight and combat schistosoma infection
by the host as after the penetration of cercariae, and before the onset
of egg deposition the immune response is primarily of Th1 type
(promoting cellular immunity) and directed against worm antigens. At
the onset of egg production, the immune response switches to Th2 type,
promoting an antibody response directed preferentially against highly
immunogenic egg glycans.[35]
Collectively we
suggest that the potent antioxidative activity of the Hp1-1 phenotype
may neutralize the effects of reactive oxygen and nitrogen species and
therefore support the early stage of Schistosoma parasite in evading immune killing and eventually facilitated the establishment of Schistosoma
infection compared to individuals with Hp2-1 or Hp2-2 phenotype.
Suppression of Th2 response by Hp1-1 could contribute as well to
infection and disease progression.
Conclusions
Our finding demonstrates that individuals with Hp1-1 phenotype may at higher risk of Schistosoma
infection acquisition compared to individuals with Hp2-1 or Hp2-2
phenotype. More studies are needed in the future to validate the
insight mechanism of Hp1-1 in schistosoma infection.
Acknowledgements
We would like to
thank all participants for agreed to be involved in this study. Our
gratitude extended to the Department of Immunology and Biotechnology
members of TMRI. This work was supported by the National Centre for
Research (NCR), Ministry of Higher Education and Scientific Research-
Sudan.
References
- Alayash AI. Haptoglobin: Old protein with new functions. Clinica Chimica Acta. 2011; Vol. 412: 493-8. https://doi.org/10.1016/j.cca.2010.12.011 PMid:21159311

- Fagoonee
S, Gburek J, Hirsch E, Marro S, Moestrup SK, Laurberg JM, et al. Plasma
protein haptoglobin modulates renal iron loading. Am J Pathol
2005;166(4):973-83. https://doi.org/10.1016/S0002-9440(10)62319-X

- Langlois
MR, Delanghe JR. Biological and clinical significance of haptoglobin
polymorphism in humans. Clinical Chemistry 1996; Vol. 42:1589-600.
PMid:8855140

- Carter
K, Worwood M. Haptoglobin: A review of the major allele frequencies
worldwide and their association with diseases. International Journal of
Laboratory Hematology 2007; Vol. 29:92-110. https://doi.org/10.1111/j.1751-553X.2007.00898.x PMid:17474882

- Constans
J, Viau M, Gouaillard C, Clerc a. Haptoglobin polymorphism among
Saharian and West African groups. Haptoglobin phenotype determination
by radioimmunoelectrophoresis on Hp O samples. Am J Hum Genet.
1981;33(4):606-16. PMid:7258189 PMCid:PMC1685091

- Speeckaert
R, Brochez L, Lambert J, van Geel N, Speeckaert MM, Claeys L, et al.
The haptoglobin phenotype influences the risk of cutaneous squamous
cell carcinoma in kidney transplant patients. J Eur Acad Dermatol
Venereol. 2012;26(5):566-71. https://doi.org/10.1111/j.1468-3083.2011.04112.x PMid:21575065

- Kasvosve
I, Speeckaert MM, Speeckaert R, Masukume G, Delanghe JR. Haptoglobin
polymorphism and infection. Advances in clinical chemistry. 2010; Vol.
50: 23-46. https://doi.org/10.1016/S0065-2423(10)50002-7

- Mahmud
SM, Koushik A, Duarte-Franco E, Costa J, Fontes G, Bicho M, et al.
Haptoglobin phenotype and risk of cervical neoplasia: A case-control
study. Clin Chim Acta. 2007;385(1-2):67-72. https://doi.org/10.1016/j.cca.2007.06.020 PMid:17706188

- Napolioni
V, Giannì P, Carpi FM, Concetti F, Lucarini N. Haptoglobin (HP)
polymorphisms and human longevity: A cross-sectional association study
in a Central Italy population. Clin Chim Acta. 2011;412(7-8):574-7. https://doi.org/10.1016/j.cca.2010.12.006 PMid:21147083

- Sadrzadeh
SM, Bozorgmehr J. Haptoglobin phenotypes in health and disorders.
American journal of clinical pathology. 2004. Vol. 121 Suppl: S97-104. https://doi.org/10.1309/8glx5798y5xhq0vw

- Arredouani
M, Matthijs P, Van Hoeyveld E, Kasran A, Baumann H, Ceuppens JL, et al.
Haptoglobin directly affects T cells and suppresses T helper cell type
2 cytokine release. Immunology. 2003;108(2):144-51. https://doi.org/10.1046/j.1365-2567.2003.01569.x PMid:12562322 PMCid:PMC1782886

- Moreira
L, Miranda-Vilela A, Silva I, Akimoto A, Klautau-Guimarães M, Grisolia
C, et al. Antioxidant effect of haptoglobin phenotypes against DNA
damage induced by hydrogen peroxide in human leukocytes. Genet Mol Res
Mol Res. 2009;8(81):284-90. https://doi.org/10.4238/vol8-1gmr569

- Deganello,
R., Cruciani, M., Beltramello, C., Duncan, O., Oyugi, V. and Montresor
A. Schistosoma haematobium and Schistosoma mansoni among children,
Southern Sudan. Emerg Infect Dis. 2007;13:1-5.

- Elhag
SM, Abdelkareem EA, Yousif AS, Frah EA, Mohamed AB. Detection of
schistosomiasis antibodies in urine patients as a promising diagnostic
maker. Asian Pac J Trop Med. 2011;4(10):773-7. https://doi.org/10.1016/S1995-7645(11)60192-2

- Sleigh
A, Xueming Li, Jackson S, Huang K. Eradication of schistosomiasis in
Guangxi, China. Part 3. Community diagnosis of the worst-affected areas
and maintenance strategies for the future. Bull World Health Organ.
1998;76(6):581-90. PMid:10191554 PMCid:PMC2312487

- Ibrahim N, Baleela R. Association of Hp 1-1 with liver disorders among Sudanese patients. Am J Sci Ind Res. 2012;3(6):403-5. https://doi.org/10.5251/ajsir.2012.3.6.403.405

- Rania
M. H. Baleela; Nada E. Ibrahim; Omran F. Osman and Atif A. Elagib2.
Distribution of Haptoglobin Phenotypes among Patients with HIV/AIDS,
Hepatitis B, Liver Cirrhosis and Chronic Renal Failure in Sudan. Sudan
J Sci. 2012;5(1):1-6.

- Elagib
AA, Kider AO, Åkerström B, Elbashir MI. Association of the haptoglobin
phenotype (1-1) with falciparum malaria in Sudan. Trans R Soc Trop Med
Hyg. 1998;92(3):309-11. https://doi.org/10.1016/S0035-9203(98)91025-2

- Yousif
AS, Abdelkareem E a., Elhag SM, Elgimeaabi L a., Ahmed M a., Frah E a.,
et al. Circulating antigens of Schistosoma parasites in urine of
schistosomiasis patients in Central Sudan. J Infect Dis Immun.
2009;1(2):011-5.

- Davis.
I. and Orenstein. J. DISC electrophporesis, Acrylamide gel columns.
Methods in immunology and immunochemistry. 1968; Vol. 2: 38-47.

- Linke
RP. Typing and subtyping of haptoglobin from native serum using disc
gel electrophoresis in alkaline buffer: Application to routine
screening. Anal Biochem. 1984;141(1):55-61. https://doi.org/10.1016/0003-2697(84)90424-X

- Chapman SJ, Hill A V. Human genetic susceptibility to infectious disease. Nat Rev Genet. 2012;13(3):175-88. https://doi.org/10.1038/nrg3114

- Brouwer
MC, de Gans J, Heckenberg SG, Zwinderman AH, van der Poll T, van de
Beek D. Host genetic susceptibility to pneumococcal and meningococcal
disease: a systematic review and meta-analysis. The Lancet Infectious
Diseases. 2009; Vol. 9:31-44. https://doi.org/10.1016/S1473-3099(08)70261-5

- Speeckaert
R, Colebunders B, Boelaert JR, Brochez L, Van Acker J, Van Wanzeele F,
et al. Association of haptoglobin phenotypes with the development of
Kaposis sarcoma in HIV patients. Arch Dermatol Res. 2011;303:763-9. https://doi.org/10.1007/s00403-011-1161-9 PMid:21748360

- Vitalis
Z, Altorjay I, Tornai I, Palatka K, Kacska S, Palyu E, et al.
Phenotypic polymorphism of haptoglobin: A novel risk factor for the
development of infection in liver cirrhosis. Hum Immunol.
2011;72(4):348-54. https://doi.org/10.1016/j.humimm.2011.01.008 PMid:21262313

- Mundaray Fernández N, Fernández-Mestre M. The Role of haptoglobin genotypes in Chagas disease. Dis Markers. 2014;2014:1-6. https://doi.org/10.1155/2014/793646 PMid:25147423 PMCid:PMC4134794

- Perdijk
O, Arama C, Giusti P, Maiga B, Troye-Blomberg M, Dolo A, et al.
Haptoglobin phenotype prevalence and cytokine profiles during
Plasmodium falciparum infection in Dogon and Fulani ethnic groups
living in Mali. Malar J. 2013;12(1):432. https://doi.org/10.1186/1475-2875-12-432 PMid:24274254 PMCid:PMC4225596

- Quaye
IKE, Ekuban FA, Goka BQ, Adabayeri V, Kurtzhals JAL, Gyan B, et al.
Haptoglobin 1-1 is associated with susceptibility to severe Plasmodium
falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(2):216-9. https://doi.org/10.1016/S0035-9203(00)90281-5

- Tseng CF, Lin CC, Huang HY, Liu HC, Mao SJT. Antioxidant role of human haptoglobin. In: Proteomics. 2004; 2221-8. PMid:15274115

- Minang
JT, Gyan BA, Anchang JK, Troye-Blomberg M, Perlmann H, Achidi EA.
Haptoglobin phenotypes and malaria infection in pregnant women at
delivery in western Cameroon. Acta Trop. 2004;90(1):107-14. https://doi.org/10.1016/j.actatropica.2003.10.016 PMid:14739029

- Mungatana
NWK, Kariuki S, Yole DS and Ngure R. Assessment Of The Acute Phase
Response In Experimental Infection Of Mice With Schistosoma mansoni.
The Internet Journal of Tropical Medicine. 2005; Volume 3 Number 1.

- Mungatana
NWK, Ngure RM, Shitandi AA, Mungatana CK and Yole DS. Effect of
experimental Schistosomiasis mansoni infection on serum levels of iron,
zinc and copper in the olive baboon (Papio anubis). Afri. J. Biochem.
Res. 2009; Vol.3 (4):107-113.1

- Mungatana
NWK, Ngure RM and Yole DS. Acute Phase Response of Albumin and
Haptoglobin in Experimental Infection of the Olive Baboon, Papio
Anubis, with Schistosoma mansoni. Scand. J. Lab. Anim. Sci. 2007; Vol.
34 No. 2.

- Cook
RM, Carvalho-Queiroz C, Wilding G, LoVerde PT. Nucleic acid vaccination
with Schistosoma mansoni antioxidant enzyme cytosolic superoxide
dismutase and the structural protein filamin confers protection against
the adult worm stage. Infect Immun. 2004;72(10):6112-24. https://doi.org/10.1128/IAI.72.10.6112-6124.2004 PMid:15385516 PMCid:PMC517585

- Pearce
EJ, Kane CM, Sun J, Taylor JJ, McKee AS, Cervi L. Th2 response
polarization during infection with die helminth parasite Schistosoma
mansoni. Immunol Rev. 2004;201:117-26. https://doi.org/10.1111/j.0105-2896.2004.00187.x PMid:15361236

[TOP]





































