Elisabetta Metafuni, Patrizia
Chiusolo, Luca Laurenti, Federica Sorà, Sabrina Giammarco, Andrea
Bacigalupo, Giuseppe Leone and Simona Sica.
Hematology Department, Fondazione Policlinico Universitario Agostino Gemelli, Rome, Italy.
Corresponding
author: Prof. Simona Sica. Hematology
Department, Fondazione Policlinico Agostino Gemelli, Largo Agostino
gemelli, 8 00168, Rome, Italy. Tel. 0039 0630155300/6016 Fax: 0039
063017319. E-mail:
simona.sica@unicatt.it
Published: January 1, 2018
Received: October 10, 2017
Accepted: Dicember 1, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018005 DOI
10.4084/MJHID.2018.005
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Therapy
related myeloid neoplasms (t-MN) occur due to direct mutational events
of chemotherapeutic agents and radiotherapy. Disease latency,
mutational events and prognosis vary with drugs categories.
Methods:
We describe a cohort of 30 patients, 18 females and 12 males, with
median age of 52.5 years (range, 20 to 64), submitted to allogeneic
stem cell transplantation (HSCT) in our department between September
1999 and March 2017. Patients had a history of solid tumour in 14
cases, haematological disease in 15 cases and both of them in one case.
After a median of 36.5 months (range, 4 to 190) from first neoplasm,
patients developed t-AML in 19 cases and t-MDS in 11 cases. Molecular
abnormalities were detected in 5 patients, while karyotype aberrations
were found in 17 patients. Patients received conventional chemotherapy
in 14 cases, azacitidine in 10 cases and both of them in one case. Five
patients were submitted to HSCT without previous treatment except for
supportive therapy.
Results:
Seventeen patients obtained sustained CR after SCT, while 8 patients
showed resistant or relapsed disease. The remaining five patients died
early after SCT. At follow up time (May 2017) 13 patients were alive
with a median OS of 48 months (range 3-195), while 17 patients died
after a median of 4 months (range 1-27) by relapse mortality in 6 cases
and non-relapse mortality in the other 11 patients.
Conclusions: Global OS was 43%. After SCT, 72.2% of patients with t-MN maintained a sustained CR.
|
Introduction
Therapy-related
myeloid neoplasms are recognized as a separate entity in the World
Health Organization (WHO) classification of haematological diseases.[1]
The incidence of therapy-related myeloid neoplasms (t-MN) continue to
rise due to the relative prolongation of survival and cure related to
chemo- and radio-therapy for primary malignancies, mostly breast cancer
and lymphoproliferative diseases.[2-7]The peak occurrence time of
therapy-related acute myeloid leukemia/myelodysplastic syndrome is 3 to
5 years after prior cytotoxic treatment, while the risk decreases
markedly after the first decade.[8] At present, t-MN account for 10–20%
of all malignant myeloid diseases.[9] Factors associated with an
increased risk of t-MN include exposure to alkylating agents,
topoisomerase II inhibitors, radiation therapy,[10-15] and older age at
treatment, in addition to genetic susceptibility.[16-21] t-MN after
anthracyclines and/or topoisomerase II inhibitors are associated with
occurrence of MLL translocation at 11q23 or RUNX1/AML1 at 21q22 after a
median latency of 1 to 3 years without a prodromal phase. t-MN after
alkylating agents have a median latency of 4 to 10 years and are often
preceded by myelodysplasia. It is associated with unbalanced chromosome
5 and 7 abnormalities, complex karyotypes, and/or TP53 mutations. After
radiation treatment, the highest risk for t-MN occurrence is registered
at 2 years and appears to normalize after 10 to 15 years.[22-24]
Particularly, patients who received radiation to chest, pelvis and
vertebrae for stomach, colorectal, liver, breast, endometrial,
prostate, and kidney cancers seem to be at a significantly higher risk
of developing t-MN.[24,25] More recently, it came to light the
potential role of various germline genetic factors in an individual’s
susceptibility to t-MN, particularly for those variants that alter drug
metabolism such as gene NQ01, glutathione-S-transferase,[9,18,19,26] as
well as those involved in DNA repair pathway such as BRCA, TP53 and
MDM2.[20,21]
Clonal cytogenetic abnormalities are found in 75–90%
of t-MN, and 46-70% of them are adverse karyotype including complex
karyotype, deletion or loss of chromosome 5 and/or 7.[3,4] Cytogenetics
assessment is the principal prognostic factor for relapse rate and
overall survival (OS).[27-29] The heterogeneous treatments of
therapy-related myeloid neoplasms, ranging from best supportive care to
intensive chemotherapy, hypomethylating agents, and allogeneic stem
cell transplantation, do not allow definite conclusions on the best
treatment choice, particularly for elderly patients.[30,31] Treatment
of t-MN with conventional therapy is associated with a poor outcome in
terms of survival (6 months),[8,32] remission rate (28% to 50%) and
duration of the remission.[33-35] On the other hand, conventional
chemotherapy might be a reasonable option for t-MN with favourable
karyotype such as inv(16), t(16;16), t(15;17) or t(8;21), since the
reported remission rate and the disease free survival are similar to
those seen for the de novo counterpart.[35,36] The introduction of new
drugs such as azacitidine and decitabine has shown promising results in
the management of t-MN with an acceptable toxicity profile also for
frail patients, and with an overall response rate of approximately
40%.[4,30,31,37-44]
Allogeneic Stem Cell Transplantation for t-MN
Allogeneic
haematopoietic stem cell transplantation (HSCT) represents the only
potentially curative strategy, but it is not feasible for all patients
due to age, comorbidities in elderly patients, poor organ reserve and
high non-relapse mortality (NRM).[4,45] The haematopoietic cell
transplantation-specific comorbidity index (HCT-CI) was developed as a
sensitive tool to measure the burden of comorbidities before HSCT and
to predict both the risks of NRM and the probabilities of survival
after HSCT.[46] As reported by ElSawy et al.[47] in the HCT-CI
validation study, the three HCT-CI risk groups with score 0, 1–2, and
≥3 result in a NRM of 14%, 23%, and 39% with a survival of 74%, 61%,
and 39%, respectively. Therefore, HSCT should be offer as a reliable
option to fit patients with good performance status, intermediate and
poor risk karyotype with suitable and available donor.[9,27,28,48-50]
With particularly interest to t-MN, the Center of International Bone
Marrow Transplantation Research (CIBMTR) and the European Group for
Bone and Marrow Transplantation (EBMT) extrapolated pre-transplant
factors predicting post-HSCT outcome in these patients from larges
study cohorts. CIBMTR conducted a large study cohorts on t-MN and
proposed a prediction model of survival after allogeneic HSCT using the
following four risk factors: age older than 35 years, poor-risk
cytogenetics, t-AML not in remission or advanced t-MDS, donor other
than an HLA-identical sibling or a partially or well-matched unrelated
donor. Five-year survival for subjects with none, 1, 2, 3, or 4 of
these risk factors was 50%, 26%, 21%, 10%, and 4%, respectively.[27]
Also the EBMT group[28] reported that disease stage at transplant
different from complete remission, abnormal cytogenetics (excluding
t(8;21), inv(16) and t(15;17)) and patients’ age >40 years are the
most significant factors predicting survival, relapse rate,
disease-free survival (DFS) and NRM dividing patients into three risk
groups: low, intermediate and high. Overall survival for the
above-mentioned groups was 62%, 33% and 24%, respectively; DFS was 58%
(low), 32% (intermediate) and 20% (high); NRM was 22% (low), 37%
(intermediate) and 38% (high); finally, relapse rate was 20% (low), 31%
(intermediate) and 32% (high) respectively.
We performed a
review of the literature on therapy-related AML/MDS submitted to
allogeneic stem cell transplantation excluding AML secondary to MDS
progression. Detailed results concerning cohort size, median follow up,
overall survival, NRM incidence, and relapse rate are depicted in Table 1.
The reported outcomes for patients submitted to HSCT for
therapy-related AML/MDS are very heterogeneous. Median OS ranges from
22% to 66%, with a NRM of 21 to 58% and a relapse rate of 26% to
42%.[2,27,28,38,51-62]
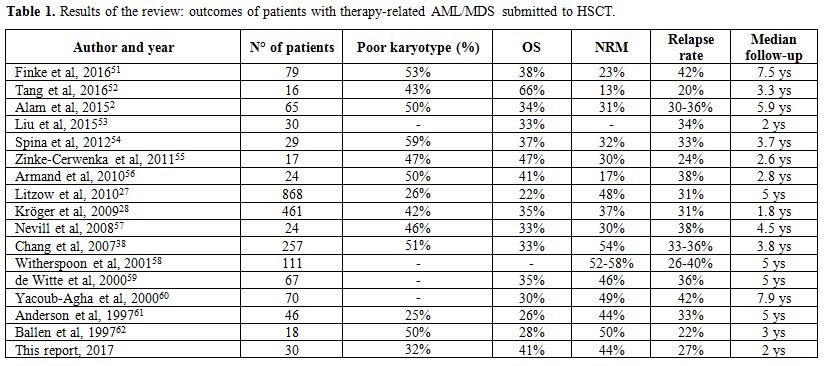 |
Table
1. Results of the review: outcomes of patients with therapy-related AML/MDS submitted to HSCT. |
Monocentric Observational Study
Patients and disease characteristics.
We retrospectively analyzed patients submitted to HSCT in our
department and identified 30 patients with a diagnosis of
therapy-related myeloid neoplasm (t-MN) transplanted between September
1999 and March 2017. Patients were 18 females (60%) and 12 males (40%)
with a median age of 52.5 years (range, 20 to 64). Secondary neoplasm
was acute myeloid leukemia (t-AML) in 19 cases (63%) and myelodysplasia
(t-MDS) in 11 cases (37%). Data were collected through retrospective
chart review and after institutional review board approval. The median
time occurred from primary disease to t-MN occurrence was of 36.5
months (range, 4 to 190). Primary disease was hematologic in 15 cases
(50%): Hodgkin’s disease (n=2), non-Hodgkin’s lymphoma (n=9), acute
lymphoblastic leukemia (n=1), chronic lymphocytic leukemia (n=2) and
acute myeloid leukemia (n=1). Fourteen patients (50%) had a previous
diagnosis of solid tumor: medulloblastoma (n=1), breast (n=8), Ewing
sarcoma (n=1), thyroid (n=1), bladder (n=2) and vagina/anus (n=1). One
patient had a history of both haematological (non-Hodgkin's lymphoma)
and solid tumor (breast). Twelve patients (40%) had been previously
treated with chemotherapy, 8 patients (26.7%) with chemotherapy and
autologous transplantation, 2 (6.7%) patients with radiotherapy, one
patient (3.3%) with radioiodine therapy and 7 patients (23.3%) with a
combination of chemo- and radiotherapy. At t-MN diagnosis all patients
had received a median of 2 lines of therapy (range, 1 to 6) for their
primary malignancy. All patients were free of their primary
malignancies at the time of transplantation.
Revised International
Prognostic Scoring System (IPSS-R)[63] was used to classify
cytogenetics of t-MDS, while European Leukemia Net AML risk
stratification by cytogenetics was used for AML.[64] Karyotype was
available for 28 out of 30 patients. Eleven patients (36.7%) had normal
karyotype, three patients (10%) had a favourable karyotype, 5 patients
(16.7%) had an intermediate-risk karyotype and 9 patients (30%) had an
adverse-risk karyotype. Molecular cytogenetics analyses were available
for 14 out of 30 patients: FLT3/ITD+ (n=2), CBFB/MYH11 (n=1), NPM1+
(n=1), NPM1 and FLT3/ITD double positivity (n=1), no abnormalities
(n=9). A detailed description of primary neoplasms, treatment for
primary neoplasm and t-HN is reported in Table 2. Transplant features and outcomes are depicted in Table 3.
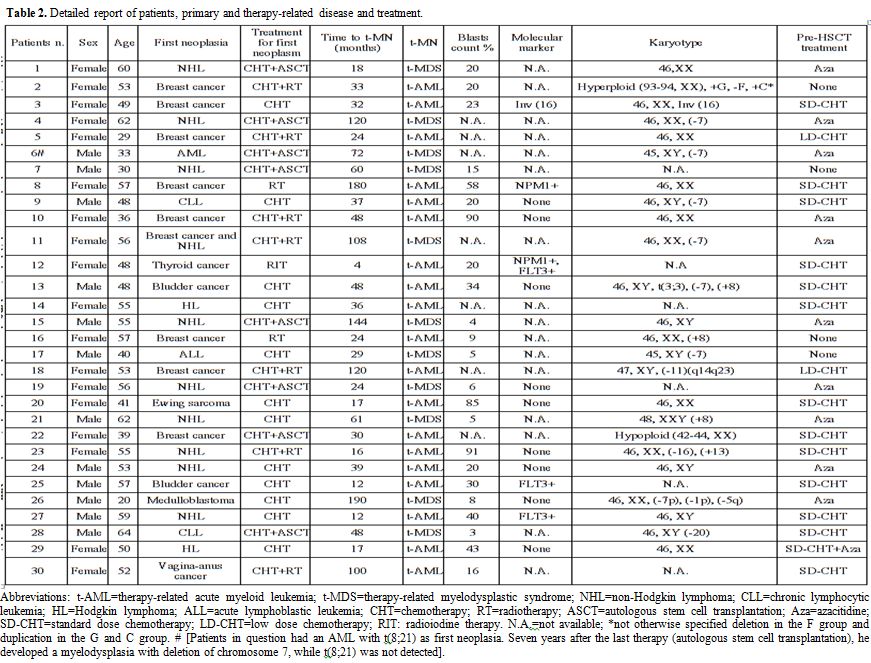 |
Table 2.
Detailed report of patients, primary and therapy-related disease and treatment. |
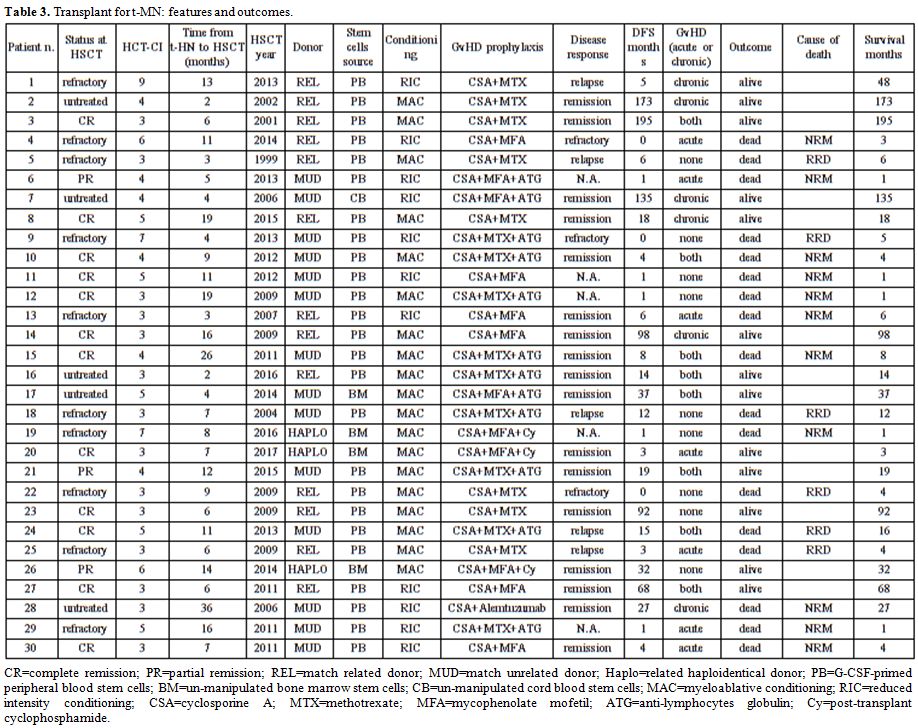 |
Table 3. Transplant for t-MN: features and outcomes. |
Statistical analysis.
Overall survival and disease-free survival (DFS) were estimated using
Kaplan-Meier product method, while for curves comparison log-rank test
was applied. χ2 test and Fisher’s exact test were used to assess
associations between categorical variables and OS, NRM, RRD, DFS. A
competing risk analysis was performed to calculate the cumulative
incidence of relapse-related death (RRD) and non-relapse mortality
(NRM). For NRM, relapse was the competing event, and for relapse, NRM
was the competing event. Fine and Gray’s method for cumulative
incidence of RRD and NRM were used to compare different groups.
Statistical analysis was realized using NCSS 10. A p-value ≤ 0.05 was
considered statistically significant. Results
Engraftment and GvHD. White blood cells count of ≥1.0x109/L and stable platelets count ≥20.0x109/L
were reached at median day +21 (range, 11 to 130) and median day +15
(range, 10 to 45), respectively. Three patients died early before
achieving stable engraftment.
Acute GvHD (aGvHD)[65] occurred in
15 patients (50%) and global grading was as follows: grade I (n=3),
grade II (n=5), grade III (n=6), and grade IV (n=1). Among them, three
patients died because of aGvHD. Chronic GvHD (cGvHD)[66] was diagnosed in
14 out of 23 patients surviving after day +100 (65%) and global scoring
was as follows: mild (n=3), moderate (n=7) and severe (n=4). One of
them died for cGvHD-related complications.
Response.
Morphological bone marrow cytology was performed on day +30 after HSCT
only in 25 patients because of early death in the others five. Three
patients (12%) had a persistence of the underlying disease, whereas
twenty-two patients achieved a CR (88%) on day +30. Among them, 5
patients (22.7%) experienced a relapse after a median time of 6 months
(range, 3 to 15), while 17 patients (77.3%) maintained a CR after a
median time of 27 months (range, 3 to 195). Median 2-ys DFS after HSCT
was of 72.2% (95% CI 51.1 to 93.3) (Figure 1A).
Overall survival, NRM and RRD.
At the follow up data fixed on May 2017, 13 patients were alive after a
median time of 48 months (range, 3 to 195), while 17 patients died
after a median time of 4 months (range, 1 to 27). The causes of death
were as follows: underlying disease (n=6), GvHD (n=3), EBV-related
post-transplant lymphoproliferative disease (PTLD) (n=1) and infectious
complications (n=7). The overall survival at 2 years after HSCT was of
40.5% (95% CI 22.1 to 58.9), whereas the cumulative incidence of NRM
and RRD at 2 years was of 44.4% (95% CI 27.6 to 71.2) and 29.6% (95% CI
15 to 58.6), respectively (Figures 1B, 1C and 1D).
No differences in terms of OS, NRM, RRD and DFS were seen stratifying
patients according to underlying disease, disease status at transplant,
previous treatment received, karyotype risk, patients and donor
characteristics, stem cell source. An association was identified
between OS and cGvHD development after HSCT, as well as between OS and
relapse occurrence. Overall survival was higher in the group with cGvHD
than those detected in the group without this complication (68% vs.
22%, p=0.018). Median OS was of 6 months (range, 4.6 to 7.4) in the
group without cGvHD, while it was not reached in the group with cGvHD
(p=0.0002, Figure 1E). An
higher mortality was recorded in the group of patients who experienced
a relapse of the underlying disease as compared with patients who did
not relapsed after HSCT (67% vs. 13%, p=0.011). Median OS in the group
relapsed after HSCT was of 5 months (range, 2.2 to 7.8) as compared to
patients without relapse, for whom a median OS was not reached
(p=0.004, Figure 1F).
Relatively to NRM, an association was identified with the conditioning
regimen: surprisingly, NRM was higher for patients who had received a
reduced intensity conditioning as compared to those who had received a
myeloablative one (p=0.046). Two-years cumulative incidence of NRM was
of 74% (95% CI 49 to 100) after RIC transplant and 24% (95% CI 10 to
58) after ABL transplant (p=0.022, Figure 1G).
Finally, also for RRD an association was found with cGvHD development
after HSCT: among patients with cGvHD, a minor number of RRD was
recorded as compared to patients who had not developed this
complication (p=0.018). The cumulative incidence of RRD at 2 years
after HSCT was of 9% (95% CI 1 to 59) for patients with cGvHD and 65%
(95% CI 38 to 100) for patients without cGvHD (p=0.004, Figure 1H).
Two
patients (6.7%) experienced a third tumor, in particular a breast
cancer occurred thirteen years after HSCT and an EBV-related PTLD of
the brain occurred eight months after HSCT.
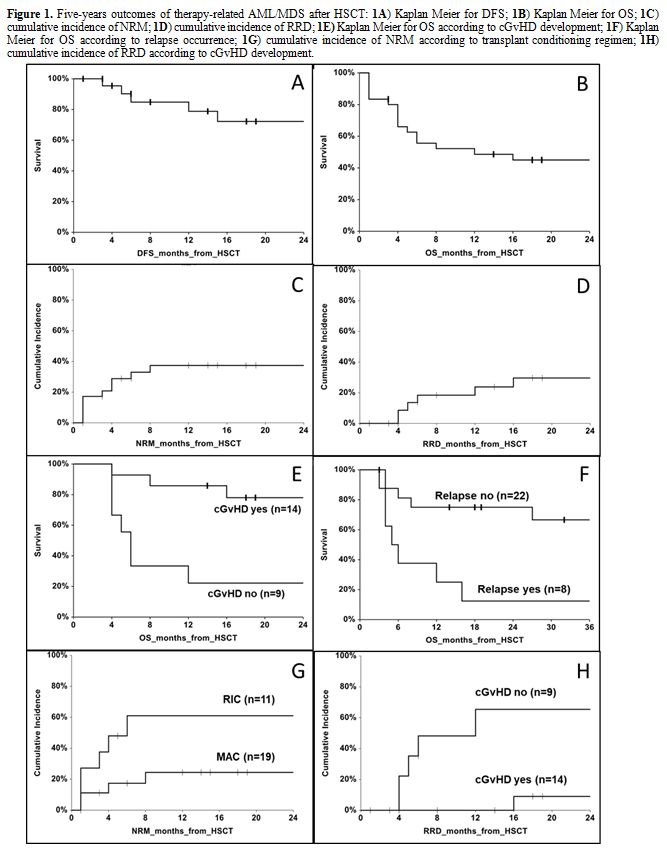 |
Figure 1. Five-years outcomes of
therapy-related AML/MDS after HSCT: 1A)
Kaplan Meier for DFS; 1B) Kaplan
Meier for OS; 1C) cumulative
incidence of NRM; 1D) cumulative
incidence of RRD; 1E) Kaplan Meier
for OS according to cGvHD development; 1F)
Kaplan Meier for OS according to relapse occurrence; 1G) cumulative incidence of NRM according to transplant
conditioning regimen; 1H) cumulative
incidence of RRD according to cGvHD development.
|
Discussion
In
the last two decades, many authors published results concerning
different cohorts of patients with therapy-related acute myeloid
leukemia or myelodysplasia submitted to allogeneic stem cell
transplantation. An high heterogeneity in the percentage of OS (22% to
66%), NRM (21% to 58%) and relapse rate (26% to 42%) come to light from
these experience.[2,27,28,38,51-62] Each of these studies highlighted a
different key point in this transplant setting, which might affect
outcome after HSCT. The mainly predicting factor for OS resulted the
karyotype and the recipient performance status at transplant.[38,54,56]
Patients achieving a CR before transplantation showed better outcomes,
whereas multiple therapy lines increase organ damage as well as the
incidence of neutropenia, infection events and the immunosuppression of
the patient increase TRM.[54,60,61] Patients at risk for
treatment-related myeloid neoplasms should be followed closely and be
considered for stem-cell transplantation early in the course of
myelodysplasia.[38,58,61] Considering the incremented risk of relapse
according to blasts percentage, patients with secondary MDS should be
direct to transplantation before the progression into AML, and if
secondary AML occurs, they should be transplanted as soon as
possible.[61] For patients who did not achieved a CR pre-transplant,
rapid transplantation, also considering alternative donor, could offer
a reasonable outcome, reducing the risk of deterioration of the
patient’s performance status. OS after HSCT in patients aged 60 years
or above was very poor.[50,51,67,68] Reduced intensity conditioning and
conditioning with targeted busulphan dose[38,51,58] might reduce TRM,
especially for those patients with a reduced organ reserve. As reported
for patients with de novo MDS,[69] pre-transplant disease stage,
cytogenetic risk group,[57,56] type of therapy given for the original
disease, transplant conditioning regimen, and patient age[61]
significantly affect relapse-free survival among patients with
secondary MDS/t-AML.[38] Concerning to stem cell source, peripheral
blood instead of bone marrow appeared to reduce NRM38 and relapse
rate[38,57] and to improve OS.[38] On the other hand, controversial
data were reported relative to donor source impact on OS.[2,27,38,53,70]
In
our cohort, global OS appeared to fit with those reported from several
authors (40.5% vs 22-66%), whereas NRM appeared the major cause of
death, even if the NRM rate was comparable to others data (44% vs
21-58%).[2,27,28,38,51-62] Surprisingly, we observed an high DFS
(72.2%) perhaps attributable to high cGvHD rate after HSCT,
corresponding to an enhanced GvL effect. In fact, among patients with
cGvHD a reduced RRD and an increased OS were registered. Graft-versus
leukemia (GvL) effect, especially associated with chronic GvHD,
improved DFS and OS also in adverse karyotype t-MN submitted to
HSCT.[71] Probably due to the small size of our study group, no
differences in terms of post-transplant outcomes emerged dividing
patients according to recipient age, previous treatment, disease status
at transplant, karyotype, donor or stem cell source. Unexpectedly, we
found a higher NRM among patients who had received a RIC transplant as
compared to ABL, but no differences in performance status,
pre-transplant risk score or disease status existed between the two
groups.
An interesting feature revealed by our curves was that
DFS reached a plateau approximately after the first year post HSCT,
while OS reached its prolonged plateau after the second one. In fact,
no relapse was ascertained after the first year post-HSCT, so that
eighteen patients (56.7%) obtained and maintained a complete remission
after HSCT. On the other hand, no deaths were recorded after the second
year post-HSCT, with an OS of 40.5% at the follow up time.
Conclusions
The
incidence of t-MN is increasing as more individuals survive treatment
for a primary cancer diagnosis. At t-MN diagnosis,[72] physicians
should evaluate molecular and cytogenetic risk of the disease,
performance status, age and comorbidities of patients, and should start
HLA-typing to timely detect a suitable donor. Older patients with poor
performance status should be offered clinical trials or best supportive
care. For fit patients, molecular and cytogenetics stratification is
crucial. t-APL might benefit from standard first line protocols.
Favorable karyotype t-MN should be treated with standard induction
chemotherapy followed by high dose cytarabine consolidation course.
Normal karyotype t-MN could receive standard induction chemotherapy
followed by HSCT while poor molecular karyotype t-MN should be
encouraged to participate in prospective clinical trials specifically
designed and they should be considered early for allogeneic HCT.[51]
Upfront HSCT could be offered to patients with low blast count and poor
performance status.
Acknowledgements
This
study was supported by Centro di Ricerca sulle Cellule Staminali
Emopoietiche e le Terapie Cellulari, Università Cattolica del Sacro
Cuore in Rome.
References
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz
MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016
revision to the World Health Organization classification of myeloid
neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391-405. doi:
10.1182/blood-2016-03-643544. Review.
https://doi.org/10.1182/blood-2016-03-643544

- Alam
N, Atenafu EG, Kuruvilla J, Uhm J, Lipton JH, Messner HA, Kim DH,
Seftel M, Gupta V. Outcomes of patients with therapy-related
AML/myelodysplastic syndrome (t-AML/MDS) following hematopoietic cell
transplantation. Bone Marrow Transplant. 2015;50:1180-6. doi:
10.1038/bmt.2015.151. Epub 2015 Jun 29.
https://doi.org/10.1038/bmt.2015.151

- Kayser
S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, von Lilienfeld-Toal
M, Wilhelm S, Kündgen A, Götze K, Rummel M, Nachbaur D, Schlegelberger
B, Göhring G, Späth D, Morlok C, Zucknick M, Ganser A, Döhner H,
Schlenk RF; German-Austrian AMLSG. The impact of therapy-related acute
myeloid leukemia (AML) on outcome in 2853 adult patients with newly
diagnosed AML. Blood. 2011 Feb 17;117(7):2137-45. doi:
10.1182/blood-2010-08-301713. Epub 2010 Dec 2.
https://doi.org/10.1182/blood-2010-08-301713

- Smith
SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, Vardiman JW,
Rowley JD, Larson RA. Clinical-cytogenetic associations in 306 patients
with therapy-related myelodysplasia and myeloid leukemia: the
University of Chicago series. Blood. 2003 Jul 1;102(1):43-52. Epub 2003
Mar 6. https://doi.org/10.1182/blood-2002-11-3343
PMid:12623843

- Ornstein
MC, Mukherjee S, Mohan S, Elson P, Tiu RV, Saunthararajah Y, Kendeigh
C, Advani A, Kalaycio M, Maciejewski JP, Sekeres MA. Predictive factors
for latency period and a prognostic model for survival in patients with
therapy-related acute myeloid leukemia. Am J Hematol. 2014
Feb;89(2):168-73. doi: 10.1002/ajh.23605. Epub 2013 Nov 21.
https://doi.org/10.1002/ajh.23605

- Easton
DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG,
Struewing JP, Morrison J, Field H, Luben R, Wareham N, Ahmed S, Healey
CS, Bowman R; SEARCH collaborators., Meyer KB, Haiman CA, Kolonel LK,
Henderson BE, Le Marchand L, Brennan P, Sangrajrang S, Gaborieau V,
Odefrey F, Shen CY, Wu PE, Wang HC, Eccles D, Evans DG, Peto J,
Fletcher O, Johnson N, Seal S, Stratton MR, Rahman
N, Chenevix-Trench G, Bojesen SE, Nordestgaard BG, Axelsson CK,
Garcia-Closas M, Brinton L, Chanock S, Lissowska J, Peplonska B,
Nevanlinna H, Fagerholm R, Eerola H, Kang D, Yoo KY, Noh DY, Ahn SH,
Hunter DJ, Hankinson SE, Cox DG, Hall P, Wedren S, Liu J, Low YL,
Bogdanova N, Schürmann P, Dörk T, Tollenaar RA, Jacobi CE, Devilee P,
Klijn JG, Sigurdson AJ, Doody MM, Alexander BH, Zhang J, Cox A, Brock
IW, MacPherson G, Reed MW, Couch FJ, Goode EL, Olson JE,
Meijers-Heijboer H, van den Ouweland A, Uitterlinden A, Rivadeneira F,
Milne RL, Ribas G, Gonzalez-Neira A, Benitez J, Hopper JL, McCredie M,
Southey M, Giles GG, Schroen C, Justenhoven C, Brauch H, Hamann U, Ko
YD, Spurdle AB, Beesley J, Chen X; kConFab.; AOCS Management Group.,
Mannermaa A, Kosma VM, Kataja V, Hartikainen J, Day NE, Cox DR, Ponder
BA. Genome-wide association study identifies novel breast cancer
susceptibility loci. Nature. 2007 Jun 28;447(7148):1087-93.
https://doi.org/10.1038/nature05887 PMid:17529967 PMCid:PMC2714974

- Azim
HA Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic
effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011
Sep;22(9):1939-47. doi: 10.1093/annonc/mdq683. Epub 2011 Feb 2.
https://doi.org/10.1093/annonc/mdq683

- Bhatia
S. Therapy-related myelodysplasia and acute myeloid leukemia. Semin
Oncol. 2013 Dec;40(6):666-75. doi: 10.1053/j.seminoncol.2013.09.013.
https://doi.org/10.1053/j.seminoncol.2013.09.013

- Churpek
JE, Larson RA. The evolving challenge of therapy-related myeloid
neoplasms. Best Pract Res Clin Haematol. 2013 Dec;26(4):309-17. doi:
10.1016/j.beha.2013.09.001.
Review. https://doi.org/10.1016/j.beha.2013.09.001

- Krishnan
A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, Fung H,
Bhatia R, Kashyap A, Molina A, O'Donnell MR, Parker PA, Sniecinski I,
Snyder DS, Spielberger R, Stein A, Forman SJ. Predictors of
therapy-related leukemia and myelodysplasia following autologous
transplantation for lymphoma: an assessment of risk factors. Blood.
2000 Mar 1;95(5):1588-93. PMid:10688812

- Govindarajan
R, Jagannath S, Flick JT, Vesole DH, Sawyer J, Barlogie B, Tricot G.
Preceding standard therapy is the likely cause of MDS after
autotransplants for multiple myeloma. Br J Haematol. 1996
Nov;95(2):349-53.
https://doi.org/10.1046/j.1365-2141.1996.d01-1891.x
PMid:8904891

- Pedersen-Bjergaard
J, Pedersen M, Myhre J, Geisler C. High risk of therapy-related
leukemia after BEAM chemotherapy and autologous stem cell
transplantation for previously treated lymphomas is mainly related to
primary chemotherapy and not to the BEAM-transplantation procedure.
Leukemia. 1997 Oct;11(10):1654-60.
https://doi.org/10.1038/sj.leu.2400809 PMid:9324285

- Gilliland
DG, Gribben JG. Evaluation of the risk of therapy-related MDS/AML after
autologous stem cell transplantation. Biol Blood Marrow Transplant.
2002;8(1):9-16. https://doi.org/10.1053/bbmt.2002.v8.pm11846355

- Milligan
DW, Ruiz De Elvira MC, Kolb HJ, Goldstone AH, Meloni G, Rohatiner AZ,
Colombat P, Schmitz N. Secondary leukaemia and myelodysplasia after
autografting for lymphoma: results from the EBMT. EBMT Lymphoma and
Late Effects Working Parties. European Group for Blood and Marrow
Transplantation. Br J Haematol. 1999 Sep;106(4):1020-6.
https://doi.org/10.1046/j.1365-2141.1999.01627.x PMid:10520006

- Wheeler
C, Khurshid A, Ibrahim J, Elias A, Mauch P, Ault K, Antin J. Incidence
of post-transplant myelodysplasia/acute leukemia in non-Hodgkin's
lymphoma patients compared with Hodgkin's disease patients undergoing
autologous transplantation following cyclophosphamide, carmustine, and
etoposide (CBV). Leuk Lymphoma. 2001 Feb;40(5-6):499-509.
https://doi.org/10.3109/10428190109097649 PMid:11426523

- Bhatia
S, Ramsay NK, Steinbuch M, Dusenbery KE, Shapiro RS, Weisdorf DJ,
Robison LL, Miller JS, Neglia JP. Malignant neoplasms following bone
marrow transplantation. Blood. 1996 May 1;87(9):3633-9.
PMid:8611687

- André
M, Henry-Amar M, Blaise D, Colombat P, Fleury J, Milpied N, Cahn JY,
Pico JL, Bastion Y, Kuentz M, Nedellec G, Attal M, Fermé C,
Gisselbrecht C. Treatment-related deaths and second cancer risk after
autologous stem-cell transplantation for Hodgkin's disease. Blood. 1998
Sep 15;92(6):1933-40. PMid:9731050

- Larson
RA, Wang Y, Banerjee M, Wiemels J, Hartford C, Le Beau MM, Smith MT.
Prevalence of the inactivating 609C>T polymorphism in the
NAD(P)H:quinone oxidoreductase (NQO1) gene in patients with primary and
therapy-related myeloid leukemia. Blood. 1999 Jul 15;94(2):803-7.
PMid:10397748

- Ellis
NA, Huo D, Yildiz O, Worrillow LJ, Banerjee M, Le Beau MM, Larson RA,
Allan JM, Onel K. MDM2 SNP309 and TP53 Arg72Pro interact to alter
therapy-related acute myeloid leukemia susceptibility. Blood. 2008 Aug
1;112(3):741-9. doi: 10.1182/blood-2007-11-126508. Epub 2008 Apr 21.
https://doi.org/10.1182/blood-2007-11-126508

- Link
DC, Schuettpelz LG, Shen D, Wang J, Walter MJ, Kulkarni S, Payton JE,
Ivanovich J, Goodfellow PJ, Le Beau M, Koboldt DC, Dooling DJ, Fulton
RS, Bender RH, Fulton LL, Delehaunty KD, Fronick CC, Appelbaum EL,
Schmidt H, Abbott R, O'Laughlin M, Chen K, McLellan MD, Varghese N,
Nagarajan R, Heath S, Graubert TA, Ding L, Ley TJ, Zambetti GP, Wilson
RK, Mardis ER. Identification of a novel TP53 cancer susceptibility
mutation through whole-genome sequencing of a patient with
therapy-related AML. JAMA. 2011 Apr 20;305(15):1568-76. doi:
10.1001/jama.2011.473. https://doi.org/10.1001/jama.2011.473

- Schulz
E, Valentin A, Ulz P, Beham-Schmid C, Lind K, Rupp V, Lackner H,
Wölfler A, Zebisch A, Olipitz W, Geigl J, Berghold A, Speicher MR, Sill
H. Germline mutations in the DNA damage response genes BRCA1, BRCA2,
BARD1 and TP53 in patients with therapy related myeloid neoplasms. J
Med Genet. 2012 Jul;49(7):422-8. doi: 10.1136/jmedgenet-2011-100674.
Epub 2012 May 31. https://doi.org/10.1136/jmedgenet-2011-100674

- Abou
Zahr A, Kavi AM, Mukherjee S, Zeidan AM. Therapy-related
myelodysplastic syndromes, or are they?. Blood Rev. 2016 Nov 24. pii:
S0268-960X(16)30111-4. doi: 10.1016/j.blre.2016.11.002. [Epub ahead of
print] Review. https://doi.org/10.1016/j.blre.2016.11.002

- Leone
G, Fianchi L, Pagano L, Voso MT. Incidence and susceptibility to
therapy-related myeloid neoplasms. Chem Biol Interact. 2010;184:39 –
45. https://doi.org/10.1016/j.cbi.2009.12.013 PMid:20026017

- Cristy M. Active bone marrow
distribution as a function of age in humans. Phys Med Biol.
1981;26:389–400. https://doi.org/10.1088/0031-9155/26/3/003
PMid:7243876

- Sun
LM, Lin CL, Lin MC, Liang JA, Kao CH. Radiotherapy- and
chemotherapy-induced myelodysplasia syndrome: a nationwide
population-based nested case-control study. Medicine. 2015;94:e737.

- Allan
JM, Wild CP, Rollinson S, Willett EV, Moorman AV, Dovey GJ, Roddam PL,
Roman E, Cartwright RA, Morgan GJ. Polymorphism in glutathione
S-transferase P1 is associated with susceptibility to
chemotherapy-induced leukemia. Proc Natl Acad Sci U S A. 2001 Sep
25;98(20):11592-7. Epub 2001 Sep 11.

- Litzow
MR, Tarima S, Pérez WS, Bolwell BJ, Cairo MS, Camitta BM, Cutler CS, de
Lima M, Dipersio JF, Gale RP, Keating A, Lazarus HM, Luger S, Marks DI,
Maziarz RT, McCarthy PL, Pasquini MC, Phillips GL, Rizzo JD, Sierra J,
Tallman MS, Weisdorf DJ. Allogeneic transplantation for therapy-related
myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010 Mar
4;115(9):1850-7. doi: 10.1182/blood-2009-10-249128.
https://doi.org/10.1182/blood-2009-10-249128

- Kröger
N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D,
Devergie A, Ruutu T, Cornish J, Ljungman P, Gratwohl A, Cordonnier C,
Beelen D, Deconinck E, Symeonidis A, de Witte T; Myelodysplastic
Syndromes Subcommittee of The Chronic Leukaemia Working Party of
European Group for Blood and Marrow Transplantation (EBMT). Risk
factors for therapy-related myelodysplastic syndrome and acute myeloid
leukemia treated with allogeneic stem cell transplantation.
Haematologica. 2009 Apr;94(4):542-9. doi: 10.3324/haematol.2008.000927.
https://doi.org/10.3324/haematol.2008.000927

- Chang
MC, Chen TY, Tang JL, Lan YJ, Chao TY, Chiu CF, Ho HT. Leukapheresis
and cranial irradiation in patients with hyperleukocytic acute myeloid
leukemia: no impact on early mortality and intracranial hemorrhage. Am
J Hematol. 2007 Nov;82(11):976-80. https://doi.org/10.1002/ajh.20939
PMid:17636473

- Larson
RA, Le Beau MM. Prognosis and therapy when acute promyelocytic leukemia
and other "good risk" acute myeloid leukemias occur as a
therapy-related myeloid neoplasm. Mediterr J Hematol Infect Dis.
2011;3(1):e2011032. doi: 10.4084/MJHID.2011.032. Epub 2011 Jul 8.
https://doi.org/10.4084/mjhid.2011.032

- Klimek
VM. Recent advances in the management of therapy-related
myelodysplastic syndromes and acute myeloid leukemia. Curr Opin
Hematol. 2013 Mar;20(2):137-43. doi: 10.1097/MOH.0b013e32835d82e6.
Review. https://doi.org/10.1097/MOH.0b013e32835d82e6

- Kantarjian
HM, Estey EH, Keating MJ. Treatment of therapy-related leukemia and
myelodysplastic syndrome. Hematol Oncol Clin North Am. 1993;7:81-107.
PMid:7680643

- Hake
CR, Graubert TA, Fenske TS. Does autologous transplantation directly
increase the risk of secondary leukemia in lymphoma patients? Bone
Marrow Transplant. 2007 Jan;39(2):59-70. Epub 2006 Dec 4.
https://doi.org/10.1038/sj.bmt.1705547 PMid:17143301

- Takeyama
K, Seto M, Uike N, Hamajima N, Ino T, Mikuni C, Kobayashi T, Maruta A,
Muto Y, Maseki N, Sakamaki H, Saitoh H, Shimoyama M, Ueda R.
Therapy-related leukemia and myelodysplastic syndrome: a large-scale
Japanese study of clinical and cytogenetic features as well as
prognostic factors. Int J Hematol. 2000 Feb;71(2):144-52. PMid:10745624

- Klimek
VM, Tray NJ. Therapy-related myeloid neoplasms: what's in a name?. Curr
Opin Hematol. 2016 Mar;23(2):161-6. doi: 10.1097/MOH.0000000000000222.
https://doi.org/10.1097/MOH.0000000000000222

- Quesnel
B, Kantarjian H, Bjergaard JP, Brault P, Estey E, Lai JL, Tilly H,
Stoppa AM, Archimbaud E, Harousseau JL, Bauters F, Fenaux P.
Therapy-related acute myeloid leukemia with t(8;21), inv(16), and
t(8;16): a report on 25 cases and review of the literature. J Clin
Oncol. 1993 Dec;11(12):2370-9.
https://doi.org/10.1200/JCO.1993.11.12.2370 PMid:8246025

- Fianchi
L, Criscuolo M, Lunghi M, Gaidano G, Breccia M, Levis A, Finelli C,
Santini V, Musto P, Oliva EN, Leoni P, Aloe Spiriti A, D'Alò F, Hohaus
S, Pagano L, Leone G, Voso MT. Outcome of therapy-related myeloid
neoplasms treated with azacitidine. J Hematol Oncol. 2012 Aug 1;5:44.
doi: 10.1186/1756-8722-5-44. https://doi.org/10.1186/1756-8722-5-44

- Chang
C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, Sandmaier
BM, Witherspoon RP, Nash RA, Sanders JE, Bedalov A, Hansen JA, Clurman
BE, Storb R, Appelbaum FR, Deeg HJ. Hematopoietic cell transplantation
in patients with myelodysplastic syndrome or acute myeloid leukemia
arising from myelodysplastic syndrome: similar outcomes in patients
with de novo disease and disease following prior therapy or antecedent
hematologic disorders. Blood. 2007 Aug 15;110(4):1379-87.
https://doi.org/10.1182/blood-2007-02-076307 PMid:17488876
PMCid:PMC1939908

- Garcia-Manero
G, Huang X, Cabrero M, Di Nardo CD, Pemmaraju N, Daver NG, Borthakur G,
Wierda WG, Kadia T, Alvarado Y, Cortes JE. A bayesian phase II
randomized trial of azacitidine versus Azacitidine Vorinostat in
patients with newly diagnosed AML or high risk MDS with poor
performance status, organ dysfunction, or other comorbidities. Blood.
2014;124:3277.

- Bally
C, Thépot S, Quesnel B, Vey N, Dreyfus F, Fadlallah J, Turlure P, de
Botton S, Dartigeas C, de Renzis B, Itzykson R, Fenaux P, Adès L.
Azacitidine in the treatment of therapy related myelodysplastic
syndrome and acute myeloid leukemia (tMDS/AML): a report on 54 patients
by the Groupe Francophone Des Myelodysplasies (GFM). Leuk Res.
2013;37:637-40. doi:10.1016/j.leukres.2013.02.014.
https://doi.org/10.1016/j.leukres.2013.02.014

- Duong
VH, Lancet JE, Alrawi E, AlAli NH, Perkins J, Field T, Epling Burnette
PK, Zhang L, List AF, Komrokji RS. Outcome of azacitidine treatment in
patients with therapy-related myeloid neoplasms with assessment of
prognostic risk stratification models. Leuk Res. 2013;37: 510-5.
doi:10.1016/j.leukres.2012.12.012.
https://doi.org/10.1016/j.leukres.2012.12.012

- Kantarjian
H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, Di Persio J, Klimek V,
Slack J, de Castro C, Ravandi F, Helmer R, Shen L, Nimer SD, Leavitt R,
Raza A, Saba H. Decitabine improves patient outcomes in myelodysplastic
syndromes: results of a phase III randomized study. Cancer.
2006;106:1794-803. doi:10.1002/cncr.21792.
https://doi.org/10.1002/cncr.21792

- Steensma
DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G, Albitar
M, Larsen JS, Arora S, Cullen MT, Kantarjian H. Multicenter study of
decitabine administered daily for 5 days every 4 weeks to adults with
myelodysplastic syndromes: the alternative dosing for outpatient
treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842-8.
doi:10.1200/JCO.2008.19.6550. https://doi.org/10.1200/JCO.2008.19.6550

- Klimek
VM, Dolezal EK, Tees MT, Devlin SM, Stein K, Romero A, Nimer SD.
Efficacy of hypomethylating agents in therapy-related myelodysplastic
syndromes. Leuk Res. 2012;36:1093-7. doi:10.1016/j.leukres.2012.04.025.
https://doi.org/10.1016/j.leukres.2012.04.025

- Fianchi
L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, Criscuolo M,
Specchia G, Maria Pogliani E, Maurillo L, Aloe-Spiriti MA, Mecucci C,
Niscola P, Rossetti E, Mansueto G, Rondoni M, Fozza C, Invernizzi R,
Spadea A, Fenu S, Buda G, Gobbi M, Fabiani E, Sica S, Hohaus S, Leone
G, Voso MT. Characteristics and outcome of therapy-related myeloid
neoplasms: Report from the Italian network on secondary leukemias. Am J
Hematol. 2015 May;90(5):E80-5. doi: 10.1002/ajh.23966. Epub 2015 Mar 3.
https://doi.org/10.1002/ajh.23966

- Sorror
ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B.
Hematopoietic cell transplantation (HCT)-specific comorbidity index: a
new tool for risk assessment before allogeneic HCT. Blood. 2005 Oct
15;106(8):2912-9. https://doi.org/10.1182/blood-2005-05-2004
PMid:15994282 PMCid:PMC1895304

- ElSawy
M, Storer BE, Pulsipher MA, Maziarz RT, Bhatia S, Maris MB, Syrjala KL,
Martin PJ, Maloney DG, Sandmaier BM, Storb R, Sorror ML. Multi-centre
validation of the prognostic value of the haematopoietic cell
transplantation- specific comorbidity index among recipient of
allogeneic haematopoietic cell transplantation. Br J Haematol.
2015;170:574–583. doi: 10.1111/bjh.13476.
https://doi.org/10.1111/bjh.13476

- Armand
P, Deeg HJ, Kim HT, Lee H, Armistead P, de Lima M, Gupta V, Soiffer RJ.
Multicentre validation study of a transplantation-specific cytogenetics
grouping scheme for patients with myelodysplastic syndromes. Bone
Marrow Transplant. 2010 May;45(5):877-85. doi: 10.1038/bmt.2009.253.
Epub 2009 Sep 28. https://doi.org/10.1038/bmt.2009.253

- Poiré
X, Labopin M, Cornelissen JJ, Volin L, Richard Espiga C, Veelken JH,
Milpied N, Cahn JY, Yacoub-Agha I, van Imhoff GW, Michallet M, Michaux
L, Nagler A, Mohty M. Outcome of conditioning intensity in acute
myeloid leukemia with monosomal karyotype in patients over 45 year-old:
A study from the acute leukemia working party (ALWP) of the European
group of blood and marrow transplantation (EBMT). Am J Hematol. 2015
Aug;90(8):719-24. doi: 10.1002/ajh.24069. Erratum in: Am J Hematol.
2016 May;91(5):E301. https://doi.org/10.1002/ajh.24069

- Koenecke
C, Göhring G, de Wreede LC, van Biezen A, Scheid C, Volin L, Maertens
J, Finke J, Schaap N, Robin M, Passweg J, Cornelissen J, Beelen D,
Heuser M, de Witte T, Kröger N; MDS subcommittee of the Chronic
Malignancies Working Party of the EBMT. Impact of the revised
International Prognostic Scoring System, cytogenetics and monosomal
karyotype on outcome after allogeneic stem cell transplantation for
myelodysplastic syndromes and secondary acute myeloid leukemia evolving
from myelodysplastic syndromes: a retrospective multicenter study of
the European Society of Blood and Marrow Transplantation.
Haematologica. 2015 Mar;100(3):400-8. doi:
10.3324/haematol.2014.116715. Epub 2014 Dec 31.
https://doi.org/10.3324/haematol.2014.116715

- Finke
J, Schmoor C, Bertz H, Marks R, Wäsch R, Zeiser R, Hackanson B.
Long-term follow-up of therapy-related myelodysplasia and AML patients
treated with allogeneic hematopoietic cell transplantation. Bone Marrow
Transplant. 2016 Jun;51(6):771-7. doi: 10.1038/bmt.2015.338. Epub 2016
Jan 11. https://doi.org/10.1038/bmt.2015.338

- Tang
FF, Huang XJ, Zhang XH, Chen H, Chen YH, Han W, Chen Y, Wang FR, Wang
Y, Wang JZ, Yan CH, Sun YQ, Mo XD, Liu KY, Xu LP. Allogeneic
hematopoietic cell transplantation for adult patients with treatment-related
acute myeloid leukemia during first remission: Comparable to de novo
acute myeloid leukemia. Leuk Res. 2016 Aug;47:8-15. doi:
10.1016/j.leukres.2016.05.005. Epub 2016 May 12.
https://doi.org/10.1016/j.leukres.2016.05.005

- Liu
N, Ning HM, Hu LD, Jiang M, Xu C, Hu JW, Wang J, Li YH, Li BT, Lou X,
Yang F, Chen JL, Su YF, Li M, Wang HY, Ren J, Feng YQ, Zhang B, Wang
DH, Chen H. Outcome of myeloablative allogeneic peripheral blood
hematopoietic stem cell transplantation for refractory/relapsed AML
patients in NR status. Leuk Res. 2015 Dec;39(12):1375-81. doi:
10.1016/j.leukres.2015.10.011. Epub 2015 Oct 19.
https://doi.org/10.1016/j.leukres.2015.10.011

- Spina
F, Alessandrino PE, Milani R, Bonifazi F, Bernardi M, Luksch R, Fagioli
F, Formica C, Farina L. Allogeneic stem cell transplantation in
therapy-related acute myeloid leukemia and myelodysplastic syndromes:
impact of patient characteristics and timing of transplant. Leuk
Lymphoma. 2012 Jan;53(1):96–102. doi:10.3109/10428194.2011.603445.
https://doi.org/10.3109/10428194.2011.603445

- Zinke-Cerwenka
W, Valentin A, Posch U, Beham-Schmid C, Groselj-Strele A, Linkesch W,
Wölfler A, Sill H. Reduced-intensity allografting in patients with
therapy-related myeloid neoplasms and active primary malignancies. Bone
Marrow Transplant. 2011 Dec;46(12):1540-4. doi:10.1038/bmt.2011.165.
Epub 2011 Aug 22. https://doi.org/10.1038/bmt.2011.165

- Armand
P, Kim HT, Mayer E, Cutler CS, Ho VT, Koreth J, Alyea EP, Antin JH, and
Soiffer RJ. Outcome of allo-SCT for women with MDS or AML occurring
after breast cancer therapy. Bone Marrow Transplant. 2010
November;45(11):1611–7. doi:10.1038/bmt.2010.20.
https://doi.org/10.1038/bmt.2010.20

- Nevill
TJ, Hogge DE, Toze CL, Nantel SH, Power MM, Abou Mourad YR, Song KW,
Lavoie JC, Forrest DL, Barnett MJ, Shepherd JD, Nitta JY, Wong S,
Sutherland HJ, Smith CA. Predictors of outcome following myeloablative
allo-SCT for therapy-related myelodysplastic syndrome and AML. Bone
Marrow Transplant. 2008 Nov;42(10):659-66. doi: 10.1038/bmt.2008.226.
Epub 2008 Aug 4. https://doi.org/10.1038/bmt.2008.226

- Witherspoon
RP, Deeg HJ, Storer B, Anasetti C, Storb R, Appelbaum FR. Hematopoietic
stem-cell transplantation for treatment-related leukemia or
myelodysplasia. J Clin Oncol. 2001 Apr 15;19(8):2134-41.
https://doi.org/10.1200/JCO.2001.19.8.2134 PMid:11304765

- de
Witte T, Hermans J, Vossen J, Bacigalupo A, Meloni G, Jacobsen N, Ruutu
T, Ljungman P, Gratwohl A, Runde V, Niederwieser D, van Biezen A,
Devergie A, Cornelissen J, Jouet JP, Arnold R, Apperley J.
Haematopoietic stem cell transplantation for patients with
myelo-dysplastic syndromes and secondary acute myeloid leukaemias: a
report on behalf of the Chronic Leukaemia Working Party of the European
Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000
Sep;110(3):620-30.
https://doi.org/10.1046/j.1365-2141.2000.02200.x
PMid:10997974

- Yakoub-Agha
I, de La Salmonière P, Ribaud P, Sutton L, Wattel E, Kuentz M, Jouet
JP, Marit G, Milpied N, Deconinck E, Gratecos N, Leporrier M, Chabbert
I, Caillot D, Damaj G, Dauriac C, Dreyfus F, François S, Molina L,
Tanguy ML, Chevret S, Gluckman E. Allogeneic bone marrow
transplantation for therapy-related myelodysplastic syndrome and acute
myeloid leukemia: a long-term study of 70 patients-report of the French
society of bone marrow transplantation. J Clin Oncol. 2000
Mar;18(5):963-71. https://doi.org/10.1200/JCO.2000.18.5.963
PMid:10694545

- Anderson
JE, Gooley TA, Schoch G, Anasetti C, Bensinger WI, Clift RA, Hansen JA,
Sanders JE, Storb R, Appelbaum FR. Stem cell transplantation for
secondary acute myeloid leukemia: evaluation of transplantation as
initial therapy or following induction chemotherapy. Blood. 1997 Apr
1;89(7):2578-85. Review. PMid:9116305

- Ballen
KK, Gilliland DG, Guinan EC, Hsieh CC, Parsons SK, Rimm IJ, Ferrara JL,
Bierer BE, Weinstein HJ, Antin JH. Bone marrow transplantation for
therapy-related myelodysplasia: comparison with primary myelodysplasia.
Bone Marrow Transplant. 1997 Nov;20(9):737-43.
https://doi.org/10.1038/sj.bmt.1700971 PMid:9384475

- Scharz
J, Tuchler H, Sole F, Mallo M, Lu-o E, Cervera J, Granada I,
Hildebrandt B, Slovak ML, Ohyashiki K, Steidl C, Fonatsch C,
Pfeilstöcker M, Nösslinger T, Valent P, Giagounidis A, Aul C, Lübbert
M, Stauder R, Krieger O, Garcia-Manero G, Faderl S, Pierce S, Le Beau
MM, Bennett JM, Greenberg P, Germing U, Haase D. New comprehensive
cytogenetic scoring system for primarymyelodysplastic syndromes (MDS)
and oligoblastic acute myeloid leukemia after MDS derived from an
international database merge. J Clin Oncol. 2012;30(8):820–829.
https://doi.org/10.1200/JCO.2011.35.6394 PMid:22331955 PMCid:PMC4874200

- Döhner
H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H,
Ebert BL, Fenaux P, Larson RA, Levine RL, Lo Coco F, Naoe T,
Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF,
Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in
adults: 2017 ELN recommendations from an international expert panel.
Blood. 2017 Jan 26;129(4):424-447. doi: 10.1182/blood-2016-08-733196.
Review. https://doi.org/10.1182/blood-2016-08-733196

- Glucksberg
H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas
ED. Clinical manifestations of graft-versus-host disease in human
recipients of marrow from HL-A-matched sibling donors. Transplantation.
1974 Oct; 18(4):295-304.
https://doi.org/10.1097/00007890-197410000-00001 PMid:4153799

- Jagasia
MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J,
Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF,
McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D,
Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler
CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME. National Institutes
of Health Consensus Development Project on Criteria for Clinical Trials
in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging
Working Group Report. Biol Blood Marrow Transplant. 2015 March; 21(3):
389–401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001 PMid:25529383
PMCid:PMC4329079

- Fang
M, Storer B, Estey E, Othus M, Zhang L, Sandmaier BM, Appelbaum FR.
Outcome of patients with acute myeloid leukemia with monosomal
karyotype who undergo hematopoietic cell transplantation. Blood. 2011
Aug 11;118(6):1490-4. doi: 10.1182/blood-2011-02-339721. Epub 2011 Jun
16. https://doi.org/10.1182/blood-2011-02-339721

- Medeiros
BC, Othus M, Fang M, Roulston D, Appelbaum FR. Prognostic impact of
monosomal karyotype in young adult and elderly acute myeloid leukemia:
the Southwest Oncology Group (SWOG) experience. Blood. 2010 Sep
30;116(13):2224-8. doi: 10.1182/blood-2010-02-270330. Epub 2010 Jun 18.
https://doi.org/10.1182/blood-2010-02-270330

- Sierra
J, Pérez WS, Rozman C, Carreras E, Klein JP, Rizzo JD, Davies SM,
Lazarus HM, Bredeson CN, Marks DI, Canals C, Boogaerts MA, Goldman J,
Champlin RE, Keating A, Weisdorf DJ, de Witte TM, Horowitz MM. Bone
marrow transplantation from HLA-identical siblings as treatment for
myelodysplasia. Blood. 2002 Sep 15;100(6):1997-2004. PMid:12200358

- Gupta
V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, Khoury HJ, Klumpp T,
Koreth J, Lazarus HM, Marks DI, Martino R, Rizzieri DA, Rowe JM,
Sabloff M, Waller EK, DiPersio JF, Bunjes DW, Weisdorf DJ. Comparable
survival after HLA-well-matched unrelated or matched sibling donor
transplantation for acute myeloid leukemia in first remission with
unfavorable cytogenetics at diagnosis. Blood. 2010 Sep
16;116(11):1839-48. doi: 10.1182/blood-2010-04-278317. Epub 2010 Jun
10. https://doi.org/10.1182/blood-2010-04-278317

- Cornelissen
JJ, Breems D, van Putten WL, Gratwohl AA, Passweg JR, Pabst T, Maertens
J, Beverloo HB, van Marwijk Kooy M, Wijermans PW, Biemond BJ, Vellenga
E, Verdonck LF, Ossenkoppele GJ, Löwenberg B. Comparative analysis of
the value of allogeneic hematopoietic stem-cell transplantation in
acute myeloid leukemia with monosomal karyotype versus other
cytogenetic risk categories. J Clin Oncol. 2012 Jun 10;30(17):2140-6.
doi: 10.1200/JCO.2011.39.6499. Epub 2012 May 7
https://doi.org/10.1200/JCO.2011.39.6499

- Zahid
MF, Parnes A, Savani BN, Litzow MR, Hashmi SK. Therapy-related myeloid
neoplasms - what have we learned so far? World J Stem Cells. 2016 Aug
26;8(8):231-42. doi: 10.4252/wjsc.v8.i8.231.
https://doi.org/10.4252/wjsc.v8.i8.231













































































