Idit Lachover Roth1, Boaz Lachover*, Guy Koren**, Carina Levin2,3, Luci Zalman4 and Ariel Koren2,3
1 Allergy and Clinical Immunology Unit, Meir Medical Center, Kfar Saba, Israel.
2 Pediatric Hematology Unit, Emek Medical Center, Afula, Israel.
3 The Ruth and Baruch Faculty of Medicine, Technion – Israel Institute of Technology, Haifa, Israel.
4 Hematology Laboratory, Emek Medical Center, Afula, Israel.
* Passed away before manuscript submission.
** Personal collaboration.
Corresponding
author: Ariel Koren MD. Pediatric Hematology Unit, Emek Medical Center,
Afula, Israel. Tel: +972-4-6495615, Fax: +972-4-6495237. E-mail:
koren_a@clalit.org.il
Published: January 1, 2018
Received: September 10, 2017
Accepted: December 8, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018008 DOI
10.4084/MJHID.2018.008
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: β-thalassemia
major is a severe disease with high morbidity. The world prevalence of
carriers is around 1.5–7%. The present study aimed to find a reliable
formula for detecting β-thalassemia carriers using an extensive
database of more than 22,000 samples obtained from a homogeneous
population of childbearing age women with 3161 (13.6%) of β-thalassemia
carriers and to check previously published formulas.
Methods:
We applied a mathematical method based on the support vector machine
(SVM) algorithm in the search for a reliable formula that can
differentiate between thalassemia carriers and non-carriers, including
normal counts or counts suspected to belong to iron-deficient women.
Results:
Shine's formula and our SVM formula showed >98% sensitivity and
>99.77% negative predictive value (NPV). All other published
formulas gave inferior results.
Conclusions:
We found a reliable formula that can be incorporated into any automatic
blood counter to alert health providers to the possibility of a woman
being a β-thalassemia carrier. A further simple hemoglobin
characterization by HPLC analysis should be performed to confirm the
diagnosis, and subsequent family studies should be carried out. Our SVM
formula is currently limited to women of fertility age until further
analysis in other groups can be performed.
|
Introduction
β-thalassemia
is considered the world's most widespread genetic disease. Between 1.5
and 7% of the world population carries one of the genes that cause
hemoglobinopathies, and about 60,000 a year are diagnosed as
β-thalassemia patients.[1,2] In Israel, the incidence of β-thalassemia
carriers is around 20% among Kurdish Jews and between 5 and 10% among
the Arab population.[3] Thalassemia patients require regular blood
transfusions and suffer from severe iron overload, which is the main
cause of morbidity and mortality. Despite an actual treatment, which
needs significant medical and financial resources,[4] quality of life
and life expectancy are still lower than in the general population. The
disease's severity, high cost and high prevalence in developing
countries with low financial capability justify the implementation of
prevention programs in those countries.
Different strategies are
implemented to cope with social and religious beliefs. In Israel, a
screening program was initiated in 1987.[5] The blood count in
β-thalassemia carriers shows low mean corpuscular volume (MCV) and low
mean corpuscular hemoglobin (MCH). These parameters, which are easily
measured by automated blood cell counters, can indicate suspicion of a
carrier state. The MCV and MCH are in the same range in β-thalassemia
carriers and patients with iron deficiency anemia (IDA), but the red
blood cell (RBC) count and red cell distribution width (RDW) can
differentiate between the two.
The gold standard for the diagnosis
of β-thalassemia carriers is electrophoresis or HPLC analysis of
hemoglobin (Hgb). Automated blood count results that suggest
β-thalassemia carrier status can significantly improve the recognition
of carriers and consequently of couples at risk. Those couples can be
referred for further genetic counseling. A reliable and inexpensive
method for mass screening of the population is needed to enable the
selection of samples for further HPLC analysis to confirm the diagnosis.
Several
investigators have tried to use blood count parameters for the
detection of suspected β-thalassemia carriers compared to patients with
IDA or normal individuals by determining cutoff points.[6] The first
attempts to use mathematical formulas were made in the early
1970s.[7-9] Those studies were performed on small samples. The only
published large-scale research was performed by Shine and Lal in
1977.[10] They developed a new formula and checked its reliability on
25,000 blood samples which included a small sample of only 138
β-thalassemia carriers (0.55%).[10] In the last few decades, more
attempts have been made using conventional mathematical methods to find
a reliable formula, but with no success.[11-35] None of the published
formulas are currently in use in daily practice because they were not
proven to be reliable enough for routine screening and detection of
thalassemia carriers. A summary of the most common published formulas
is presented in Table 1.
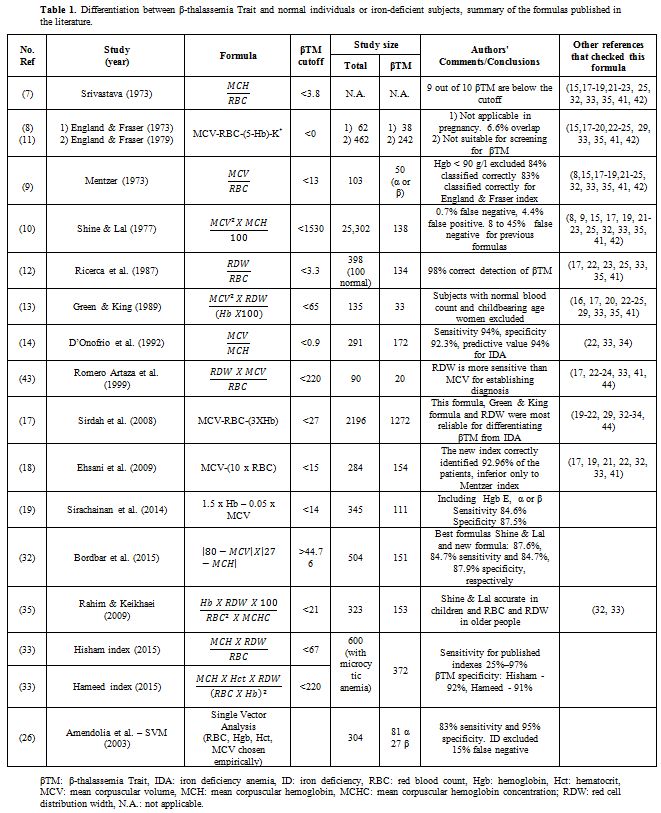 |
Table
1. Differentiation between β-thalassemia Trait and normal individuals
or iron-deficient subjects, summary of the formulas published in the
literature. |
A
sample used to validate a mathematical formula for inclusion in routine
use needs to be sufficiently large to be considered reliable. Very high
specificity and minimal false negative results are required to confirm
the formula's reliability. The aim of the present study was to find a
reliable formula using a large database of more than 22,000 samples
obtained from a relatively homogeneous population of childbearing age
woman with a relatively large percentage of β-thalassemia carriers,
3161 samples, (13.6%). The secondary aim was to use our extensive
database to check and potentially validate the previously published
formulas. We found an innovative formula that can be included as part
of the routine analysis in automated blood counters and will give an
automatic warning when a β-thalassemia carrier is suspected.
Methods
Study population.
As part of the screening program for β-thalassemia, routinely
implemented by the Israel Ministry of Health since 1987, blood samples
are collected from all childbearing age women in the northeast of
Israel.[5] The ethnic origin of the population covered by this program is equally distributed between Jews and Arabs.
Laboratory tests.
Blood samples were analyzed by blood count and by Hgb electrophoresis
(until 1999) and HPCL (afterward). HPLC analysis included determination
of Hgb A, A2, and F as well as other pathological hemoglobin.
All
the samples were analyzed at the same laboratory at the Emek Medical
Centre. From the beginning of the survey and until the early nineties
the analyses were performed using the Technicon H1 or H2 machine, and
from this time until now the Siemens Advia 2120i Hematology System was
used. Throughout all the years the hematology laboratory has always had
a regular external quality control and qualified by the ISO certificate.
We
only included blood samples from childbearing age women to establish a
homogeneous population. Just β-thalassemia carriers confirmed by Hgb
electrophoresis or HPLC and hemograms with normal Hemoglobin
electrophoresis or HPLC were included in the analysis. Blood counts
with other abnormal hemoglobin results were excluded. The whole
database included more than 80,000 samples.
Our data included all samples collected as part of the β-thalassemia screening program from 1987 to 2013. As shown in Figure 1,
64,586 samples were initially included in the study. 57,089 blood
counts were from healthy individuals in whom the β-thalassemia trait
was ruled out by hemoglobin electrophoresis or HPLC analysis. The
second group of 7497 samples belongs to proven β-thalassemia trait.
After selection of only women and samples for which all red cell
indices were included, there were 22,340 samples in our study,
including 19,179 (85.85%) samples from healthy women or women with IDA
(non-β-thalassemia cohort) and 3,161 (13.6%) samples from female
β-thalassemia carriers.
Insufficient data for SVM analysis were found in 17,143 samples.
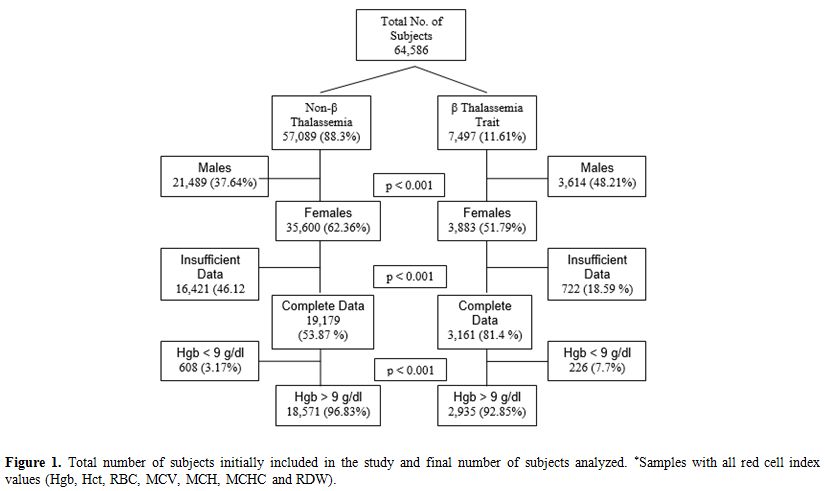 |
Figure
1. Total number of subjects initially included in the study and final
number of subjects analyzed. *Samples with all red cell index values
(Hgb, Hct, RBC, MCV, MCH, MCHC and RDW). |
From the 22,340
samples, 21,506 had Hgb level above 90 g/l, and of these, 18,571
(86.35%) belonged to the non-β-thalassemia group and 2935 (13.64%) to
the β-thalassemia carriers. The proportion of β-thalassemia carriers
was higher in the group of women with Hgb level below 90 g/l (p <
0.001).
Since the combination of being a β-thalassemia carrier
and having IDA, a frequent state in childbearing age women, can cause
more severe anemia than each condition alone, we analyzed the data from
blood counts with Hgb level above or below 90 g/l separately. Plasma
Iron or ferritin levels were not routinely analyzed as part of the
screening program.
Mathematical formulas.
We applied the blood count parameters of our collected data to the
formulas published in the literature. After this analysis, we used
another mathematical method, based on the support vector machine (SVM)
algorithm, to the same data with the aim of finding a new and reliable
formula that can differentiate between β-thalassemia carriers and
non-carriers, including normal counts and counts suspected of stemming
from women with IDA. Briefly, this SVM algorithm intends to find the
linear differentiation between two categories; in the first step the
algorithm uses measurable values for the two categories and calculates
the maximal distance that can differentiate between the two categories
with a minimal overlapping of the two series. If a linear
differentiation cannot be found, then the algorithm intends to found a
nonlinear differentiation. A more detailed explanation is beyond the
purpose of this paper and can be found in detail in the published
literature (26,36). The SVM
algorithm initially used all of the relevant data from the RBC counts,
including Hgb, Hc, RBC, MCV, MCH, MCHC, and RDW. After running the
whole database, the algorithm chooses the most relevant values and
discards the others. The cutoff of the SVM formula is "0". Any negative
number is considered as β-thalassemia carrier, and any positive number
is considered as a "noncarrier." We used the SVM method to the
samples where all the RBC indexes were available (Figure 1),
and applied the same parameters calculated for the previously published
formulas. For those formulas the originally published cutoff was used
too.
Statistical methods. Student t-test and chi-square test (χ2)
were used to calculate the differences between the β-thalassemia
carriers and non-carriers. p < 0.05 was considered
significant.
This study was approved by the local ethics committee.
Results
All
of the RBC parameters differed significantly between the β-thalassemia
carriers and non-carriers. However, for the data obtained from both
groups with Hgb < 90 g/l, the differences between the RDW values
were not significant, probably due to the presence of combined
β-thalassemia carriers and IDA in both groups (Table 2).
The formulas already published in the literature (Table 1) were applied to our whole database, and the results are given in Table 3 for Hgb > 90 g/l and in Table 4 for Hgb < 90 g/l. The SVM equation that finally used the MCV and MCH values corrected with different constants is shown.
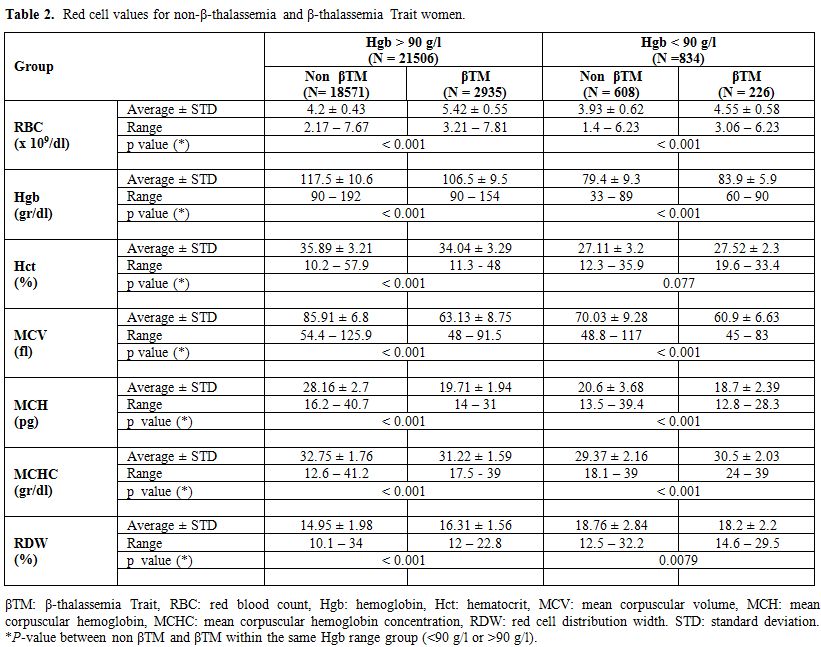 |
Table 2.
Red cell values for non-β-thalassemia and β-thalassemia Trait women. |
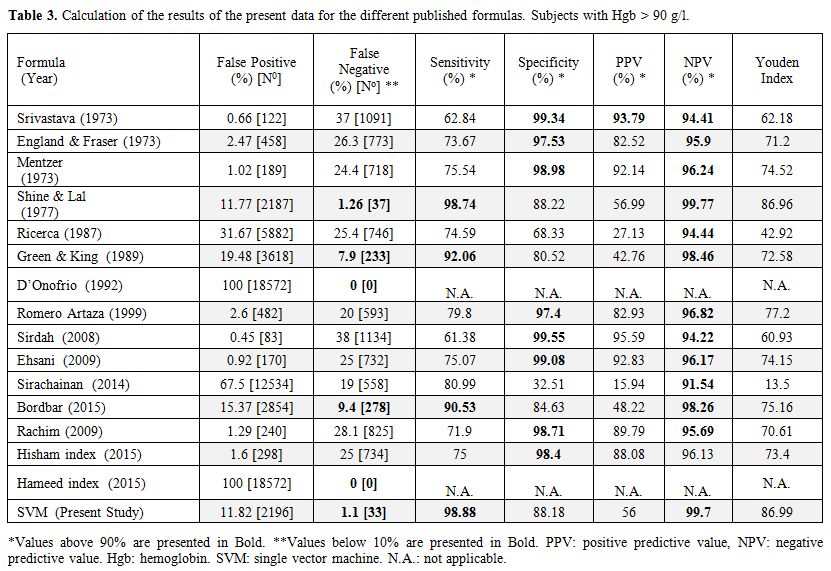 |
Table 3.
Calculation of the results of the present data for the different published formulas. Subjects with Hgb > 90 g/l. |
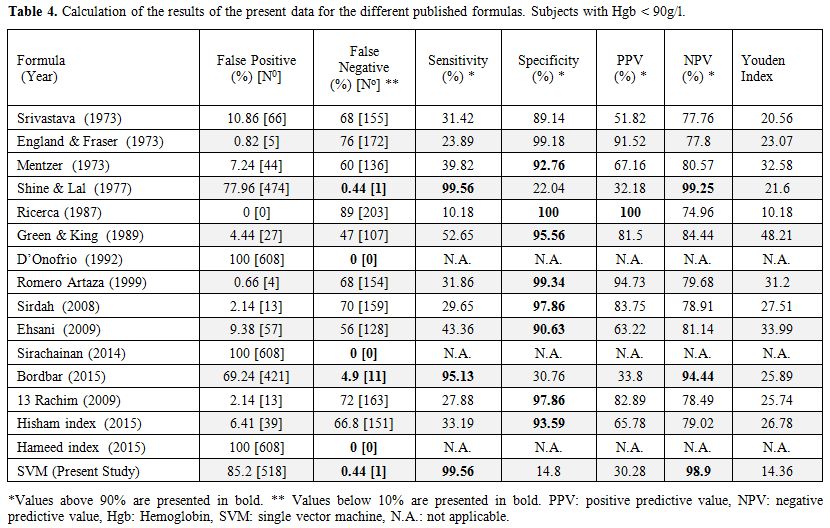 |
Table 4. Calculation of the results of the present data for the different published formulas. Subjects with Hgb < 90g/l. |
Discussion
For
the physician, RBC indices should be sufficient to raise suspicion of a
β-thalassemia carrier and therefore to perform a further evaluation.
Despite this logical rationale, most β-thalassemia carriers are only
diagnosed in population-screening programs or when a new family case is
discovered. This reasoning and the burden of β thalassemic patients for
health services have pushed many countries to develop effective
screening programs for β-thalassemia prevention.[37]
Since
the early 1970s, many investigators have tried to find a reliable
formula for β-thalassemia detection based on RBC indices, but none of
those formulas had been ever integrated as part of the routine blood
counts in automated machines. All of the studies, except one, consisted
of analyses of small numbers of patients (maximum of 2,196 samples).
The only study that analyzed a large database was published in 1977 by
Shine and Lal,[10] with 25,302 samples, but only 138 (0.55%) β-thalassemia carriers.
Kiss et al.[27]
combined low MCV and ethnic background to create an algorithm that
predicts the probability of detecting β-thalassemia carriers. Their
database included 789 patients with MCV < 80 fl and only 31 patients
diagnosed as β-thalassemia carriers. They concluded that by using MCV
and ethnic background it was sufficient to detect β-thalassemia
carriers, but that study was performed in a population where
β-thalassemia is prevalent in a specific ethnic community and might not
be applicable when the incidence of β-thalassemia carriers is dispersed
throughout the whole population.[27,28] Amendolia et al.[26]
were the first to use separation algorithms, including SVM, to
differentiate α and β-thalassemia carriers from healthy subjects, but
they excluded IDA patients. They found 83% sensitivity with the SVM
algorithm. Since the SVM analysis used all the possible relevant
parameters, in our study, we exclude from the analysis all the samples
were some of the RBC indexes are missing. The calculation for all the
other formulas was based on the same cohort that the SVM formula was
calculated. Urrechaga et al.[30] used multiple
discriminant analysis to differentiate IDA from β and α thalassemia
carriers; they did not intend to separate thalassemia carriers from
healthy subjects. Sargolzaie and Miri-Moghaddam[29]
recently described the use of binary logistic regression analysis to
find the best equation for a group of 100 β-thalassemia carriers and 77
IDA patients, in this study healthy subjects were not included. The
most recent attempts to find a reliable formula or algorithm were
limited to discriminating subjects with microcytic anemia, excluding
people with low Hgb and including small control groups.[31,35] In 2015, Bordbar et al.[32]
applied five previously published formulas and a new one on the blood
count data from 504 patients, 151 of them β-thalassemia carriers. The
highest sensitivity and specificity found for detection of
β-thalassemia carriers was 87%. A meta-analysis examined the most
frequently used indices and concluded that MCV and MCH are the most
important ones, but the sensitivity and specificity of the published
formulas were not high enough to make a definitive diagnosis of
thalassemia carriers.[34]
In a reliable formula,
a negative predictive value (NPV) higher than 99% is enough to
recognize a formula reliable for daily use. A program that intends to
become safe for mass population screening should miss as few false
negative samples as possible.
In our study, we applied all of
the published formulas to our vast database that included more than
22,000 samples with 3161 (13.6%) β-thalassemia carriers. Only one of
these formulas was reliable enough to differentiate between
β-thalassemia carriers and healthy or IDA patients: that published by
Shine and Lal.[10] This formula showed only 37 false
negative results among a total number of 2936 samples, representing a
sensitivity of 98.74% and an NPV of 99.77%. Our new SVM formula gave
similar results: 34 false negative results, a sensitivity value of
98.84% and NPV of 99.79%. All 34 false negative samples detected with
the SVM algorithm were also found to be false negative with Shine and
Lal's formula. In all of those false negative results, the MCV was
≥77.6 fl. A high MCV is known to be characteristic of specific
mutations with milder disease.[38] The specificity of those formulas was also high, 88.22% for Shine and Lal's formula and 88.15% for our SVM formula.
The
accuracy of the HPLC analysis for detection of β-thalassemia carriers
might be limited if the Hgb level is below 90 g/l. For patients with
Hgb < 90 g/l, it is recommended to correct the iron deficiency
before performing the HPLC analysis. We analyzed the results for a
group with Hgb level below 90 g/l that included 834 samples. Among
those samples we found a significant reduction of the false negative
results, 1/226 in Shine and Lal and in the SVM formula, but we had more
cases false positive, which mean that those formulas became more
sensitive but less specific. When we applied the Mentzer index to the
group with low Hgb, the results found a sensitivity of only 75.54%, a
high specificity of 98.98% but an NPV of 96.24, which is lower than the
Shine and Lal and SVM formulas.[9] Since the screening
program does not include analysis of iron status, we cannot prove which
proportion of samples with Hgb level below 90 g/l belong to
β-thalassemia carriers or IDA or both.
Although the prevention programs implemented in some countries[5,39]
have succeeded to lower the prevalence of giving birth to affected
children, they have several limitations, financial and operative.
Efficient utilization of the RBC indices' automatic alerts can increase
the number of detected β-thalassemia carriers without the need for
special attention from the medical staff. The HPLC test costs more than
a routine blood count, and if we can routinely include an automatic
alert signal in any routine blood count analyzed by the automated
machines, the referring physician or nurse can get an indication of
whether they need to perform further studies and genetic counseling.
As
far as we know, our study is the first to exam all of the formulas
present in the current literature. Our group found a new formula on a
numerous database of more than 22,000 samples, including a large group
of β-thalassemia carriers, 3161 samples (13.6%) compared to Shine study
that included a small percentage of carriers.
Our strategy for
β-thalassemia screening is based on choosing childbearing age women.
The selection of blood samples from only these women does not reduce
the reliability of our formulas or the ability to use them on a routine
basis. This choice made our study database much more homogeneous and
helped us to obtain more reliable results.
The goal of a
reliable screening test is to get as close as possible to zero false
negative results with a minimal percentage of false positive results.
We did not succeed in getting zero false negative results, but only
Shine and Lal's formula[10] and our SVM formula had
similar very low false negative results, in a range that is acceptable
for population screenings. All of the other published formulas had too
many false negative which made them insufficiently reliable as a
screening tool. Only Shine and Lal's and our SVM formulas succeeded to
detect β-thalassemia carriers with normal, or near normal blood count
indices. We succeeded in identifying 1074 β-thalassemia carriers with
Hgb ≥ 110 g/l, 67 with MCV ≥ 75 fl and even 44 women who had Hgb ≥ 110
g/l and MCV ≥ 75 fl.
The finding of 11% false positive results
meant that all those suspected of being carriers would require further
evaluation by HPLC test. We think that this is a very “small” price to
pay considering the number of carriers that we can discover in a
routine blood sample by increasing the NPV to over 99% and lowering the
undetected false negative cases (Table 3).
Integrating
a formula into a routine blood count to detect samples that raise
suspicion of being β-thalassemia carriers means that we can add an
alert in the test result that says: “suspected β-thalassemia carrier,
please refer to HPLC test.” In this way, the detection of β-thalassemia
carriers will no longer depend only on the alertness of the health-care
staff.
The accuracy of the HPLC analysis for blood samples with
low Hgb is lower than the results when Hgb is above 90 g/l. In
β-thalassemia carriers with IDA, Hgb A2
levels can be lower than in non-IDA β-thalassemia carriers and even in
the normal range, and in patients with severe IDA, it is recommended
that the iron deficiency is treated first, and then the Hgb
electrophoresis or HPLC test performed.[40] To the
best of our knowledge, this is the first study to analyze samples with
Hgb below 90 g/l. In this group, the sensitivity was still very high
(99.56%) with less than 0.5% of false negative results.
 |
Figure 2. Single vector machine algorithm calculated formula. |
Conclusions
We
found a reliable formula that can be incorporated into any automatic
blood counter and advice health providers when women are suspected of
being β-thalassemia carriers. Our SVM formula is currently limited to
women of fertility age until further analysis of other groups can be
performed. A similar study should be conducted in a large number of α
thalassemia carriers to prove the reliability of the formula in this
group. Of course, the diagnosis of α thalassemia carriers needs to be
confirmed by molecular analysis.
Acknowledgements
This
paper is dedicated to the memory of Boaz Lachover, Idit Lachover Roth's
husband, who was killed in a cycling "hit and run" accident before this
study was completed.
References
- Modell B, Darlison M. Global epidemiology of
haemoglobin disorders and derived service indicators. Bull World Health
Organ. 2008;86(6):480-7. https://doi.org/10.2471/BLT.06.036673 PMid:18568278 PMCid:PMC2647473

- De
Sanctis V, Kattamis C, Canatan D, Soliman AT, Elsedfy H, Karimi M, et
al. beta-Thalassemia Distribution in the Old World: an Ancient Disease
Seen from a Historical Standpoint. Mediterr J Hematol Infect Dis.
2017;9(1):e2017018. https://doi.org/10.4084/mjhid.2017.018 PMid:28293406 PMCid:PMC5333734

- Filon
D, Oron V, Krichevski S, Shaag A, Shaag Y, Warren TC, et al. Diversity
of beta-globin mutations in Israeli ethnic groups reflects recent
historic events. Am J Hum Genet. 1994;54(5):836-43. PMid:8178823
PMCid:PMC1918256

- Koren
A, Profeta L, Zalman L, Palmor H, Levin C, Zamir RB, et al. Prevention
of beta Thalassemia in Northern Israel - a Cost-Benefit Analysis.
Mediterr J Hematol Infect Dis. 2014;6(1):e2014012. https://doi.org/10.4084/mjhid.2014.012 PMid:24678389 PMCid:PMC3965716

- Koren
A, Zalman L, Palmor H, Ekstein E, Schneour Y, Schneour A, et al. [The
prevention programs for beta thalassemia in the Jezreel and Eiron
valleys: results of fifteen years experience]. Harefuah.
2002;141(11):938-43, 1210. PMid:12476624

- Cazzavillan
M, Barbui T, Franchi F, Chisesi T, Dini E. Comparison of Price-Jones
curves obtained with an electronic corpuscle counter in normal subjects
and in patients with thalassaemia and iron deficiency. Haematologia
(Budap). 1973;7(3):333-7

- Srivastava PC. Differentiation of thalassaemia minor from iron deficiency. Lancet. 1973;2(7821):154-5. PMid:4124080

- England
JM, Fraser PM. Differentiation of iron deficiency from thalassaemia
trait by routine blood-count. Lancet. 1973;1(7801):449-52. https://doi.org/10.1016/S0140-6736(73)91878-3

- Mentzer WC, Jr. Differentiation of iron deficiency from thalassaemia trait. Lancet. 1973;1(7808):882. https://doi.org/10.1016/S0140-6736(73)91446-3

- Shine I, Lal S. A strategy to detect beta-thalassaemia minor. Lancet. 1977;1(8013):692-4. https://doi.org/10.1016/S0140-6736(77)92128-6

- England
JM, Fraser P. Discrimination between iron-deficiency and
heterozygous-thalassaemia syndromes in differential diagnosis of
microcytosis. Lancet. 1979;1(8108):145-8. https://doi.org/10.1016/S0140-6736(79)90532-4

- Ricerca
BM, Storti S, d'Onofrio G, Mancini S, Vittori M, Campisi S, et al.
Differentiation of iron deficiency from thalassaemia trait: a new
approach. Haematologica. 1987;72(5):409-13. PMid:3121463

- Green
R, King R. A new red cell discriminant incorporating volume dispersion
for differentiating iron deficiency anemia from thalassemia minor.
Blood Cells. 1989;15(3):481-91; discussion 92-5.
PMid:2620095

- d'Onofrio
G ZG, Ricerca BM, Mancini S, Mango G. Automated measurement of red
blood cell microcytosis and hypochromia in iron deficiency and
beta-thalassemia trait. Archives of Pathology & Laboratory
Medicine. 1992;116(1):84-9. PMid:1734838

- Sonati
Mde F, Grotto HZ, Kimura EM, Costa FF. [Differentiation between
heterozygotic beta-thalassemia and iron deficiency anemia]. Rev Assoc
Med Bras. 1993;39(4):221-3. PMid:8162086

- Lima
CS, Reis AR, Grotto HZ, Saad ST, Costa FF. Comparison of red cell
distribution width and a red cell discriminant function incorporating
volume dispersion for distinguishing iron deficiency from beta
thalassemia trait in patients with microcytosis. Sao Paulo Med J.
1996;114(5):1265-9. https://doi.org/10.1590/S1516-31801996000500005 PMid:9239926

- Sirdah
M, Tarazi I, Al Najjar E, Al Haddad R. Evaluation of the diagnostic
reliability of different RBC indices and formulas in the
differentiation of the beta-thalassaemia minor from iron deficiency in
Palestinian population. Int J Lab Hematol. 2008;30(4):324-30. https://doi.org/10.1111/j.1751-553X.2007.00966.x PMid:18445163

- Ehsani
MA, Shahgholi E, Rahiminejad MS, Seighali F, Rashidi A. A new index for
discrimination between iron deficiency anemia and beta-thalassemia
minor: results in 284 patients. Pak J Biol Sci. 2009;12(5):473-5. https://doi.org/10.3923/pjbs.2009.473.475 PMid:19579993

- Sirachainan
N, Iamsirirak P, Charoenkwan P, Kadegasem P, Wongwerawattanakoon P,
Sasanakul W, et al. New mathematical formula for differentiating
thalassemia trait and iron deficiency anemia in thalassemia prevalent
area: a study in healthy school-age children. Southeast Asian J Trop
Med Public Health. 2014;45(1):174-82. PMid:24964667

- Miri-Moghaddam
E, Sargolzaie N. Cut off Determination of Discrimination Indices in
Differential Diagnosis between Iron Deficiency Anemia and beta-
Thalassemia Minor. Int J Hematol Oncol Stem Cell Res. 2014;8(2):27-32.
PMid:24800036 PMCid:PMC4003440

- Sahli
CA, Bibi A, Ouali F, Fredj SH, Dakhlaoui B, Othmani R, et al. Red cell
indices: differentiation between beta-thalassemia trait and iron
deficiency anemia and application to sickle-cell disease and
sickle-cell thalassemia. Clin Chem Lab Med. 2013;51(11):2115-24. https://doi.org/10.1515/cclm-2013-0354 PMid:23800659

- Vehapoglu A, Ozgurhan G, Demir AD, Uzuner S, Nursoy MA, Turkmen S,
et al. Hematological indices for differential diagnosis of Beta
thalassemia trait and iron deficiency anemia. Anemia. 2014;2014:576738.
https://doi.org/10.1155/2014/576738 PMid:24818016 PMCid:PMC4003757

- Beyan
C, Kaptan K, Ifran A. Predictive value of discrimination indices in
differential diagnosis of iron deficiency anemia and beta-thalassemia
trait. Eur J Haematol. 2007;78(6):524-6. https://doi.org/10.1111/j.1600-0609.2007.00853.x PMid:17419742

- Ntaios
G, Chatzinikolaou A, Saouli Z, Girtovitis F, Tsapanidou M, Kaiafa G, et
al. Discrimination indices as screening tests for beta-thalassemic
trait. Ann Hematol. 2007;86(7):487-91. https://doi.org/10.1007/s00277-007-0302-x PMid:17476506

- Rathod
DA, Kaur A, Patel V, Patel K, Kabrawala R, Patel V, et al. Usefulness
of cell counter-based parameters and formulas in detection of
beta-thalassemia trait in areas of high prevalence. Am J Clin Pathol.
2007;128(4):585-9. https://doi.org/10.1309/R1YL4B4BT2WCQDGV PMid:17875509

- Amendolia
SR, Cossu, G., Ganadu, M.L., Golosio, B., Masala, G.L., Mura, G.M. A
comparative study of K-Nearest Neighbour, Support Vector Machine and
Multi-Layer Perceptron for Thalassemia screening. Chemometrics and
Intelligent Lasboratory Systems. 2003;69:13-20. https://doi.org/10.1016/S0169-7439(03)00094-7

- Kiss
TL, Ali MA, Levine M, Lafferty JD. An algorithm to aid in the
investigation of thalassemia trait in multicultural populations. Arch
Pathol Lab Med. 2000;124(9):1320-3. PMid:10975930

- Lafferty
JD, Crowther MA, Ali MA, Levine M. The evaluation of various
mathematical RBC indices and their efficacy in discriminating between
thalassemic and non-thalassemic microcytosis. Am J Clin Pathol.
1996;106(2):201-5. https://doi.org/10.1093/ajcp/106.2.201 PMid:8712174

- Sargolzaie
N, Miri-Moghaddam E. A local equation for differential diagnosis of
beta-thalassemia trait and iron deficiency anemia by logistic
regression analysis in Southeast Iran. Hemoglobin. 2014;38(5):355-8. https://doi.org/10.3109/03630269.2014.948187 PMid:25155260

- Urrechaga
E, Aguirre U, Izquierdo S. Multivariable discriminant analysis for the
differential diagnosis of microcytic anemia. Anemia. 2013;2013:457834. https://doi.org/10.1155/2013/457834 PMid:24093062 PMCid:PMC3777209

- Zaghloul
A, Al-Bukhari TA, Bajuaifer N, Shalaby M, Al-Pakistani HA, Halawani SH,
et al. Introduction of new formulas and evaluation of the previous red
blood cell indices and formulas in the differentiation between beta
thalassemia trait and iron deficiency anemia in the Makkah region.
Hematology. 2016;21(6):351-8. https://doi.org/10.1080/10245332.2015.1133753 PMid:26907523

- Bordbar
E, Taghipour M, Zucconi BE. Reliability of Different RBC Indices and
Formulas in Discriminating between beta-Thalassemia Minor and other
Microcytic Hypochromic Cases. Mediterr J Hematol Infect Dis.
2015;7(1):e2015022. https://doi.org/10.4084/mjhid.2015.022 PMid:25745549 PMCid:PMC4344165

- Hisham
A.Getta1 HAY, Hameed M. Said Hi & Ha, are new indices in
differentiation between Iron deficiency anemia and beta-Thalassaemia
trait /A Study in Sulaimani City-Kurdistan/Iraq Journal of Dental and
Medical Sciences 2015;14(7):67-72.

- Hoffmann
JJ, Urrechaga E, Aguirre U. Discriminant indices for distinguishing
thalassemia and iron deficiency in patients with microcytic anemia: a
meta-analysis. Clin Chem Lab Med. 2015;53(12):1883-94. https://doi.org/10.1515/cclm-2015-0179 PMid:26536581

- Schoorl
M, Schoorl M, van Pelt J, Bartels PC. Application of Innovative
Hemocytometric Parameters and Algorithms for Improvement of Microcytic
Anemia Discrimination. Hematol Rep. 2015;7(2):5843. https://doi.org/10.4081/hr.2015.5843 PMid:26331001 PMCid:PMC4508552

- Burges C. Data mining and knowledge discovery. A tutorial on support vector machines for pattern recognition. 1998:121-67.

- Angastiniotis
M, Eleftheriou A, Galanello R, Harteveld CL, Petrou M,
Traeger-Synodinos J, et al. In: Old J, ed. Prevention of Thalassaemias
and Other Haemoglobin Disorders: Volume 1: Principles. 2nd ed. Nicosia,
Cyprus; 2013.
- Rund D, Filon D, Strauss
N, Rachmilewitz E, Oppenheim A. Mean corpuscular volume of
heterozygotes for beta-thalassemia correlates with the severity of
mutations. Blood. 1992;79(1):238-43. PMid:1728311

- Weatherall
DJ, Clegg JB. Inherited haemoglobin disorders: an increasing global
health problem. Bull World Health Organ. 2001;79(8):704-12.
PMid:11545326 PMCid:PMC2566499

- Wasi
P, Disthasongchan P, Na-Nakorn S. The effect of iron deficiency on the
levels of hemoglobins A2 and E. J Lab Clin Med. 1968;71(1):85-91.
PMid:5635011

- Zaghloul
A, Al-Bukhari TA, Bajuaifer N, Shalaby M, Al-Pakistani HA, Halawani SH,
et al. Introduction of new formulas and evaluation of the previous red
blood cell indices and formulas in the differentiation between beta
thalassemia trait and iron deficiency anemia in the Makkah region.
Hematology. 2016. https://doi.org/10.1080/10245332.2015.1133753 PMid:26907523

- Rahim
F, Keikhaei B. Better differential diagnosis of iron deficiencyanemia
from beta-thalassemia trait. Turk J Haematol. 2009;26(3):138-45.
PMid:27265497

- Romero
Artaza J, Carbia CD, Ceballo MF, Diaz NB. [Red cell distribution width
(RDW): its use in the characterization of microcytic and hypochromic
anemias]. Medicina (B Aires). 1999;59(1):17-22.

- Ismail
M, Patel Nisar G. Evaluation of the Diagnostic Accuracy of Twelve
Discrimination Indices for Differentiating ß-thalassemia Trait from
Iron Deficiency Anemia. Indian Journal of Public Health Research &
Development. 2016;7(1):104. https://doi.org/10.5958/0976-5506.2016.00021.8



















































