Motunrayo Oluwabukola Adekunle, Adeola Barakat Animasahun, Ijeoma Nnenna Diaku-Akinwumi and Olisamedua Fidelis Njokanma 1
Department of Paediatrics, Lagos State University Teaching Hospital, Ikeja, Lagos. Nigeria
Corresponding
author: Motunrayo Oluwabukola Adekunle. 1-5 Oba Akinjobi Lane, Ikeja, Lagos, Nogeria. Tel: +2348036000219. E-mail:
motunbamm@yahoo.com
Published: August 14, 2017
Received: March 23, 2017
Accepted: July 3, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017050 DOI
10.4084/MJHID.2017.050
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Cerebrovascular
accident (CVA) is a common, devastating neurological complication of
sickle cell disorder (SCD) with a high recurrent and mortality rate.
The Stroke Prevention Trial in Sickle Cell Anaemia study (STOP)
recommends routine screening with transcranial Doppler ultrasonography
in children aged two to sixteen years with SCD. The present study
assessed cerebral blood flow velocities of children with SCD in
accordance with the recommendation of routine screening by the STOP
study.
Methods:
Transcranial Doppler ultrasonography was done for children with SCD
that attended Sickle Cell Foundation, Nigeria between July and November
2015.
Results: In all, 388
subjects were screened within the study period (360 HbSS and 28 HbSC).
The prevalence of abnormal Time-Averaged Maximum Mean Velocity (TAMMV)
of at least 200 cm/second was 10.8%: this was seen solely in HbSS
subjects. The mean Time-averaged mean of the maximum (TAMM) velocity
were 163±25 cm/sec, 162±30 cm/sec and 150±30 cm/sec for children less
than five years, five to ten years and eleven to sixteen years
respectively.
Conclusion:
The prevalence of abnormal TAMM velocity in children with HbSS is
10.8%. Identification of subjects at risk helped in primary CVA
prevention by prompt therapy institution.
|
Introduction
Sickle cell disorder (SCD) is one of the commonest genetic diseases in the world.[1]
Of the world’s population, 5.2% carry a significant variant of sickle
cell gene of which over 70% occur in sub-Saharan Africa.[2] Nigeria has the largest burden of sickle cell disorder in Africa with a prevalence of 3% in newborns.[2]
Cerebrovascular
accident (CVA) is one of the most devastating complications of SCD that
causes high morbidity and mortality in children; approximately 11% of
children with SCD have a CVA before the age of twenty years[3] with a
recurrence rate as high as 85% with the first three years of the first
episode.[4] The reported prevalence of CVA in children with SCD in Nigeria varied between 4.3% and 6.8%,[5–7] the recurrence rate was as high as 61.5% in one of the studies.[6]
Children
at risk of CVA can be identified using transcranial Doppler
ultrasonography which enables evaluation of cerebral artery blood flow
velocity with a sensitivity of ninety percent and specificity of one
hundred percent when compared with cerebral angiography.[8]
The Stroke Prevention Trial in Sickle Cell Anaemia study (STOP)
recommends that yearly TCD screening should be done for children with
SCD between the ages of two years and sixteen years with a repeat
within three months for those children with abnormal results.[9] Following identified risk for a cerebrovascular accident in the anterior cerebral vessels in the extended STOP trial study,[10] the vessels were insonated.
Early
identification of children at risk of CVA with cerebral blood flow
velocity of at least 200 cm/second and prompt interventions help to
curtail the devastating neurological complication.[8]
Few studies have reported the prevalence of abnormal cerebral blood flow velocities in children in Nigeria. Lagunju et al.[11,12]
in Ibadan, Nigeria in two studies reported 4.7% and 8.4% results of
high risk for a CVA, 22.1% of the subjects had a conditional risk for a
CVA. Oniyangi et al.[13] at Abuja, Nigeria reported that 6.9% of subjects had abnormal cerebral blood flow velocity and 81.4% had a normal study.
The
present study aimed to determine the pattern of cerebral blood flow
velocities of children with SCD that presents at Sickle Cell Foundation
Centre, Nigeria within the studied period.
Materials and Methods
It
was a cross-sectional study carried out at the Sickle Cell Foundation
Centre, Idi -Araba Lagos between July and November 2015. It is a
non-governmental organization that receives people with SCD all over
the country. The foundation offers facilities like transcranial Doppler
ultrasonography, genetic counseling, major diagnostic and research
facilities and prenatal diagnosis of sickle cell disorder. The study
population comprised children with sickle cell disorder aged two to
sixteen years in a steady state that presented to Sickle Cell
Foundation Centre. Steady state was defined as the absence of any
crisis for at least four consecutive weeks with no history of blood
transfusion in the previous four months prior to the screening.
Children with previous CVA and on hydroxyurea were excluded from the
study.
In all, 388 subjects were recruited with sample size
calculation based on the previously reported prevalence of 8.4% by
Lagunju et al.[11] Total sample size was divided into
three age strata. 130 subjects for the age group of less than five
years, and 129 subjects each for the age group of five to ten years and
eleven to sixteen years.
Approval for the present study was
obtained from the Health Research Ethics Committee of Lagos State
University Teaching Hospital.
The study was done using a
Compumedics DWL Doppler machine (FDA K051 085) which is non-imaging.
Recruited subjects TCD evaluations were done by one of the authors
(M.O.) who had learned transcranial Doppler ultrasonography previously
at the study center by a qualified trainer that does the transcranial
Doppler ultrasonography at the center.
All recruited subjects had
the Cerebral Blood Flow Velocities measured using a 2-MHz hand held
probe attached to a Doppler box according to the Stroke Prevention in
Sickle Cell Disease protocol.[9] The velocities of
blood flow in the middle cerebral artery, internal carotid artery and
anterior cerebral arteries were measured. The highest velocity in each
artery was recorded as the Time-Averaged Maximum Mean Velocity (TAMMV).
TAMMV less than 170 cm/second was considered normal, values greater or
equal 170 centimetre per second but less than 200 cm/second were
conditional risks and velocity at least 200 cm/second was considered
abnormal. Further classification as low and high Conditional Risk
according to TAMMV of 170 to 184 cm/second and 185 to 199 cm/second
respectively was done.[9]
Results
Out
of 388 subjects that had transcranial Doppler ultrasonography done, 360
were HbSS (92.8%), and 28 (7.2%) were HbSC. Their age range is two and
sixteen years respectively while the mean age was 7.66±4.2 years. The
female to male ratio is 1:1.4.
The minimum and maximum cerebral
velocities were recorded in the left anterior cerebral velocity and
right middle cerebral velocity respectively as shown in table 1.
The
mean total TAMMV was highest in subjects below five years and lowest in
subjects above ten years with a value of 161±26 and 149±31cm/sec
respectively. Amongst the HbSS subjects, the mean TAMMV of 163±25
cm/second was highest in under-5s. The mean TAMMV values were
consistently higher in all the age strata in HbSS than in HbSC subjects.
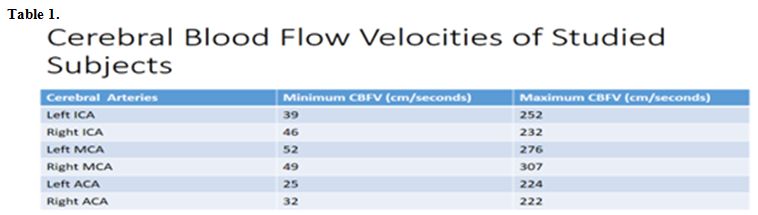 |
Table 1 |
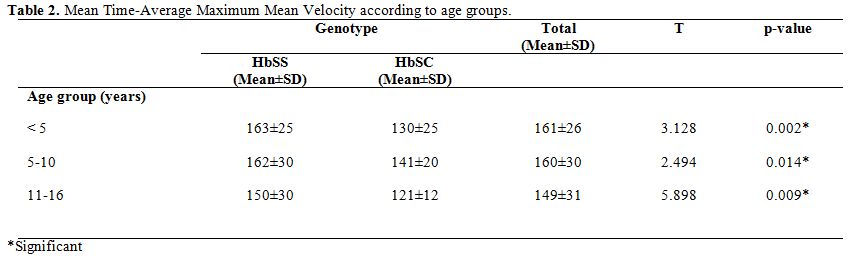 |
Table 2. Mean Time-Average Maximum Mean Velocity according to age groups. |
In Table 3,
the occurrence of abnormal TAAMV was seen only in HbSS subjects. The
prevalence of abnormal cerebral blood flow velocity was 10.8%.
Concerning HbSC subjects, all but one eight-year-old child had
Conditional Cerebral Blood Flow Velocity.
For HbSS subjects, the
frequency of normal Cerebral Blood Flow Velocity was highest in the 11
to 16-year-old age group (71.9%). The corresponding figures for younger
age groups were 59.6% for under-5s and 59.1% for those between five and
ten years. Thus, HbSS subjects in the 11 to 16-year-old age bracket had
a higher frequency of normal Cerebral Blood Flow Velocity. Subjects
within the age bracket of five and ten years had the highest prevalence
of Abnormal Cerebral Blood Flow Velocity.
Conditional velocities
were highest in subjects less than five years, intermediate in five to
ten years and lowest in subjects eleven years above. Further
classification of conditional velocities into high and low risk, eleven
of the forty subjects in children below five years had a high
conditional risk. The corresponding figure for five to ten years and
eleven to sixteen years is five and three respectively.
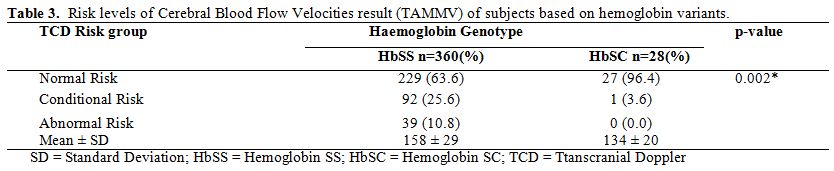 |
Table 3.
Risk levels of Cerebral Blood Flow Velocities result (TAMMV) of subjects based on hemoglobin variants. |
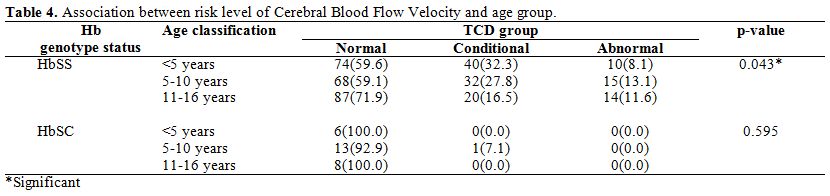 |
Table 4. Association between risk level of Cerebral Blood Flow Velocity and age group. |
Discussion
The
prevalence of abnormal Cerebral Blood Flow Velocity among HbSS subjects
in the present study was 10.8%. A similar prevalence of 10% was
reported in a retrospective study in Philadelphia by Kwiatkowski et al.[14] Adam et al.[15]
reported a similar prevalence of 9.7% in Georgia, USA. The prevalence
of abnormal Cerebral Blood Flow Velocity in the current study is
slightly higher than a prevalence of 8.4% in a Nigerian study by
Lagunju et al.[11] In another Nigerian study by Oniyangi et al.,[13]
a much lower prevalence of 6.98% Cerebral Blood Flow Velocity in
children with SCD was reported. The higher prevalence in the index
study compared to the survey by Oniyangi et al.[13]
could be as a result of differences in methodology. While the current
study used non-imaging TCD, the study by Oniyangi et al.[13]
used imaging Doppler Ultrasonography. It has been demonstrated that TCD
values from imaging studies are 10% to 15% lower than non-imaging ones.[16]
No immediate explanation can be provided for the slightly higher
prevalence of abnormal cerebral blood velocity in the present study
compared with the reported value by Lagunju et al.[11]
who also used a non-imaging Doppler machine. Studies have shown that
about 95% of Nigerians with SCD have Benin haplotype and differences in
haplotype will likely not explain the disparity. However, factors such
as coexistence of alpha thalassemia as a likely factor is not routinely
assessed in our environment, and it is beyond the scope of the current
research.[17] The high prevalence of abnormal TAMMV
in the present study underlines and supports the recommendation that
transcranial Doppler ultrasonography should be done routinely. There
have to be more efforts to make the Doppler machine more widely
available. People travel from different regions of the country to have
the screening done, and more children will benefit from routine
screening if the services are closer to them. Prevention of a primary
CVA in studied subjects was possible due to its early identification
and therapy commencement. In our region, hydroxyurea is mostly adopted
as a CVA preventive measures due to the cost of chronic blood
transfusion and management of its possible complications as well as
unavailability of blood products.[18]
The Stroke
Prevention Trial in Sickle Cell Anaemia study (STOP) was carried out in
African-American children with SCD. Values generated has been widely
used in determining the risk for a CVA as applied in the present study.
However, need for reassessment of cut-off values based on ethnicities
and haplotypes has been suggested. So studies for re-evaluating race
specific cut-off values for TAMMV are worthwhile.[19]
Younger
subjects had a higher prevalence of abnormal Cerebral Blood Flow
Velocity in the current study, similarly to the findings by Adam et al.[15] and Lagunju et al.[11]
However, there were specific differences in the age
ranges. Subjects aged five to ten years in the current study had
the highest value of abnormal velocity compared to under 5s in the
study by Lagunju et al.[11] and two peaks of two to nine years and nine to twelve years in the study by Adam et al.[15]
However, the under-5 age group in the present study had the highest
prevalence of high conditional risk. A possible explanation for the
disparity in the age with the highest prevalence could be that while
this study is cross-sectional, the study by Lagunju et al.[11]
was a longitudinal study in which the subjects had serial TCD done over
a two-year period. As noted by the authors, this allowed a number of
the subjects with conditional risk to convert to abnormal risk. Also,
the authors reported that the rate of conversion to abnormal risk was
higher among subjects with high conditional risk than those with low
conditional risk. In the current study, under-5were subjects have the
highest prevalence of high conditional risk. Thus it can be suggested
that if followed up with serial TCD this group is at significant risk
of conversion to abnormal risk. Prevalence of CVA in SCD is commonest
among children aged two to nine years, and this can account for the
finding of high abnormal velocity in this age group compared to eleven
years and above.
The mean value of Time-Averaged Maximum
Mean Velocity was lower in HbSC subjects compared to HbSS subjects. In
addition, none of the HbSC subjects had abnormal Cerebral Blood Flow
Velocity. This datum is in keeping with reported lower TCD values and
lower risk of CVA in HbSC people.[20] The explanation
for the fact that none of the HbSC subjects had abnormal velocity could
be that hemoglobin SC disorder is associated with less severe hemolysis
and the red cell life span is two times longer than HbSS. Thus they are
less prone to hemolysis related vasculopathy and consequent abnormal
TAMMV. Use of lower cut-off values of TAMMV have however been suggested
in heterozygote children with SCD.[21,22] The
occurrence of abnormal risk that was solely seen in HbSS subjects in
the current study and others implies the need of prioritizing
transcranial Doppler ultrasonography for HbSS subjects especially in
regions like Nigeria where the machine and expertise are not readily
available.
Conclusion
Prevalence
of abnormal cerebral blood flow velocity is high in Nigeria children
with SCA. There is a need for more availability of transcranial Doppler
machine for routine screening of children with SCD. This will help in
early identification of children at risk of a CVA for prompt
intervention that can avert the deadly complication.
References
- Diallo D, Tchernia G. Sickle Cell Disease in Africa. Curr Opin Haematol 2002;9:111 https://doi.org/10.1097/00062752-200203000-00005

- Odunvbun
ME, Okolo AA, Rahimy CM. Newborn screening for sickle cell disease in a
Nigerian hospital. Public Health, 2008 Oct;122(10):1111-6 https://doi.org/10.1016/j.puhe.2008.01.008 PMid:18486954.

- Ohene-Frempong
K, Weiner S, Sleeper L, Miller S, Embury S, Moohr J. Cerebrovascular
accidents in Sickle Cell Disease: Rates and Risk Factors. Blood.
1998;91(1):288-94. PMid:9414296

- Hsu
L. Specific Problems: Neurologic symptoms and strokes. Available at:
scinfo.org/problem-oriented-clinical-guidelines/specific-problems-neurologic-symptoms-and-stroke.
Accessed December 9, 2013

- George
I, Frank-Briggs A. Stroke in Nigerian Children with Sickle Cell
Anaemia. J Public Health Epidemiol. 2011;3(9):407-9..

- Fatunde
OJ, Adamson FG, Ogunseyinde O, Sodeinde O, Familusi JB. Stroke in
Nigerian children with sickle cell disease. Afr J Med Med Sci
2005;34(2):157-60. PMid:16749340 .

- Lagunju IA, Brown BJ, Famosaya AA. Childhood stroke in sickle cell disease in Nigeria. J Pediatr Neurol. 2011;9(1):49-53.

- Adams
RJ, Nichols FT, Figueroa R, McKie V, Lott T. Transcranial Doppler
correlation with cerebral angiography in sickle cell disease. Stroke.
1992;23(8):1073-7 https://doi.org/10.1161/01.STR.23.8.1073 PMid:1636180.

- Nichols
F, Jones A, Adams R. Stroke Prevention in Sickle Cell Disease(STOP)
Study Guidelines for Transcranial Doppler Testing. J neeuroimaging.
2001;11(4):354-62 https://doi.org/10.1111/j.1552-6569.2001.tb00063.x .

- Kwiatkowski
JL, Granger S, Brambilla DJ, Brown RC, Miller st, Adams RJ, STOP Trial
Investigators. Elevated blood flow velocity in the anterior cerebral
artery and stroke risk in sickle cell disease: extended analysis from
the STOP trial. Br J Haematol. 2006; 134(3):333-9 https://doi.org/10.1111/j.1365-2141.2006.06193.x PMid:16848777 .

- Lagunju
I, Sodeinde O, Brown B, Akinbami F, Adedokun B. Transcranial doppler
ultrasonography in children with sickle cell anemia: Clinical and
laboratory correlates for elevated blood flow velocities. J Clin
Ultrasound. 2014;42(2):89-95 https://doi.org/10.1002/jcu.22099 PMid:24166013 .

- Lagunju
I, Sodeinde O, Telfer P. Prevalence of transcranial Doppler
abnormalities in Nigerian children with sickle cell disease. Am J
Hematol 2012;87(5):544-7. https://doi.org/10.1002/ajh.23152 PMid:22460323 .

- Oniyangi
O, Akano AO, Wakama TT, Oyesakin AB. Transcranial Doppler ultrasound
studies for the primary prevention of strokes among children with
sickle cell disease in Nigeria- a single tertiary center experience.
Research 2014;1:825. https://doi.org/10.13070/rs.en.1.825 .

- Kwiatkowski
JL, Hunter JV, Smith-Whitley K, Katz ML, Shults J. Transcranial doppler
ultrasonography in siblings with sickle cell disease. Br J Haematol.
2003;121:375-80 https://doi.org/10.1046/j.1365-2141.2002.01193.x-i1.

- Adams
RJ, McKie VC, Brambilla D, Carl E, Gallagher D, Nichols FT, et al.
Stroke prevention trial in sickle cell anemia. Control Clin Trials
1998;19(1):110-29 https://doi.org/10.1016/S0197-2456(97)00099-8.

- Adams
RJ, Ohene-Frempong K, Wang W. Sickle cell and the brain. American
Society of Haematology. 2001;31-46 PMCid:PMC60990.

- Hsu
LL, Miller ST, Wright E, Kutlar A, McKie V, Wang W, et al. Stroke
Prevention Trial (STOP) and the Cooperative Study of Sickle Cell
Disease (CSSCD). J Paediatr Haematol Oncol.2003 Aug;25(8):622-8 https://doi.org/10.1097/00043426-200308000-00007 PMid:12902915.

- Lagunju
IA, Brown BJ, Sodeinde OO. Chronic blood transfusion for primary and
secondary stroke prevention in Nigerian children with sickle cell
disease: a 5-year appraisal. Paediatr Blood Cancer.2013;60(12):1940-5 https://doi.org/10.1002/pbc.24698 PMid:23956197.

- Shahripour
RB, Mortazavi MM, Kristian B, Keikhaei B, Mousakhani H, Azarpazhooh MR,
et al. Can STOP Trial Velocity Criteria Be Applied to Iranian Children
with Sickle Cell Disease. JOS 2014;16(2):97-101.

- Deane
CR, Goss D, ODriscoll S, Mellor S, Pohl KR, Dick MC, et al.
Transcranial Doppler scanning and the assessment of stroke risk in
children with haemoglobin sickle cell disease. Arch Dis Child.
2008;93(2):138. https://doi.org/10.1136/adc.2007.125799 PMid:17925326 .

- Hokazono
M, Silva GS, Silva EM, Braga JA. Results from transcranial Doppler
examination on children and adolescents with sickle cell disease and
correlation between the time-averaged maximum mean velocity and
haematologic characteristics: a cross sectional analytical study. Paulo
Med J. 2011 May;129(3):134-8 https://doi.org/10.1590/S1516-31802011000300003.

- Vieira
C, de Oliveira CNC, de Figueiredo LAB, Santiago RP, Adanho CSA, Santana
SS, et al. Transcranial Doppler in haemoglobin SC disease. Paediatr
Blood & Cancer. 2017 May;64(5):e26342 https://doi.org/10.1002/pbc.26342 PMid:27957790.

[TOP]