Isabela Cristina Cordeiro Farias1, Taciana Furtado Mendonça-Belmont1,2, Andreia Soares da Silva1, Kleyton Palmeira do Ó1, Felipe Ferreira3, Fernanda Silva Medeiros1, Luydson Richardson da Silva Vasconcelos4, Marcos André Cavalcanti Bezerra3,5, Aderson da Silva Araújo5, Patricia Muniz Mendes Freire de Moura1*, Betânia Lucena Domingues Hatzlhofer3, Ana Claudia Mendonça dos Anjos5 and Maria do Socorro de Mendonça Cavalcanti1,2
1 Biological Science Institute, University of Pernambuco Pernambuco, Brazil.
2 Post-Graduation Program in Biotechnology (RENORBIO), Federal Rural University of Pernambuco, Brazil.
3 Federal University of Pernambuco, Brazil.
4 Research Center Aggeu Magalhães (CPqAM), Oswaldo Cruz Foundation (Fiocruz), Brazil.
5 Hematology and Hemotherapy Foundation of Pernambuco (HEMOPE), Brazil.
Corresponding
author: Isabela Farias, Biomedic, Biological Science Institute,
University of Pernambuco, Arnóbio Marques ST, 310, 52051-280, Santo
Amaro, Recife, Brazil. Tel.: +55-81-3183-3510. E-mail:
isabela.c.farias@hotmail.com
Published: February 21, 2018
Received: September 13, 2017
Accepted: January 15, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018012 DOI
10.4084/MJHID.2018.012
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
The SOD2 polymorphism Val16Ala T>C
influences the antioxidative response. This study investigated the
association of the SOD2 polymorphism and superoxide dismutase (SOD)
activity with the vaso-occlusive crisis (VOC) and acute splenic
sequestration (ASS) in children with sickle cell anemia (SCA). One
hundred ninety-five children with SCA aged 1-9 years old were analyzed.
The TC and CC genotypes were associated with lower SOD activity
compared with the TT genotype (p=0.0321; p=0.0253, respectively).
Furthermore, TC and CC were more frequent in patients with VOC or ASS
(p=0.0285; p=0.0090, respectively). These results suggest that the SOD2
polymorphism associated with low SOD activity could be a susceptibility
factor for the occurrence of VOC and ASS.
Introduction
Sickle
erythrocytes trigger vaso-occlusive events such as vaso-occlusive
crisis (VOC) and acute splenic sequestration (ASS), mainly in sinuous
circulation where there is a limited terminal arterial blood supply.[1]
Recurrent ischemia-reperfusion, subsequent activation of the
endothelium and vascular injury induce continuous inflammatory
responses in sickle cell anemia (SCA).[2] Reactive
oxygen species (ROS) can cause significant damage to erythrocytes,
reducing their lifespan, especially in patients with SCA.[3]Superoxide
dismutase (SOD) is responsible for the first major cellular antioxidant
defense, and it catalyzes the dismutation of the superoxide anion into
hydrogen peroxide.[4] Manganese superoxide dismutase (MnSOD) isoform is encoded by the SOD2
gene, which is addressed to the mitochondria. Its role in the cell’s
defense against the deleterious effects of ROS is evident. The
polymorphism -9 T>C (V16A) in the mitochondrial targeting sequence of the gene results in the substitution of valine by alanine.[5] Studies in different diseases and populations revealed that the variant allele C of SOD2 reduces MnSOD catalytic activity.[4,6]Patients with SCA (HbSS) presented reduced SOD activity when compared to the control (HbAA).[7] In addition, Schacter et al.[7] observed that patients with SCA presenting severe manifestations had lower SOD activity.Because SOD2 has a wide range of allele frequencies depending on ethnicity,[8] and the Brazilian population has mixed ancestry, our study aimed to investigate the frequency of SOD2 polymorphisms in the controls and in patients with SCA. Materials and Methods
The
study included 173 children with SCA (HbSS), aged 1-9 years old with a
median age of 4 years; 52% were male. These patients were followed up
at neonatal screening, from 2002 until December 2012, and diagnosed at
the Hematology Hospital of the HEMOPE Foundation. Clinical data were
collected from the records in the medical files. The clinical events
considered were ASS and VOC, which consisted of painful crisis (severe
pain in the abdomen and/or chest), dactylitis (severe pain that affects
the bones of the hands and/or the feet) and acute thoracic syndrome
(which was confirmed by radiographic pulmonary infiltrates after the
occurrence of thoracic pain). The control group included 172 blood
donor volunteers without SCA and sickle cell trait (HbAS), aged 18 to
56 years, with a median age of 33 years; 60% were male.
The
patients selected for SOD determination were included randomly during
the routine consultations at HEMOPE, and they were clinically stable,
without the use of erythropoietin or hydroxyurea and/or without
transfusion history in the last three months.
SOD2 polymorphism analysis (rs4880) was performed by real time PCR using the Rotor Gene 6000TM
apparatus (Corbett Research Mortlake, Sydney, Australia) and Taqman
Genotyping Assays (ID: C___8709053_10). SOD catalytic activity was
determined in the plasma of patients using the Superoxide Dismutase
Assay KIT according to the manufacturer’s protocol (Cayman Chemical,
Ann Arbor, MI, USA).
The Hardy-Weinberg equilibrium test was
performed using ARLEQUIN software (Geneva, Switzerland), and the
differences of frequencies were analyzed using the Chi-square test with
Yates’ correction using 2×2 contingency tables. The T-Student was used
to see differences in SOD activity. The statistical analyses were
performed using EPinfo (CDC, Atlanta, USA), with p<0.05 considered
significant.
Results
Genotypic frequencies of the SOD2 polymorphism in Table 1 showed no difference between the control group and the patients (p=0.7915). The populations were in Hardy-Weinberg equilibrium.
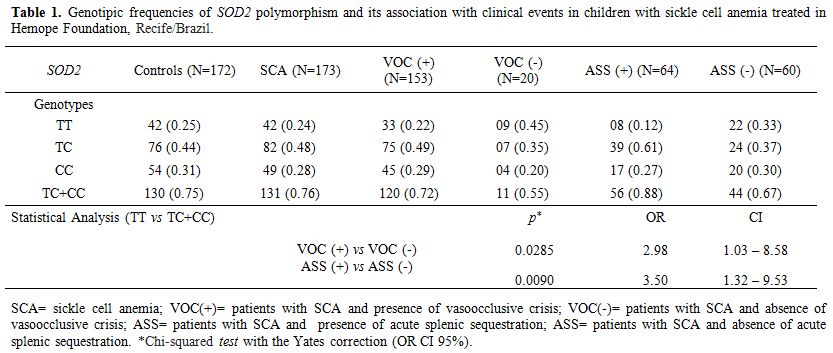 |
Table 1. Genotipic frequencies of SOD2
polymorphism and its association with clinical events in children with
sickle cell anemia treated in Hemope Foundation, Recife/Brazil. |
SOD activity was associated with the SOD2 polymorphism. TC (p=0.0321) and CC (p=0.0253) genotypes were related to decreased activity compared with TT (Figure 1).
However, the SOD activity was not associated with VOC or ASS (p=0.7603;
p=0.6909, respectively). On the other hand, a positive association was
found between the genotypes (TC+CC) of SOD2,
related to lower SOD activity, and the presence of one or more than one
VOC or ASS episodes (p=0.0285; p=0.0090, respectively) (Table 1).
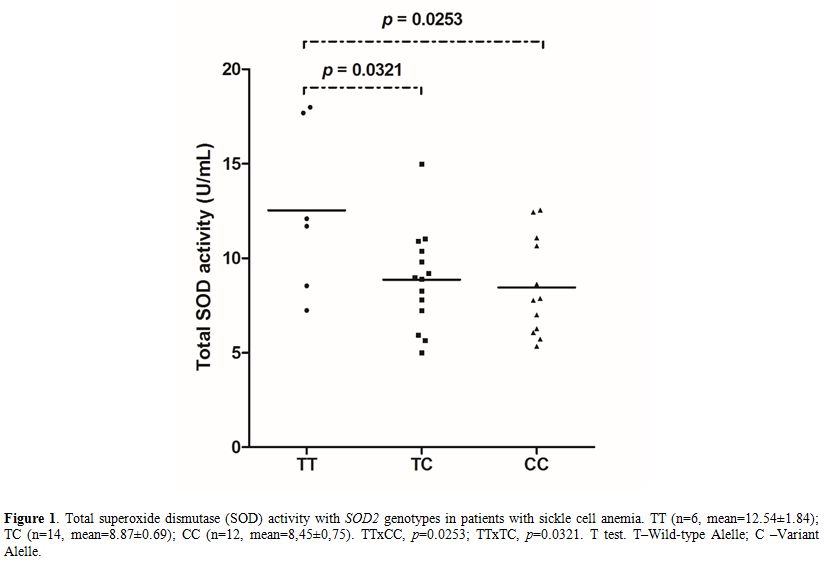 |
Figure 1. Total superoxide dismutase (SOD) activity with SOD2
genotypes in patients with sickle cell anemia. TT (n=6,
mean=12.54±1.84); TC (n=14, mean=8.87±0.69); CC (n=12, mean=8,45±0,75).
TTxCC, p=0.0253; TTxTC, p=0.0321. T test. T–Wild-type Alelle; C –Variant Alelle. |
Discussion
The frequency of SOD2
genotypes was associated with VOC and ASS in children with SCA. Our
study determined, for the first time, the frequency of the SOD2
polymorphism in Brazilian blood donor volunteers as well as in patients
with SCA. The C allele frequency in the blood donors was 0.53, similar
to healthy Caucasians (0.50);[8] however, it was higher than the Japanese population (0.015).[9] In contrast, the allele C frequency in patients with SCA was 0.35 in the Turkish population,[10] while it was 0.52 in our study. The Turkish study, as well as ours, showed no difference in SOD2 frequency when comparing patients with SCA to the healthy individuals.
The present study found an association between SOD2
genotypes (TC+CC) and the presence of VOC and ASS. The association
between the genotypes of decreased SOD activity (TC+CC) with ASS could
be explained by the fact that macrophages in the spleen of patients
with SCA would be highly activated promoting the phagocytosis of
sickled cells, however, presenting diminished ability to control
oxidative stress. Consequently, these macrophages could deregulate the
homeostasis, contributing to the production of pro-inflammatory
cytokines such as TNF-α found during VOC episodes,[11] that induces SOD2 expression to protect cells from the TNF-α pro-inflammatory effects.[12]
Human
MnSOD precursor variants imported into rat liver mitochondria showed
that the C allele precursor generated higher activity when compared to
the T allele.[5] It was demonstrated that the C allele
seems to allow efficient import of MnSOD into the mitochondrial matrix,
while the variant T allele appears to cause a partial arrest of the
precursor within the inner membrane and a decreased formation of the
active MnSOD homotetramer in the mitochondrial matrix.[5]
On the other hand, Martin et al.[4] showed that the C allele of the SOD2 polymorphism was associated with lower catalytic activity of MnSOD in cryopreserved hepatocytes. Bastaki et al.[6]
also found an association between the allele C and low activity of
MnSOD in erythrocyte isolates of 231 healthy volunteers. In part, these
reports are in accordance with our results, except that we determined
the total SOD activity in the present work. The divergent effects of
the allelic variants of SOD2
could be related to the experimental conditions of each study,
indicating that the impact of the polymorphism could differ in regards
to the species, tissue and organ compartment, such as plasma, spleen or
liver.
Studies about the effects of SOD2 as marker of oxidative stress suggest a protective role of the T allele. Hong et al.[13]
observed increased formation of 8-OHdG, a common biomarker of DNA
damage induced by ROS, in individuals carrying MnSOD TC/CC genotypes.
Accordingly, Park et al.[14] found that TC/CC
genotypes modulate the effect of 1-OHP (a biomarker of exposure to
PAHs), resulting in increased oxidative damage compared with the TT
genotype. Thus, although these studies did not measure the functional
enzyme activity, they support the idea that the T allele could be a
marker for ROS protection.
Manfredini et al.[15]
showed increased SOD activity in hemolysates of patients with SCA when
compared to the healthy individuals, indicating the activation of
chronic oxidative stress in the patients with SCA. However, Schacter et
al.[7] showed association of lower SOD activity with
severe manifestations in patients with SCA. Therefore, the individuals
presenting genetic predisposition to have lower SOD activity would have
difficulties to compensate the ROS triggered by sickling cells.
Our findings indicate that the SOD2
C allele may affect the SOD activity hampering innate cell response to
oxidative stress, which plays an essential role in the occurrence of
VOC and ASS. However, further functional studies should be conducted to
clarify the role of the SOD2 polymorphism and its influence in SCA.
Therefore, the polymorphism of SOD2
could be considered as a genetic marker for predisposition of VOC and
ASS. However, the genetic frequencies of SNPs should be limited to the
studied population due to the small sample size. In addition, the
combined analysis of SNPs and serum levels strongly suggests that these
SNPs could have a significant influence in the variation of serum
levels, which could influence the occurrence of VOC and ASS in SCA.
Acknowledgments
We acknowledge the staff Hospital HEMOPE for the cooperation in monitoring the patients and FACEPE for the financial support.
.
References
- Hebbel RP. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Invest 1997;100:83-86.

- Conran
N, Franco-Penteado CF, Costa FF. Newer aspects of the pathophysiology
of sickle cell disease vaso-occlusion. Hemoglobin 2009;33:1-16. https://doi.org/10.1080/03630260802625709 PMid:19205968 .

- Amer
J, Fibach E. Chronic oxidative stress reduces the respiratory burst
response of neutrophils from beta-thalassaemia patients. Br J Haematol
2005;129:435-441. https://doi.org/10.1111/j.1365-2141.2005.05463.x PMid:15842669 .

- Martim
RCG, Li Y, Liu Q, Jensen NS, Barker F, Doll MA, Hein DW. Manganese
superoxide dismutase V16A single-nucleotide polymorphism in the
mitochondrial targeting sequence is associated with reduced enzymatic
activity in cryopreserved human hepatocytes. DNA Cell Biol 2009;28:3-7.
https://doi.org/10.1089/dna.2008.0788 PMid:18821846 PMCid:PMC2851837.

- Sutton
A, Imbert A, Igoudjil A, Descatoire V, Cazanave S, Pessayre D, Degoul
F. The manganese superoxide dismutase Ala16Val dimorphism modulates
both mitochondrial import and mRNA stability. Pharmacogenet Genomics
2005;15:311-319. https://doi.org/10.1097/01213011-200505000-00006 PMid:15864132 .

- Bastaki
M, Huen K, Manzanillo P, Chande N, Chen C, Balmes JR, Tager IB,
Hollanda N. Genotype-activity relationship for Mn-superoxide dismutase,
glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics
2006;16:279-286. https://doi.org/10.1097/01.fpc.0000199498.08725.9c PMid:16538174 .

- Schacter
L, Warth JA, Gordon EM, Prasad A, Klein BL. Altered amount and activity
of superoxide dismutase in sickle cell anemia. FASEB J 1988;2:237-243. https://doi.org/10.1096/fasebj.2.3.3350236 PMid:3350236 .

- Ambrosone
CB, Ahn J, Singh KK, Rezaishiraz H, Furberg H, Sweeney C, Coles B,
Trovato A. Polymorphisms in genes related to oxidative stress (MPO,
MnSOD, CAT) and survival after treatment for breast cancer. Cancer Res
2005;65:1105-1111. PMid:15705913.

- Iida
R, Tsubota E, Takeshita H, Yasuda T. Multiplex single base extension
method for simultaneous genotyping of non-synonymous SNP in the three
human SOD genes. Electrophoresis 2008;29:4788-4794. https://doi.org/10.1002/elps.200800332 PMid:19016244 .

- Sogut
S, Yonden Z, Kaya H, Oktar S, Tutanc M, Yilmaz HR, Yigit A, Ozcelik N,
Gali E. Ala-9Val polymorphism of Mn-SOD gene in sickle cell anemia.
Genet Mol Res 2011;10:828-833. https://doi.org/10.4238/vol10-2gmr1106 PMid:21574139 .

- Croizat H. Circulating cytokines in sickle cell patients during steady state. Br J Haematol 1994;87:592-597. https://doi.org/10.1111/j.1365-2141.1994.tb08318.x PMid:7527647 .

- Wang
SS, Davis S, Cerhan JR, Hartge P, Severson RK, Cozen W, Lan Q, Welch R,
Chanock SJ, Rothman N. Polymorphisms in oxidative stress genes and risk
for non-Hodgkin lymphoma. Carcinogenesis 2006;27:1828-1834. https://doi.org/10.1093/carcin/bgl013 PMid:16543247.

- Hong
YC, Lee KH, Yi CH, Ha EH, Christiani DC. Genetic susceptibility of term
pregnant women to oxidative damage. Toxicol Lett 2002;129:255-262. https://doi.org/10.1016/S0378-4274(02)00014-0 .

- Park
SY, Lee KH, Kang D, Lee KH, Ha EH, Hong YC. Effect of genetic
polymorphisms of MnSOD and MPO on the relationship between PAH exposure
and oxidative DNA damage. Mutat Res 2006;593:108-115. https://doi.org/10.1016/j.mrfmmm.2005.06.022 PMid:16084535 .

- Manfredini
V, Lazzaretti LL, Griebeler IH, Santin AP, Brandão VD, Wagner S, Castro
SM, Peralba MdoC, Benfato MS. Blood antioxidant parameters in sickle
cell anemia patients in steady state. J Natl Med Assoc
2008;100:897-902. https://doi.org/10.1016/S0027-9684(15)31402-4 .

[TOP]

















