Alessandro Corso and Silvia Mangiacavalli
Clinica Ematologica, Fondazione IRCCS Policlinico San Matteo, Pavia.
Published: August 18, 2017
Received: May 19, 2017
Accepted: July 28, 2017
Mediterr J Hematol Infect Dis 2017, 9(1): e2017053 DOI
10.4084/MJHID.2017.053
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Non-secretory
myeloma is a rare myeloma subtype whose diagnosis, until a few years
ago, was established by demonstration of monoclonal plasma cells
≥10% in the bone marrow and by negative results on serum and urine
electrophoresis and immunofixation studies. However, this type of
myeloma could be misdiagnosed if the workup does not include an
accurate study of serum free light chain test since some of the
patients diagnosed as non-secretory could be light chain only with
small amounts monoclonal proteinuria. Due to this limit in
classification, all the information available today, generally coming
from retrospective studies including patients studied completely and
incompletely, could be misleading. A new definition is, thus, needed to
distinguish between the true non-secretory, with a possible better
prognosis, and the other forms of oligo-secretory myeloma with a
prognosis more similar to the secretory form of myeloma. With all the
data of the literature, the availability of laboratory and radiological
tools, times are mature to depict a new definition of nonsecretory
myeloma that deserves a peculiar work up and different response
evaluation and, may be, a different therapeutic approach.
|
Introduction
Multiple
myeloma (MM) is a malignancy of plasma cells defined by infiltration of
bone marrow, and presence of CRAB feature (skeletal lesions, anemia,
bone pain, renal insufficiency, hypercalcemia) as well as 3 specific
biomarkers: clonal bone marrow plasma cells ≥60%, serum free light
chain (FLC) ratio ≥100 (provided involved FLC level is ≥100 mg/L), and
more than one focal lesion on magnetic resonance imaging (MRI).[1-2]
In the USA this hematologic malignancy accounts for approximately 10%
of all hematologic neoplasm and 1% of all malignant disease, being
twice as common in African-Americans compared with Caucasians and
lowest among the Chinese and Japanese.[1,2] In Italy data are similar, MM accounting of 1% of all cancers and 13% of all hematologic malignancies.[3]
MM cells represent the neoplastic counterpart of normal plasma cells,
and thus the hallmark of most neoplastic plasma cells is the persistent
production of clonal immunoglobulin, albeit completely non-functional,
either complete (heavy and light chain) or as part of immunoglobulins
(heavy chain or light chain). The availability of this protein in the
blood or urine for quantitative assessment using serum protein
electrophoresis (SPEP), urine protein electrophoresis (UPEP), or the
serum free light chain assay allows easy monitoring of response in most
cases of myeloma.[4-5]
Monoclonal component (MC) typically can be detected in serum and/or urine as:
1) high concentrations of a full Ig molecule consisting of heavy and light chains bound together;
2)
elevated levels of the complete Ig molecule plus high concentrations of
light chains unbound to heavy chain (free light chains [FLCs]);
3)
primarily FLC in the presence of minimal amounts or even no complete
Immunoglobulin (Ig) whatsoever which is rare;
4)
a fourth entity, characterized by the absence of detectable MC either
in the serum or the urine, represents a very small subset of the
myeloma population.
The incidence of these non-secretory multiple myelomas (NSMMs) ranges from 3% to 5% of the total MM population.[6-8]
However, advances in the detection of serum FLCs have demonstrated that
most of these previously defined NSMMs are probably oligo secretors,[9]
namely producing primarily or solely serum FLC in the absence of heavy
chain. Thus, the proportion of true NSMM, meaning MM that secretes no
measurable monoclonal heavy or light chains at all, is closer to 1–2%
of all MMs.[1,10]
Non-secretory
myeloma is classically defined as clonal bone marrow plasma cells ≥10%
or biopsy proven plasmacytoma, evidence of end-organ damage that can be
attributed to the underlying plasma cell proliferative disorder,
specifically hypercalcemia, renal insufficiency, anemia, or bone
lesions, and lack of serum and urinary monoclonal protein on
electrophoresis and immunofixation.[8-9] Clinically,
patients who present with true non-secretory disease at diagnosis
behave differently from patients who present with the oligo-secretory
disease, as well as from those who progress from having secretory
disease at diagnosis to oligo-secretory or non-secretory disease at the
time of relapse.
In this article, we review all the information
available on this particular entity trying to outline a possible
definition of different subsets of non-secretory myeloma.
Biological Basis
Non-secretory
myeloma patients can be divided into several groups. The true
non-secretory myeloma should be considered only the group of
non-producers patients, whose tumors have a defect in immunoglobulin
synthesis, resulting in no measurable protein in the blood or urine,
although they still have a significant plasma cell burden in the bone
marrow and evidence of end-organ damage.[11] In these patients, even the use of the FLC assay will not reveal measurable disease as currently defined.[12]
The next category of non-secretory myeloma patients consists of those
cases whose neoplastic plasma cells produce an altered MC but have
defects in secretion. The exact mechanisms that prevent either
production or secretion of monoclonal Ig by NSMM remain poorly
understood. One hypothesis argues that true NSMMs arise from a
consecutive loss of secretion, firstly of heavy chains and then light
chains.[13] It has been demonstrated in vitro that a
single amino acid substitution in a light chain can potentially block
secretion outside the cell and that a mutation in the immunoglobulin
gene can account for the lack of secretion in a patient with
non-secretory myeloma.[14] On the other hand,
patients presenting only immunoglobulin light chains in serum and
urine, and then affected by light chain MM, never displayed a
functional IgH recombination.[15] The absence of
legitimate IgH rearrangement at the DNA level, reflecting possible
abnormalities in the IgH gene recombinations during B-cell maturation,
permits the secretion in the abnormal plasma cells of the only light
chains.[15] One study in 2002 found that 11 out of 14
NSMM patients had a t(11;14)(q13;q32) rearrangement, which the authors
postulated gave the cells a more “lymphoplasmacytic morphology” with a
lower secreting capacity than MM cells without the translocation.[16]
Interestingly, the same translocation was detected in the MM case
report detailed earlier that also demonstrated the frameshift mutation
in the gene coding the light-chain constant region, functionally
preventing secretion of the kappa light chain.[14]
These data, taken together, suggest that the “evolution” of NSMM
cells may be stepwise from fully secretory MM to MM that loses
production of the heavy chain and then in a subsequent step fails
production of the light chain.
Among patients whose tumors have
defects in Ig production, there is a subset of patients who have
impaired secretion but can produce a small amount of light chains.
These are patients who met criteria for oligo-secretory “free light
only” myeloma, since their protein secretion may not be as high as that
seen in typical myeloma, but it can be measured using current
technology, in particular, serum FLC assay.[17]
Oligo-secretory multiple myeloma is often characterized by serum
protein of < 1.0 g/dL, urine protein of < 200 mg/24 hrs, and free
light chain values of < 100 mg/L (or 10 mg/dL).[11]
Clinically, patients who present with true non-secretory disease at
diagnosis behave differently from patients who present with
oligo-secretory disease at the onset, as well as from those who
progress from having secretory disease at diagnosis to oligo-secretory
or non-secretory disease at the time of relapse (free light chain
“escape”). These latter patients typically have high-risk myeloma,
genomic instability, and rapid clonal evolution.[18-19]
The
International Myeloma Working Group still defines NSMM as MM lacking
monoclonal protein by serum or urine immunofixation, which can include
light-chain MM with quite high levels of monoclonal FLCs detected
solely by the SFLC assay.[11,17]
However, this definition is probably not sufficient, since the MM
indeed is actively secreting a component of Ig. Thus, cases of NSMM can
more accurately be subclassified into at least four distinct categories
with separate molecular mechanisms:
1) Oligo-secretors/FLC-restricted MMs: as discussed most of these cases can be followed by sFLC assay.[17]
2) Non-producers:
MM is non-secretory due to a complete, real absence of any Ig
production whatsoever. Such rare patients would not be able to be
monitored by either traditional methods or intracellular
immunofluorescence, which can be used to detect monoclonal Ig in the
cytoplasm of many cases of NSMM. It is hypothesized that the mechanism
of non-production is the loss of sFLC secretion by MM clones, which
were initially FLC secretors, although this has not been definitively
proven.
3) True non-secretors: these MM cells
produce Ig molecules but are unable to secrete them (the variety of
mechanisms by which this occurs is discussed in detail in the following)
4)
False non-secretors: MM variants or related plasma cell diseases that
had measurable intracellular Ig by immunofluorescence but no measurable
extracellular component by conventional testing. A straight
pathological evidence that they are secreted (such as Ig deposits found
in renal biopsies) can be accessed as part of the recently described
entity monoclonal gammopathy of renal significance).[12,20-21]
Furthermore, some data are suggesting that these Igs are secreted in
vesicles via budding off of the cell membrane, rendering them
undetectable in the serum. This would represent a challenge for
detection and treatment, too.
Workup and Prognosis
The
standard workup for any patients with known or suspected non-secretory
myeloma as recommended by the 2003 consensus statement from the
International Myeloma Workshop[11] includes SPEP, UPEP, and serum free light chain assay, in addition to imaging survey (Table 1).
All patients with suspected MM, including NSMM, should undergo bone
marrow aspiration (or biopsy of suspected plasmacytomas) completed by
flow cytometry and CD138-enriched fluorescent in situ hybridization
testing. If true NSMM is suspected, samples should also be stained for
intracellular Ig. As in all other forms of symptomatic MM, NSMM
requires the presence of any myeloma-defining events and/or evidence of
MM-mediated end-organ damage such as hypercalcemia, anemia, or bone
lesions to differentiate an asymptomatic MM precursor from actual MM.[2]
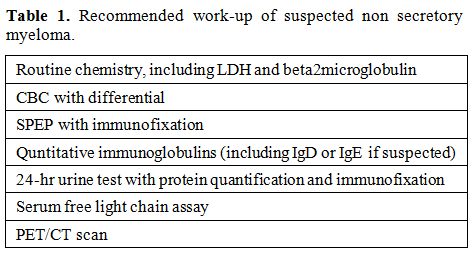 |
Table 1. Recommended work-up of suspected non secretory myeloma. |
Patients
with light chain myeloma may have only a serum free light chain
abnormality, although these patients should not be considered to have
right non-secretory myeloma. The group of true NSMM does not show
measurable disease with no serum/urine monoclonal component, or free
light chain assay abnormalities. In these patients, who are typically
characterized by the absence of any easily measurable parameter, a
skeletal survey is performed with a novel more sensitive and functional
methods. In particular positron emission tomography (PET)/CT scan bone
survey, along with marrow plasmacytosis, can serve as a relatively
objective assay to assess the extension of disease at presentation and
the level of disease response. PET/CT imaging can help identify sites
of bone disease and to distinguish between active and quiescent lesions
at treatment completion and during follow-up.[22]
Given
the rarity of NSMM in the overall MM population, its clinical course
and prognosis are still not thoroughly characterized. Moreover, since
monitoring of the Ig is essential to evaluate response to therapy and
to detect relapse, NSMM patients are usually excluded from clinical
trials. Results on the characteristics and the outcome of NSMM are not
univocal. In a series from France, it was reported that there was a
higher proportion of patients with the t(11;14) translocation among
patients with non-secretory myeloma.[16] The
frequency of this translocation in non-secretory myeloma patients was
83% in a cohort of 24 patients. In a group of 127 myeloma patients from
the UK who had undergone transplantation, 6 were found to be patients
with the non-secretory disease. The overall survival (OS) and
progression-free survival (PFS) of this small group of patients were
found to be superior to those of the patients with a traditional
secretory myeloma phenotype (36 vs 23 months).[23] A
possible hypothesis for this could be that there is a lower frequency
of high-risk genetic alterations in the non-secretory patients, which
allows their improved outcomes respect to patients with IgG, IgA, or
light chain myelomas.[16] In 1986, Smith et al.
released a case series that included 13 NSMM patients, in which NSMM
patients had a median survival of 46 months compared to 22 months for
secretors. At that time, ELISA-based SFLC testing was not commercially
available, and therefore it is unclear how many of the NSMM patients
had light-chain oligo-secretory MMs.[24]
By
contrast, Kyle et al., in their 1,027 patients cohort, report an
outcome for patients with non-secretory myeloma similar to that of
patients with secretory myeloma (OS 38 vs 33.4 months).[25]
Similarly, no difference in PFS or OS was observed in a series from the
Center for International Blood & Marrow Transplant Research
(CIBMTR), among 110 patients with non-secretory myeloma compared with
matched controls in a 4:1 fashion.[26] However, the
number of true non-secretors vs those with the oligo-secretory disease
was not available. Finally, Chawla et al. retrospectively examined the
survival and prognosis of a group of NSMM patients. The study included
124 patients with non-secretory myeloma treated in a period from 1973
until 2012. Around two third of patients (88 pts) have been addressed
before 2001 with conventional therapy (mainly chemotherapy) and
one-third (36 pts) after 2001 when novel agents entered in routine
clinical practice. The median follow-up was 102 months; the median PFS
after initial therapy was 28.6 months and overall survival 49.3 months.
They observed a significant improvement after 2001 (99 vs. 43 months),
as also reported in general for myeloma. However, while survival before
2001 was similar in non-secretory and secretory patients (3.6 vs. 3.5
yrs), interestingly after 2001 non-secretory myeloma showed a
significantly higher overall survival respect to secretory ones (8.3
vs. 5.4 yrs, p=0.03). Several factors were evaluated on survival, in
multivariate analysis only age and the time-period of diagnosis were
significantly correlated with a better outcome.[27]
Since FLC assay was available only for 29 out of 124 entering the
analysis, despite this study was performed on a very large group of
patients, the percentage of patients who could be better defined as
oligo-secretory MM was not determinable.
Actually, with all data available from the literature, there is no evidence for poor prognosis associated with NSMM phenotype (CFR Table 2).
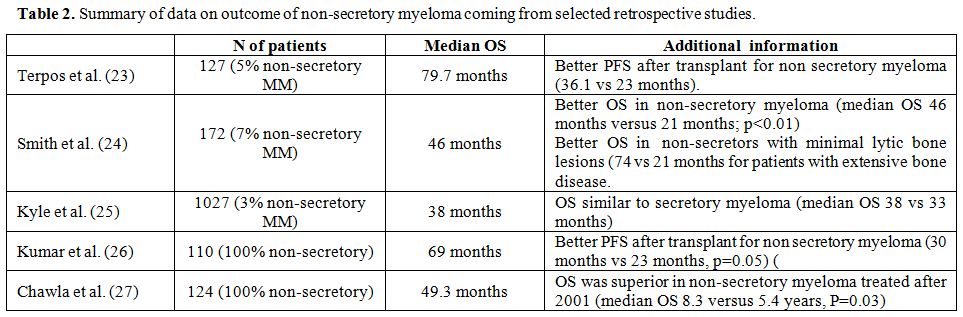 |
Table 2. Summary of data on outcome of non-secretory myeloma coming from selected retrospective studies. |
Treatment and Response Assessment
Although
non-secretory myeloma usually is not included in protocols since the
difficulty in monitoring the response, the few data available seems not
to suggest that NSMM responds differently to standard MM treatments.
Thus a standard approach including when possible autologous stem cell
transplantation (ASCT) may do equally well if not better than secretory
MM.[23,27]
In a study on
patients receiving lenalidomide, bortezomib, and dexamethasone (RVD)
induction followed by early or late transplant, Nooka et al., reported
a similar 3-year OS of > 85%, in all analyzed patients, secretory
and non-secretory.[28] Terpos et al. as well, in a
larger series of patients provided similar results, suggesting that the
gains in outcomes associated with the use of new agents were similar
for secretory and non-secretory myeloma patients.[23]
Thus, until new evidence suggests other pathways, treatment of NSMM
should follow the same guidelines as those provided for secretory MM.
Monitoring
response of NSMM is a challenging. Serial bone marrow studies could be
the gold standard, but the cost, time, and patient discomfort
associated with frequent bone marrow aspirations and/or biopsies make
them less feasible in real life. Also, routine marrow histology and
routine flow cytometry are notoriously inaccurate, due in large part to
the patchy nature of marrow involvement, which entails that the extent
of marrow involvement at different sites can be heterogeneous within a
single patient.[29] A possible solution can come from
the use of multiparametric flow cytometry (MPF), which allows
evaluating the marrow better. Moreover, the minimal residual disease
(MRD), measured with MPF, has not only predictive but also
prognostic implications in the setting of disease assessment
post-transplant.[30] However, although the
significant improvement of this technique over conventional flow
cytometry or histologic assessment of plasma cell number, MPF need a
partner to assess total body myeloma burden better. Therefore, the
pairing of imaging and more sensitive marrow assessment represents an
optimal procedure to evaluate response to therapy and MRD in
non-secretory patients in whom the inability to use SPEP/UPEP/FLC tests
limits response assessment. Since no data are available directly on
non-secretory myeloma, information is extrapolated from a study on
secretory MM, where magnetic resonance imaging (MRI) and positron
emission tomography (PET) are most adopted. In a systematic review,
Regelink et al. observed, using X-rays as the gold standard, that both
MRI and PET had a sensitivity of 90% (i.e. MRI and PET individually
detected abnormalities in 90% of patients who had abnormal findings on
X-ray). Furthermore, both methods identified a higher total number of
lesions than X-rays, suggesting that both techniques were more
sensitive than the standard.[31] Several studies have
demonstrated the diagnostic and prognostic role of PET and that a lack
of a post-transplant normalization of standard uptake value activity
strongly predicts a short duration of responses.[32-35]
On the other hand, of patients showing focal marrow lesions on MRI,
only 33.5% of them achieving a very good partial response or better
response by standard response criteria[36] had shrinkage of these lesions, suggesting inadequate sensitivity for detecting the response.[37-38]
Hence, MRI, although very sensitive for detecting lesions at diagnosis,
is insufficient for monitoring, due to the practical limitations and
the relatively static nature of bone despite tumor killing.
Thus,
in the clinical practice, in NSMM patients with detectable lesions at
diagnosis on PET/CT, this will be performed at intervals decided based
on the duration of treatment cycles and the clinical circumstances. An
aggressive disease and/or lack of other reliable clinical indicators of
response suggest a more frequent checking with PET/CT, whereas an
indolent disease and/or the presence of other clinical indicators, such
as improvement in symptoms or cell counts permit a less frequent one.
Even for patients in remission and undergoing long-term monitoring, the
timing of PET/CT will be established in relation to the depth of
response obtained and to the characteristics of patients before
treatment. In these sets of patients, it is convenient to associate
also a bone marrow evaluation with biopsies or MPF when available. In
patients that cannot be followed by PET/CT, monitoring of disease will
be based only on serial bone marrow aspirations and biopsies with the
same criteria reported above (Table 3), associated to Rx.
 |
Table 3. Recommended tests to assess response and disease status in a patient with non-secretory myeloma. |
Conclusions
Given
the availability of higher sensitive methods for monoclonal component
identification and quantification, particularly with the introduction
of serum free light chain assay, the subset of patients meeting
criteria for true non-secretory MM has become more rare, with an
estimated incidence closer to 1-2% of all MM diagnosis.
In the
absence of any laboratory test easily measurable during therapy and
follow-up, new cross sectional imaging modalities, in particular,
PET-CT represents a useful tool in clinical practice for disease
monitoring, at least in those fraction of patients with detectable
lesions at the onset. In the absence of radiologically detectable
lesions, serial bone marrow examinations for quantification of
neoplastic plasma cell infiltration remains the only way for disease
monitoring.
Due to the small proportion of patients encountering
criteria for NSMM and the systematic exclusion of these patients from
the clinical trials, it is not possible to define if the prognosis of
these patients is significantly different from secretory ones. Limited
data available from the literature seem to show that the presence of a
not secretory phenotype at the onset gives no additional risk for the
outcome, unlike from what happens when an oligo or no secretory
phenotype is acquired at relapse with the previously described
phenomenon of free light chain escape. In the absence of more extensive
data, NSMM deserves similar treatment of secretory MM. More studies ad
hoc are needed to define the course and the outcome of this entity
better.
References
- Rajkumar SV. Multiple myeloma: 2016 update on
diagnosis, risk-stratification, and management. Am J Hematol. 2016;
91(7):719-34. doi: 10.1002/ajh.24402. https://doi.org/10.1002/ajh.24402

- Rajkumar
SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working
Group updated criteria for the diagnosis of multiple myeloma. Lancet
Oncol. 2014;15:e538-e548. https://doi.org/10.1016/S1470-2045(14)70442-5

- AIRTUM
Working Group, Busco S, Buzzoni C, Mallone S, Trama A, Castaing M,
Bella F, et al. Italian cancer figures--Report 2015: The burden of rare
cancers in Italy Epidemiol Prev. 2016 Jan-Feb;40(1 Suppl 2):1-120

- Rajkumar
SV, Harousseau JL, Durie B, et al. Consensus recommendations for the
uniform reporting of clinical trials: report of the International
Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691-5. https://doi.org/10.1182/blood-2010-10-299487 PMid:21292775 PMCid:PMC3710442

- Kyle
RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R,
Rajkumar SV, Offord JR, Larson DR, Plevak MF, Therneau TM, Greipp PR.
Review of 1,027 patients with newly diagnosed multiple myeloma. Mayo
Clinic Proc. 2003; 78: 21-33 https://doi.org/10.4065/78.1.21 PMid:12528874

- Blade
J, Kyle RA. Nonsecretory myeloma, immunoglobulin D myeloma, and plasma
cell leukemia. Hematol Oncol Clin North Am. 1999; 13(6): 1259-72 https://doi.org/10.1016/S0889-8588(05)70125-8

- Middela S, Kanse P. Nonsecretory multiple myeloma. Indian J Orthop. 2009;43(4):408-411 https://doi.org/10.4103/0019-5413.55979 PMid:19838394 PMCid:PMC2762556

- Cavo
M, Galieni P, Gobbi M, et al. Nonsecretory multiple myeloma. Presenting
findings, clinical course and prognosis. Acta Haematol 1985;74(1):27-30
https://doi.org/10.1159/000206159 PMid:3934904

- Drayson
M, Tang LX, Drew R, Mead GP, Carr-Smith H, Bradwell AR. Serum free
light-chain measurements for identifying and monitoring patients with
nonsecretory multiple myeloma. Blood. 2001;97(9):2900-2902 https://doi.org/10.1182/blood.V97.9.2900 PMid:11313287

- Chawla
SS, Kumar SK, Dispenzieri A, et al. Clinical course and prognosis of
non-secretory multiple myeloma. Eur J Haematol. 2015;95(1):57-64 https://doi.org/10.1111/ejh.12478 PMid:25382589

- The
International Myeloma Working Group. Criteria for the classification of
monoclonal gammopathies, multiple myeloma and related disorders: a
report of the International Myeloma Working Group. Br J Haematol. 2003;
121: 749-57 https://doi.org/10.1046/j.1365-2141.2003.04355.x

- Decourt
C, Galea HR, Sirac C, Cogne M. Immunologic basis for the rare
occurrence of true nonsecretory plasma cell dyscrasias. J Leukoc Biol.
2004;76:528-36 https://doi.org/10.1189/jlb.0803382 PMid:15155772

- Preud
Homme JL, Hurez D, Danon F, Brouet JC, Seligmann M. Intra¬cytoplasmic
and surface-bound immunoglobulins in nonsecretory and Bence-Jones
myeloma. Clin Exp Immunol. 1976;25(3):428-436 PMid:822974
PMCid:PMC1541419

- Coriu D, Weaver K, Schell M, et al. A molecular basis for nonsecretory myeloma. Blood. 2004;104:829-31 https://doi.org/10.1182/blood-2004-02-0477 PMid:15090444

- On,
et al. Light-chain only multiple myeloma is due to the absence of
functional (productive) rearrangement of the IgH gene at the DNA level.
Blood. 2004;103(10):3869-3875 https://doi.org/10.1182/blood-2003-07-2501 PMid:14715636

- Avet-Loiseau
H, Garand R, Lodé L, Harousseau JL, Bataille R; Intergroupe Francophone
du Myélome. Translocation t(11;14)(q13;q32) is the hallmark of IgM,
IgE, and nonsecretory multiple myeloma variants. Blood.
2002;101(4):1570-1571 https://doi.org/10.1182/blood-2002-08-2436 PMid:12393502

- Lonial S, Kaufman JL. Non-secretory myeloma: a clinician's guide. Oncology. 2013; 27(9): 924-8, 30

- Brioli
A, Giles H, Pawlyn C, Campbell JP, Kaiser MF, Melchor L, Jackson GH,
Gregory WM, Owen RG, Child JA, Davies FE, Cavo M, Drayson MT, Morgan
GJ. Serum free immunoglobulin light chain evaluation as a marker of
impact from intraclonal heterogeneity on myeloma outcome Blood. 2014
May 29;123(22):3414-9 https://doi.org/10.1182/blood-2013-12-542662 PMid:24733348

- Tacchetti
P, Cavo M, Rocchi S, Pezzi A, Pantani L, Brioli A, Testoni N, Terragna
C, Zannetti BA, Mancuso K, Marzocchi G, Borsi E, Martello M, Rizzello
I, Zamagni E. Prognostic impact of serial measurements of serum-free
light chain assay throughout the course of newly diagnosed multiple
myeloma treated with bortezomib- based regimens. Leuk Lymphoma. 2016
Sep;57(9):2058-64 https://doi.org/10.3109/10428194.2015.1124994 PMid:26763357

- Turesson
I, Grubb A. Non secretory or low secretory myeloma with intracellular
kappa chains. Report of six cases and review of the literature. Actamed
Scan. 1978; 204(6):445-451

- Leung
N, Bridoux F, Hutchinson CA, et al. Monoclonal gammopathy of renal
significance; when MGUS is no longer undetermined or insignificant.
Blood, 2012; 120: 4292-95 https://doi.org/10.1182/blood-2012-07-445304 PMid:23047823

- Zamagni E, Cavo M. The role of imaging techniques in the management of multiple myeloma. Br J Haematol. 2012;159:499-513 https://doi.org/10.1111/bjh.12007

- Terpos
E, Apperley JF, Samson D, et al. Autologous stem cell transplantation
in multiple myeloma: improved survival in nonsecretory multiple myeloma
but lack of influence of age, status at transplant, previous treatment
and conditioning regimen. A single-centre experience in 127 patients.
Bone Marrow Transplant. 2003;31:163-70 https://doi.org/10.1038/sj.bmt.1703818 PMid:12621476

- Smith
DB, Harris M, Gowland E, Chang J, Scarffe JH. Non-secretory multiple
myeloma: a report of 13 cases with a review of the literature. Hematol
Oncol. 1986;4(4):307-313 https://doi.org/10.1002/hon.2900040407 PMid:3549511

- Kyle
RA, Gertz MA, Witzig TE, et al. Review of 1027 patients with newly
diagnosed multiple myeloma. Mayo Clin Proc. 2003;78:21-33 https://doi.org/10.4065/78.1.21 PMid:12528874

- Kumar
S, Perez WS, Zhang MJ, et al. Comparable outcomes in non-secretory and
secretory multiple myeloma after autologous stem cell transplantation.
Biol Blood Marrow Transplant, 2008;14:1134-40 https://doi.org/10.1016/j.bbmt.2008.07.011 PMid:18804043 PMCid:PMC2634851

- Chawla
SS, Kumar SK, Dispenzieri A, et al. Clinical course and prognosis of
non-secretory multiple myeloma. Eur J Haematol. 2015;95(1):57-64 https://doi.org/10.1111/ejh.12478 PMid:25382589

- Nooka
A, Langston A, Waller EK, et al. Early versus delayed autologous stem
cell transplant (ASCT) in patients receiving induction therapy with
lenalidomide, bortezomib, and dexamethasone (RVD) for newly diagnosed
multiple myeloma (MM). Presented at ASCO 2013 Annual Meeting; 2013;
Abstract 8540
- Paiva
B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation,
serum free light chain, and immunophenotyping for response evaluation
and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627-33 https://doi.org/10.1200/JCO.2010.33.1967 PMid:21402611

- Paiva
B, Vidriales MB, Cervero J, et al. Multiparameter flow cytometric
remission is the most relevant prognostic factor for multiple myeloma
patients who undergo autologous stem cell transplantation. Blood.
2008;112:4017-23 https://doi.org/10.1182/blood-2008-05-159624 PMid:18669875 PMCid:PMC2581991

- Regelink
JC, Minnema MC, Terpos E, et al. Comparison of modern and conventional
imaging techniques in establishing multiple myeloma-related bone
disease: a systematic review. Br J Haematol. 2013;162(1):50-61 https://doi.org/10.1111/bjh.12346 PMid:23617231

- Zamagni
E, Nanni C, Patriarca F, et al. A prospective comparison of
18F-fluorodeoxyglucose positron emission tomography-computed
tomography, magnetic resonance imaging and whole-body planar
radiographs in the assessment of bone disease in newly diagnosed
multiple myeloma. Haematologica. 2007;92(1):50-55 https://doi.org/10.3324/haematol.10554 PMid:17229635

- Caers
J, Withofs N, Hillengass J, et al. The role of positron emission
tomography-computed tomography and magnetic resonance imaging in
diagnosis and follow up of multiple myeloma. Haematologica.
2014;99(4):629-637 https://doi.org/10.3324/haematol.2013.091918 PMid:24688111 PMCid:PMC3971072

- Zamagni
E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT
in newly diagnosed multiple myeloma patients treated with up-front
autologous transplantation. Blood. 2011;118(23):5989-5995 https://doi.org/10.1182/blood-2011-06-361386 PMid:21900189

- Walker
R, Barlogie B, Haessler J, et al. Magnetic resonance imaging in
multiple myeloma: diagnostic and clinical implications. J Clin Oncol.
2007;25(9):1121-1128 https://doi.org/10.1200/JCO.2006.08.5803 PMid:17296972

- Durie
BGM, Harousseau JL, Miguel JS, et al; International Myeloma Working
Group. International uniform response criteria for multiple myeloma.
Leukemia. 2007;21(5):1134 https://doi.org/10.1038/sj.leu.2404582

- Lin
C, Luciani A, Belhadj K, et al. Multiple myeloma treatment response
assessment with whole-body dynamic contrast-enhanced MR imaging.
Radiology. 2010;254(2):521-531 https://doi.org/10.1148/radiol.09090629 PMid:20093523

- Bannas
P, Hentschel HB, Bley TA, et al. Diagnostic performance of whole-body
MRI for the detection of persistent or relapsing disease in multiple
myeloma after stem cell transplantation. Eur Radiol.
2012;22(9):2007-2012 https://doi.org/10.1007/s00330-012-2445-y PMid:22544292

[TOP]









































