Rossana Mineri1, Jacopo Mariotti2, Barbara Sarina2, Lucio Morabito2, Roberto Crocchiolo2, Stefania Bramanti2, Tiziana Sarno1, Federica Tordato3, Carmelo Carlo-Stella2, Armando Santoro2,4 and Luca Castagna2.
1 Molecular
Biology Section, Clinical Investigation Laboratory, Humanitas Clinical
and Research Center, Via Manzoni 56, Rozzano 20089, Italy.
2 Hematology Unit, Humanitas Cancer Center, Humanitas Clinical and Research Center, Via Manzoni 56, Rozzano 20089, Italy.
3 Infectious Disease Unit, Humanitas Clinical and Research Center, Via Manzoni 56, Rozzano 20089, Italy.
4 Humanitas University, Via Alessandro Manzoni 113, 20089 Rozzano (Mi), Italy.
Corresponding
author: Rossana Mineri, PhD. Molecular Biology Section, Clinical
Investigation Laboratory, Humanitas Clinical and Research Center,
Via Manzoni 56, 20089 Rozzano, Italy. Tel. 0039 02 28844748. E-mail:
rossana.mineri@humanitas.it
Published: February 15, 2018
Received: October 3, 2017
Accepted: January 31, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018013 DOI
10.4084/MJHID.2018.013
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
The monitoring of Human Herpesvirus 6
(HHV-6) after allogeneic stem cell transplantation has proven to be
useful in preventing life-threatening complications; however, the
pathogenic role of HHV-6 after autologous transplantation is not
well-characterized, although viral reactivation might be responsible
for significant complications even after this type of transplant. Here
we report, for the first time to our knowledge, the case of a patient
with chromosomally integrated HHV-6 (ciHHV-6), presenting with high
titers of HHV-6 DNA copies after autologous transplantation, mimicking
HHV-6 reactivation. The presence of viral DNA in the follicle bulb
confirmed the ciHHV-6 and allowed for the discontinuation of the
antiviral treatment. Due to the increasing awareness of HHV-6 potential
pathogenicity and the fact that ciHHV-6 is expected in 1-2% of the
population, such a case might be helpful in recognizing ci HHV-6, thus
avoiding unnecessary and potentially toxic antiviral therapy once the
viral genomic integration is confirmed.
|
Introduction
Human
herpesvirus 6 (HHV-6) reactivation may be responsible for severe side
effects after allogeneic hematopoietic stem cell transplantation
(allo-HSCT) with early post-transplant mortality.[1-3]
HHV-6 reactivation is typically detected 2 to 4 weeks after allo-HSCT
and is associated with hepatitis, pneumonitis, CMV reactivation, fever,
skin rash, myelosuppression, encephalitis, acute graft-versus-host
disease; therefore, its regular monitoring is recommended after those
allo-HSCTs with a higher risk of reactivation, such as cord blood or
haploidentical HSCT.[4-5] At our center, the control
of HHV-6 is routinely performed after cord or haploidentical HSCT,
whereas for all the other transplant settings (i.e., allo-HSCT from
HLA-identical donor, or autologous stem cell transplantation, ASCT) the
search for HHV-6 is made only in the presence of clinical conditions
evoking a potential role of the virus, such as prolonged aplasia or
cutaneous rash.[6] Recently, it has been discovered
that 1% to 2% of the population have chromosomally integrated HHV-6
(ciHHV-6), that is the presence of HHV-6 in every somatic cell leading
to high copy numbers of the virus in the absence of any viral
reactivation or disease.[7-10]
Methods
Here
we present a case of a patient affected by non-Hodgkin’s lymphoma
undergoing high-dose chemotherapy followed by ASCT, with ciHHV-6
(detected through CMV HHV6,7,8 R-gene®, bioMérieux) mimicking viral
reactivation early after transplant.
Results
A
66-years old man was diagnosed with stage IVA diffuse large B-cell
lymphoma in December 2015 following the appearance of hepatomegaly,
leading to the detection of a 7-cm hepatic mass by abdominal
ultrasound. Complete staging showed sub- and supradiaphragmatic nodal
and extranodal disease (liver, lung), with a Revised-International
Prognostic Index (R-IPI)=4 (stage, LDH higher than the average range,
age> 60 years old and extranodal disease) and an NCCN IPI=5. The
patient's history disclosed myocardial infarction 25 years before and
concurrent HCV positivity. He received six cycles of full-dose R-CHOP
with concomitant central nervous system prophylaxis, from April to July
2016. A new disease assessment in August 2016 showed metabolic complete
remission. Due to the high-risk features at diagnosis, consolidation
therapy with high-dose chemotherapy followed by ASCT had been planned,
and the patient was admitted to the transplantation unit at the end of
August to receive ASCT, after conditioning chemotherapy with FEAM
(fotemustine, etoposide, cytarabine, melphalan) regimen.[11] The aplastic phase was complicated by NCI-CTC grade 3 mucositis and Aspergillus pneumonia, classified as probable, according to EORTC criteria,[12]
successfully treated with voriconazole (the patient has been receiving
prophylaxis with fluconazole). On day +15 after ASCT, a determination
of HHV-6 viral load was made because of persistent aplasia, showing
9.7x10e5 copies/mL, with a total leucocytes count of 1,380x10e6/L.
Molecular analysis by PCR was negative for CMV and EBV. Then, in the
hypothesis of HHV-6 reactivation, known to have an incidence of 8-15%
after ASCT,[13-15] antiviral therapy with foscarnet
was started, and subsequent shift to ganciclovir was realized, after
hematological recovery. However, the constant increase of HHV-6 load,
observed in conjunction of the augmentation of the leucocyte counts
(see Figure 1), led to the
alternative hypothesis of ciHHV-6, soon confirmed by the analysis of
patient’s follicle bulb at the beginning of October.
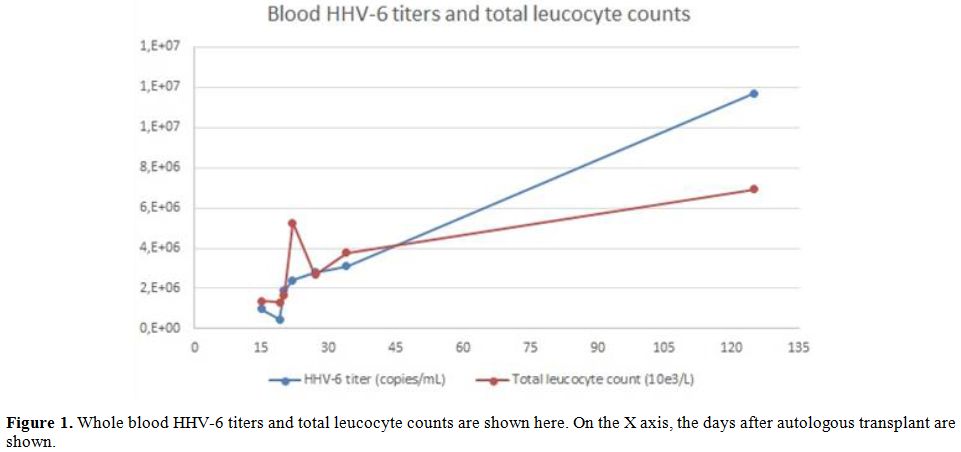 |
Figure 1. Whole blood HHV-6 titers and
total leucocyte counts are shown here. On the X axis, the days after
autologous transplant are shown. |
Indeed,
it is known that ciHHV-6 corresponds to the presence of one or more
HHV-6 copies per white blood cell, with high HHV-6 titers persisting
over time in blood, and of virus in tissues, allowing for a diagnosis
made through the analysis of follicle bulb or nails.[16]
Nonetheless, a confirmatory test is needed in the diagnostic process,
as we did in the present case. The patient was discharged in good
clinical conditions at day +28 from ASCT, and the antiviral treatment
was discontinued at the moment of hospital discharge. Finally, the
cause of the prolonged aplasia was not identified. Three months later
the patient was healthy, and a CT scan confirmed the complete remission
and a favorable evolution of the fungal infection. As expected,
leukocyte, neutrophil and platelet counts were normalized, while HHV-6
blood titer was 1.14x10e7 copies/mL with a total leucocyte count of
6,920x10e6/L in early January 2017.
Discussion
The
present clinical case shows a genomic integration of HHV-6 mimicking
reactivation early after autologous stem cell transplantation. Similar
cases of transmission of integrated HHV-6 were described in recipients
of allogeneic transplant from HLA matched or mismatched related donors;[17-18] more recently, it has been described after allogeneic, combined cord blood/haplo transplantation.[19]
However, to our knowledge, our case is the first described after
autologous stem cell transplantation. It is known from the literature
that approximately 95% of the healthy adults experienced the HHV-6
infection in childhood. After primary infection, HHV-6 persists in the
host and is detectable in multiple tissues, such as salivary glands,
brain cells, monocytes, and early bone marrow progenitor cells in a
similar way to other herpesviruses as CMV. However, ciHHV-6 represents
an alternative form of viral persistence, occurring in a subgroup of
individuals and characterized by very high viral loads in the blood
(>1x106 HHV-6 copies/ml), in other
body fluids or tissue samples. Growing evidence suggests that the
integrated viral sequences are inherited through the germline and are
therefore present in every nucleated cell. This phenomenon may confound
the laboratory diagnosis of active HHV-6 infection because whole blood
and spinal fluid from individuals with viral integration are
persistently positive for HHV-6 DNA even in the absence of independent
viral replication. Therefore, ciHHV-6 must be suspected every time
whole blood HHV-6 DNA is detected at high load at the time of
engraftment and persists at a high level after transplant, without any
adverse influence on patients outcome.
Although the frequency of HHV-6 infection is quite different between allo-HSCT and ASCT, being higher after allo-HSCT,[20]
the
recognition of these cases may lead to the avoidance of unnecessary and
potentially toxic antiviral treatments, particularly during the
delicate phase of hematological recovery in the early post-transplant
period. Currently, the screening for ciHHV-6 is not recommended, but we
believe that the awareness of this phenomenon after ASCT is of interest
for clinicians working in the field, since over 20,000 ASCTs are
reported by the European Blood and Marrow Transplantation,[21] with an estimated number of 200-400 such new cases every year only in Europe.
Acknowledgments
We
thank all the personnel of the Transplant Unit in the Oncology
Department, at Humanitas Cancer Center, Rozzano, Italy, for patient’s
assistance, clinical care and follow-up.
.
References
- http://www.eortc.org/sites/default/files/ECIL3_CMV_HHV-6_Guidelines_Update_2009.pdf
- Zerr
DM. Human herpesvirus 6 and central nervous system disease in
hematopoietic cell transplantation. J Clin Virol. 2006;37 Suppl
1:S52-56. https://doi.org/10.1016/S1386-6532(06)70012-9

- Dulery
R, Salleron J, Dewilde A, Rossignol J, Boyle EM, Gay J, de Berranger E,
Coiteux V, Jouet JP, Duhamel A, Yakoub-Agha I. Early human herpesvirus
type 6 reactivation after allogeneic stem cell transplantation: a
large-scale clinical study. Biol Blood Marrow Transplant.
2012;18:1080-1089. https://doi.org/10.1016/j.bbmt.2011.12.579 PMid:22212513

- Sashihara
J, Tanaka-Taya K, Tanaka S, Amo K, Miyagawa H, Hosoi G, Taniguchi T,
Fukui T, Kasuga N, Aono T, Sako M, Hara J, Yamanishi K, Okada S. High
incidence of human herpesvirus 6 infection with a high viral load in
cord blood stem cell transplant recipients. Blood. 2002;100:2005-2511.
PMid:12200359

- Tormo
N, Solano C, de la Cámara R, Garcia-Noblejas A, Carde-oso L, Clari MA,
Nieto J, López J, Hernández-Boluda JC, Remigia MJ, Benet I, Navarro D.
An assessment of the effect of human herpesvirus-6 replication on
active cytomegalovirus infection after allogeneic stem cell
transplantation. Biol Blood Marrow Transplant. 2010;16:653-661. https://doi.org/10.1016/j.bbmt.2009.12.003 PMid:20005968

- Fule
Robles JD, Cheuk DK, Ha SY, Chiang AK, Chan GC. Human herpesvirus types
6 and 7 infection in pediatric hematopoietic stem cell transplant
recipients. Ann Transplant. 2014;19:269-276. https://doi.org/10.12659/AOT.889995 PMid:24881673

- Tanaka-Taya
K, Sashihara J, Kurahashi H, Amo K, Miyagawa H, Kondo K, Okada S,
Yamanishi K. Human herpesvirus 6 (HHV-6) is transmitted from parent to
child in an integrated form and characterization of cases with
chromosomally integrated HHV-6 DNA. J Med Virol. 2004;73:465-473. https://doi.org/10.1002/jmv.20113 PMid:15170644

- Daibata
M, Taguchi T, Nemoto Y, Taguchi H, Miyoshi I. Inheritance of
chromosomally integrated human herpesvirus 6 DNA. Blood.
1999;94:1545-1549. PMid:10477678

- Hubacek
P, Virgili A, Ward KN, Pohlreich D, Keslova P, Goldova B, Markova M,
Zajac M, Cinek O, Nacheva EP, Sedlacek P, Cetkovsky P. HHV-6 DNA
throughout the tissues of two stem cell transplant patients with
chromosomally integrated HHV-6 and fatal CMV pneumonitis. Br J
Haematol. 2009;145:394-398. https://doi.org/10.1111/j.1365-2141.2009.07622.x PMid:19222466

- Kamble
RT, Clark DA, Leong HN, Heslop HE, Brenner MK, Carrum G. Transmission
of integrated human herpesvirus-6 in allogeneic hematopoietic stem cell
transplantation. Bone Marrow Transplant. 2007;40:563-566. https://doi.org/10.1038/sj.bmt.1705780 PMid:17637686

- Musso
M, Scalone R, Marcacci G, Lanza F, Di Renzo N, Cascavilla N, Di
Bartolomeo P, Crescimanno A, Perrone T, Pinto A. Fotemustine plus
etoposide, cytarabine and melphalan (FEAM) as a new conditioning
regimen for lymphoma patients undergoing auto-SCT: a multicenter
feasibility study. Bone Marrow Transplant. 2010;45:1147-1153. https://doi.org/10.1038/bmt.2009.318 PMid:19898504

- De
Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T,
Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson
TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler
CC, Kullberg BJ, Marr KA, Mu-oz P, Odds FC, Perfect JR, Restrepo A,
Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR,
Zaoutis T, Bennett JE; European Organization for Research and Treatment
of Cancer/Invasive Fungal Infections Cooperative Group; National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Revised definitions of invasive fungal
disease from the European Organization for Research and Treatment of
Cancer/Invasive Fungal Infections Cooperative Group and the National
Institute of Allergy and Infectious Diseases Mycoses Study Group
(EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813-1821. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227

- Chapenko,
Trociukas I, Donina S, Chistyakov M, Sultanova A, Gravelsina S,
Lejniece S, Murovska M. Relationship between beta-herpesviruses
reactivation and development of complications after autologou
peripheral blood stem cell transplantation. J Med Virol.
2012;84:1953-1960. https://doi.org/10.1002/jmv.23412 PMid:23080502

- Piukovics
K, Borbényi Z, Rajda C, Csomor A, Déak J, Terhes G. Monitoring human
herpesvirus-6 in patients with autologous stem cell transplantation. In
Vivo 2014;28:1113-1117. PMid:25398808

- Colombier
MA, Amorim S, Salmona M, Thieblemont C, Legoff J, Lafaurie M. HHV-6
reactivation as a cause of fever in autologous hematopoietic stem cell
transplant recipients. J Infect 2017;75:155-159. https://doi.org/10.1016/j.jinf.2017.05.011 PMid:28551368

- Pellett
PE, Ablashi DV, Ambros PF, Agut H, Caserta MT, Descamps V, Flamand L,
Gautheret-Dejean A, Hall CB, Kamble RT, Kuehl U, Lassner D,
Lautenschlager I, Loomis KS, Luppi M, Lusso P, Medveczky PG, Montoya
JG, Mori Y, Ogata M, Pritchett JC, Rogez S, Seto E, Ward KN, Yoshikawa
T, Razonable RR. Chromosomally integrated human herpesvirus 6:
questions and answers. Rev Med Virol 2012;22:144-155. https://doi.org/10.1002/rmv.715 PMid:22052666 PMCid:PMC3498727

- Kamble
RT, Clark DA, Leong HN, Heslop HE, Brenner MK, Carrum G. Transmission
of integrated human herpesvirus-6 in allogeneic hematopoietic stem cell
transplantation. Bone Marrow Transplant. 2007;40:563-566. https://doi.org/10.1038/sj.bmt.1705780 PMid:17637686

- Clark
DA, Nacheva EP, Leong HN, Brazma D, Li YT, Tsao EH, Buyck HC, Atkinson
CE, Lawson HM, Potter MN, Griffiths PD. Transmission of integrated
human herpesvirus 6 through stem cell transplantation: implications for
laboratory diagnosis. J Infect Dis. 2006;193:912-916. https://doi.org/10.1086/500838 PMid:16518751

- Purev
E, Winkler T, Danner RL, Fahle GA, Cook L, Zerr DM, Jerome KR, Childs
RW. Engraftment of donor cells with germ-line integration of HHV6
mimics HHV6 reactivation following cord blood/haplo transplantation.
Blood. 2014;124:1198-1199. https://doi.org/10.1182/blood-2014-06-577684 PMid:25124787 PMCid:PMC4133492

- Quintela
A, Escuret V, Roux S, Bonnafous P, Gilis L, Barraco F,
Labussière-Wallet H, Duscastelle-Leprêtre S, Nicolini FE, Thomas X,
Chidiac C, Ferry T, Frobert E, Morisset S, Poitevin-Later F, Monneret
G, Michallet M, Ader F; Lyon HEMINF Study Group. HHV-6 infection after
allogeneic hematopoietic stem cell transplantation:from chromosomal
integration to viral co-infections and T-cell reconstitution patterns.
J Infect 2016 ;72 :214-222. https://doi.org/10.1016/j.jinf.2015.09.039 PMid:26518057

- Passweg
JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, Duarte RF,
Dufour C, Kuball J, Farge-Bancel D, Gennery A, Kröger N, Lanza F,
Nagler A, Sureda A, Mohty M. Hematopoietic stem cell transplantation in
Europe 2014: more than 40000 transplants annually. Bone Marrow
Transplant. 2016;51:786-792. https://doi.org/10.1038/bmt.2016.20 PMid:26901709 PMCid:PMC4895175

[TOP]






















