Suparak Para1, Punchalee Mungkalasut1, Makamas Chanda2, Issarang Nuchprayoon3, Srivicha Krudsood4 and Chalisa Louicharoen Cheepsunthorn5*
1 Medical
Biochemistry Program, Department of Biochemistry, Faculty of Medicine,
Chulalongkorn University, Bangkok 10330, Thailand.
2 Biomedical Sciences Program, Graduate School, Chulalongkorn University, Bangkok 10330, Thailand.
3 Department of Pediatrics, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
4
Department of Tropical Hygiene and Clinical Malaria Research Unit,
Faculty of Tropical Medicine, Mahidol University, Bangkok 10400,
Thailand.
5 Department of Biochemistry, Faculty of Medicine, Chulalongkorn University, Bangkok 10330, Thailand.
Corresponding
author: Chalisa Louicharoen Cheepsunthorn. Department of Biochemistry,
Faculty of Medicine, Chulalongkorn University, 1873 Rama 4 Rd.,
Pathumwan, Bangkok 10330, Thailand. Tel: +66(0)22564482 ext. 4123, Fax:
+66(0)22564482. E-mail address:
chalisa.l@chula.ac.th
Published: February 16, 2018
Received: February 3, 2018
Accepted: August 23, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018015 DOI
10.4084/MJHID.2018.015
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: The protective effect of α-thalassemia, a common hematological disorder in Southeast Asia, against Plasmodium falciparum malaria has been well established. However, there is much less understanding of the effect of α-thalassemia against P. vivax.
Here, we aimed to investigate the proportion of α-thalassemia including
the impact of α-thalassemia and HbE on the parasitemia of P. vivax in Southeast Asian malaria patients in Thailand.
Methods:
A total of 210 malaria patients, admitted to the Hospital for Tropical
Diseases, Thailand during 2011-2012, consisting of 159 Myanmeses, 13
Karens, 26 Thais, 3 Mons, 3 Laotians, and 6 Cambodians were recruited. Plasmodium spp.
and parasite densities were determined. Group of deletion mutation
(--SEA, -α3.7, -α4.2deletion) and substitution mutation (HbCS and HbE)
were genotyped using multiplex gap-PCR and PCR-RFLP, respectively.
Results:
In our malaria patients, 17/210 homozygous and 74/210 heterozygous
-α3.7 deletion were found. Only 3/210 heterozygous -α4.2 and 2/210
heterozygous--SEA deletion were detected. HbE is frequently found with
6/210 homozygotes and 35/210 heterozygotes. The most common thalassemia
allele frequencies in Myanmar population were -α3.7 deletion (0.282),
followed by HbE (0.101), HbCS (0.013), -α4.2 deletion (0.009), and
--SEA deletion (0.003). Only density of P. vivax
in α-thalassemia trait patients (-α3.7/-α3.7, --SEA/αα, -α3.7/-α4.2)
but not in silent α-thalassemia (-α3.7/αα, -α4.2/αα, ααCS/αα) were
significantly higher compared with non-α-thalassemia patients
(p=0.027). HbE did not affect P. vivax parasitemia. The density of P. falciparum significantly increased in heterozygous HbE patients (p=0.046).
Conclusions: Alpha-thalassemia trait is associated with high levels of P. vivax parasitemia in malaria patients in Southeast Asia.
|
Introduction
Malaria
is the most prevalent parasitic disease worldwide where 214 million
patients suffer due to Plasmodium vivax and Plasmodium falciparum
infection, and more than 400,000 people die annually.[1] Both P. vivax
and P. falciparum have been the main causes of malaria on the
Thailand-Myanmar border for many years. In Thai villagers, P. vivax
infection has recently become the largest proportion of cases in
patients.[2] The result of selective malaria pressure on recent human
genome evolution is presented in the form of high frequencies of
genetic disorders of hemoglobin including thalassemias and
hemoglobinopathies in populations living in historically malarious
regions.[3-6] Such malaria-protective properties have since been
demonstrated in glucose 6-phosphate dehydrogenase (G6PD) deficiency,[7]
α-thalassemia,[4,8-9] hemoglobin C,[10-11] hemoglobin S[12] and hemoglobin
E.[13] The protective effect of thalassemia against P. falciparum malaria
has been well established.[14] However, the impact of thalassemia on P.
vivax is not well understood yet. Alpha-thalassemia is caused by the
deletion of a number of α-globin genes resulting in an imbalance of α-
and β- globin. There are several types of α-thalassemia; silent
α-thalassemia, α-thalassemia trait and HbH, which depleted one, two,
and three copy of α-globin genes, respectively. The -α3.7 and-α4.2
deletions are most common forms of silent α-thalassemia in Southeast
Asians.[15-16] Clinical symptoms of α-thalassemia traits are mild anemia
with hypochromic erythrocytes, whereas heterozygotes are
asymptomatic.[17] The meta-analysis demonstrated the protective effect of
silent α-thalassemia against P. falciparum.[14] A case-control study in
Africa and Papua New Guinea (PNG) found that silent α-thalassemia
protects against P. falciparum.[8-9,18-21] Alpha-thalassemia trait --SEA
deletion was commonly found in Thailand and Southeast Asia (SEA).[15-16]
The --SEA allele has been identified as the recent balancing selected
allele triggered by malaria.[22] However, several studies failed to
detect the association of α-thalassemia traits and parasitemia of P.
vivax.[23] Hemoglobin E (HbE) is the most common β-hemoglobinopathies in
Southeast Asia. Several studies have found that HbE confers protection
against P. falciparum.[13] However, HbE has been found to be more prone
to P. vivax.[24] This study aimed to investigate the proportion of
α-thalassemia and HbE and to clarify the effect of α-globin gene
numbers and HbE genotype on the parasitemia in Southeast Asian malaria
patients in Thailand.
Materials and Methods
Study subjects and sample collection.
The study protocol was reviewed and approved by the Institutional
Review Board of the Faculty of Medicine, Chulalongkorn University
(Bangkok, Thailand) (COA No. 040/2013 IRB No. 459/55). Malaria patients
in this cohort study were referred from many malaria-endemic provinces
including borders of Thailand: Tak (Maesod District), Kanchanaburi
(Sangkhlaburi District), Phetchaburi (Kaeng Krachan District),
Suphanburi (Dan Chang District), Ranong, Sisaket (Kantharalak District)
and Chonburi (Figure 1). Before enrolment in the study, all patients gave written informed consent. Patients who were slide-positive for Plasmodium
malaria with no history of antimalarial drug treatment within the
preceding 2 weeks, and were admitted to the Hospital for Tropical
Diseases in Thailand during 2011-2012, were recruited. G6PD deficiency,
an enzymopathy involved in protecting against malaria, which may
interfere with interpretation of the effect of α-thalassemia and HbE,
was excluded.
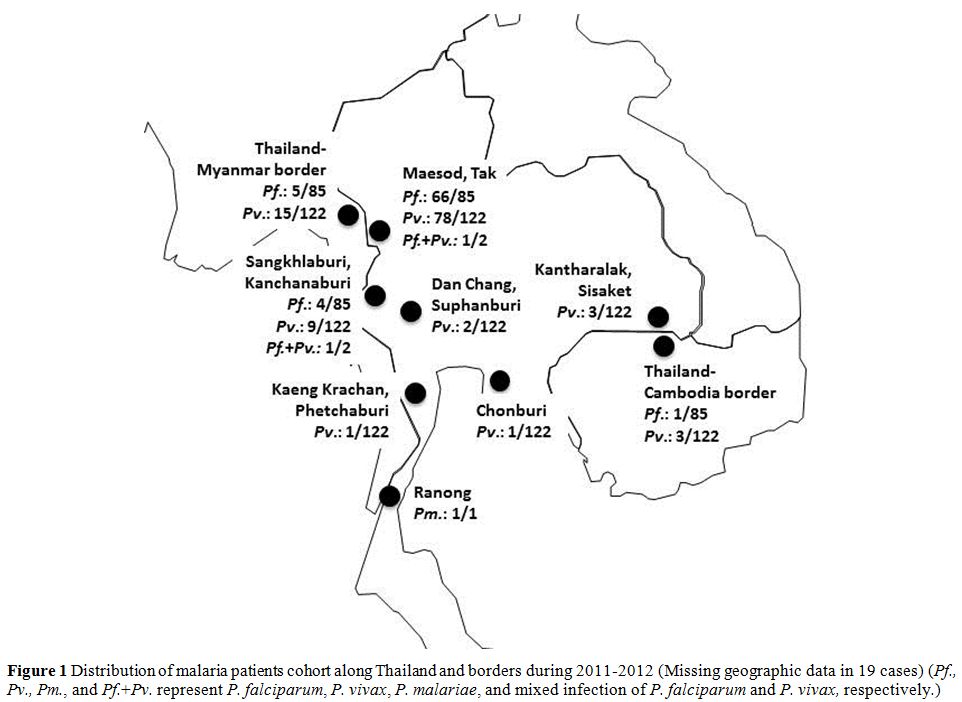 |
Figure 1. Distribution of malaria patients
cohort along Thailand and borders during 2011-2012 (Missing geographic
data in 19 cases) (Pf., Pv., Pm., and Pf.+Pv. represent P. falciparum, P. vivax, P. malariae, and mixed infection of P. falciparum and P. vivax, respectively.). |
Identify Plasmodium spp.
infection and parasite density. All blood samples from finger pricks
were Giemsa stained for thick and thin blood films. Blood smears were
tested every 12 hours from initiation of treatment until they were
negative on two consecutive occasions; after that, blood smears were
daily tested until patients were discharged. Parasite densities
(asexual parasite/microliter of blood) were examined by counting the
number per 200 leukocytes (thick film) or per 1,000 erythrocytes (thin
film). In interpretation the Plasmodium spp.,
blood smear films were read under microscope by an independent
parasitologist at the Hospital for Tropical Diseases. The species was
confirmed by polymerase chain reaction (PCR)-based analysis.
Measurement of G6PD activity.
G6PD activity assays were performed prior to treatment and weekly
repeated until patients were discharged. Quantitative test for G6PD
activity was performed using G6PD kit assay (Trinity Biotech, Bray,
County Wicklow, Ireland), which measured NADPH production at wavelength
of 340 nm. All samples were run parallel with positive and negative
control. Hemoglobin for calculation of G6PD activity was measured using
Hb201 (HemoCue, Sweden). G6PD activity <1.5 IU/g Hb classified as
G6PD deficient[25] was excluded from the study. Leftover blood samples were kept at -20°C for molecular typing.
Detection of α-thalassemia. Genomic DNA was extracted from peripheral blood using phenol-chloroform method.[26] Alpha-globin gene variants including α-thalassemia trait (--SEA deletion) and silent α-thalassemia (-α3.7, -α4.2 deletion) were investigated by multiplex gap-polymerase chain reaction (multiplex gap-PCR).[27] HbCS and HbE were genotyped using PCR-restriction fragment length polymorphism (PCR-RFLP).[28-29]
Statistical analysis. All statistical analyses were performed using the SPSS version 22.0. The main outcomes of interest were parasite densities of P. falciparum and P. vivax
malaria before treatment. Parasite density that was not normally
distributed was log-transformed prior to analysis. Parasitemia of
α-thalassemia and HbE patients were compared with that of
non-thalassemia (HbA) using unpaired T-test. In all statistical
analyses, significance levels were set at the 95% confidence interval
(CI) (P<0.05).
Results
Characteristics of the study population.
A total of 210 patients (201 males and 9 females) including 159
Myanmeses, 13 Karens, 26 Thais, 3 Mons, 3 Laotians, and 6 Cambodians
were recruited for the study. Patients were from Myanmar (N=159), Tak
(Maesod district, N=127), Kanchanaburi (Sangkhlaburi district, N=9),
Ranong (N=1), Thailand-Myanmar border (N=15), Thailand-Cambodia border
(N=1, Figure 1) and missing data (N=6). The average age of all subjects was 28.0±10.0 (range 14-60) years. Eighty-five had P. falciparum, while 122 had P. vivax infection, two had mixed infection of P. falciparum, and P. vivax and one had P. malariae.
In this study, 17 homozygous and 74 heterozygous -α3.7 deletion were found among 210 patients, while only three heterozygous -α4.2 and two heterozygous --SEA deletion were detected.
HbE was also highly prevalent, with six homozygotes and 35 heterozygotes. For HbCS, five heterozygous were detected (Table 1).
The Myanmese was the major ethnic group in this study accounting for
75% of all patients. Among these, the proportion of α- thalassemia was
48.4% (77/159), including 45.9% (73/159) of -α3.7 deletion, 1.8% (3/159) -α4.2 deletion, 0.6% (1/159) --SEA deletion, and 2.5% (4/159) ααCS whereas HbE was 20.8% (29/159). Allele frequencies were calculated for the major population. The most common was -α3.7 deletion (0.282), followed by HbE (0.101), HbCS (0.013), -α4.2 deletion (0.009), and --SEA deletion (0.003) (Table 1). Thalassemia and hemoglobinopathies were not found in 3 patients with P. malariae and mixed infection patients.
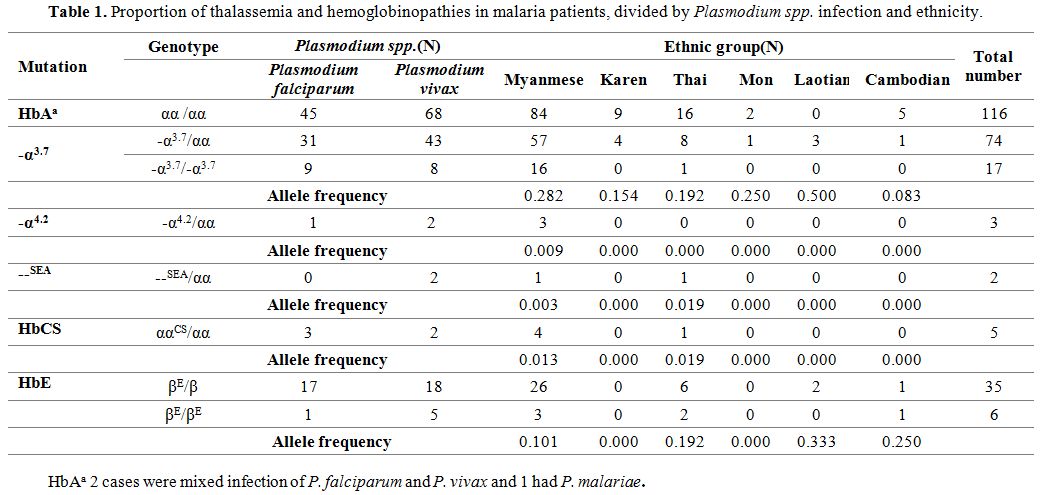 |
Table 1. Proportion of thalassemia and hemoglobinopathies in malaria patients, divided by Plasmodium spp. infection and ethnicity. |
Association of α-globin gene dosage, HbE, and parasitemia. To assess the effect of α-globin gene presence and HbE genotype on the parasitemia of P. vivax and P. falciparum,
the number of parasites in the blood of α-thalassemia and HbE genotypes
were compared with that of non-thalassemia (HbA). The results found
that P. vivax density in patients with α-thalassemia trait (-α3.7/-α3.7, --SEA/αα, -α3.7/-α4.2) was 4.21±0.32 log10 value/μl, which was significantly higher than HbA patients (3.89±0.71 log10 value/μl) (p=0.027) (Table 2). Whereas, P. vivax parasitemia was not significantly different in patients who depleted only one α-globin gene or had silent α-thalassemia (-α3.7/αα, -α4.2/αα, ααCS/αα) (3.94±0.66 log10 value/μl) (p=0.707) (Table 2). Nevertheless, HbH patient (-α3.7/--SEA) had low level of P. vivax parasitemia compared with HbA (2.08 log10 value/μl). However, there was no significant effect of the number of alpha globin gene deletions on P. falciparum parasitemia.
However, significant increases of P. falciparum density in heterozygous HbE patients was detected (4.45±0.66 log10 value/μl) (p=0.046) (Table 2). On the other hand, P. falciparum parasitemia was reduced in homozygous HbE patient (3.00 log10 value/μl) (Table 2). Nevertheless, this study could not find the effect of HbE on P. vivax parasitemia.
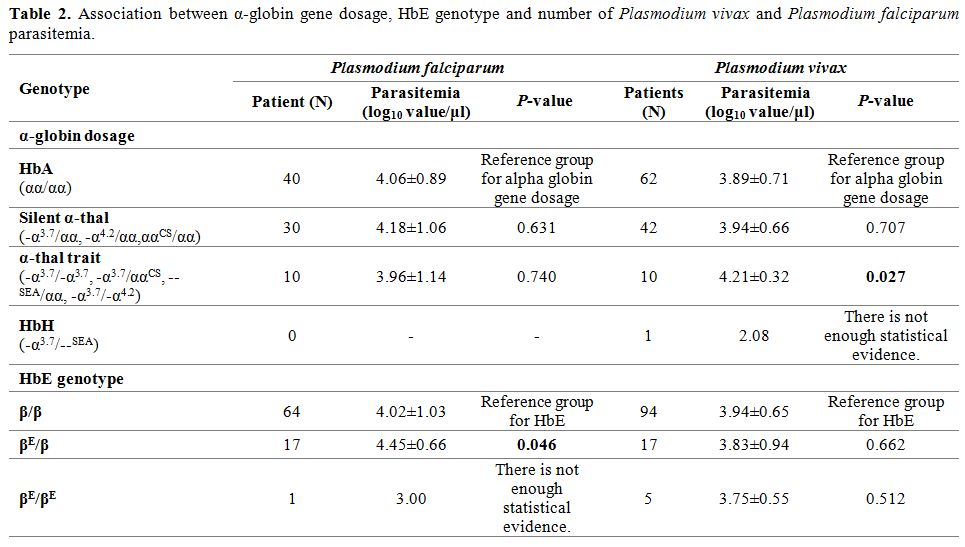 |
Table 2. Association between α-globin gene dosage, HbE genotype and number of Plasmodium vivax and Plasmodium falciparum parasitemia. |
Discussion
Our study is an association study between α-thalassemia and P. vivax density in Southeast Asia. The proportion of P. vivax infection in this study was higher than P. falciparum infection with a ratio of 1.4:1, which corresponds to the WHO World Malaria Report in 2015[1] which reported that P. vivax (54%) was detected more frequently than P. falciparum (38%) in Thailand. The distribution of P. vivax in Thailand is predominantly along the western region; Tak Province or the Thailand-Myanmar border (Figure 1), which had the highest malaria incidence.[30]
Since all patients in the study, who were referred to the Hospital for
Tropical Diseases after malaria infection, were immigrant laborers, the
ratio of males was much higher than female malaria patients. Since a
more numerous population of men had been working outdoors, it was
exposed to a higher chance of malaria infection.
The overall
frequencies of α-thalassemia and HbE in Myanmar villagers living in
malaria-endemic regions of Myanmar were 37.5% (343/916) and 20.3%
(186/916), respectively.[31] Our study is comparable to a previous study and may reflect real prevalence. From our finding and the report of Than[31] support α-thalassemia especially -α3.7
deletion and HbE are highly frequent in both malarial and non-malarial
infected Myanmar populations. While it is difficult to demonstrate the
protective effect of α- thalassemia and HbE when conducting a study
only in malaria patients, our findings of high prevalence of
thalassemia traits among malaria patients supports the conclusion that
malaria infection risk is not reduced in people with α-thalassemia and
HbE. In line with this finding, an increased frequency of uncomplicated
malaria was found in people with α+-thalassemia in the Vanuatu study.[23] The high prevalence of α-thalassemia and HbE in Southeast Asia remains unexplained.
In contrast to the Haldane hypothesis, where α-thalassemia is expected to protect from malaria, we observed higher levels of P. vivax parasitemia among people with α-thalassemia trait. Similarly, a study in Papua New Guinea also showed higher P. vivax parasitemia (but not P. falciparum) in α+-thalassemia heterozygous and homozygous children.[17] In addition, the study in Kenya also showed that α+-thalassaemia neither protected against symptomatic malaria nor reduced parasitemia.[9] However, α+-thalassaemia appeared to reduce the rate of severe anemia in falciparum malaria and had lower hospitalization.[9] The contrasting effects may be explained by the lack of P. vivax in African population, while both P. vivax and P. falciparum are prevalent in Southeast Asian region.
Despite the dosage effect of P. vivax
density where two alpha gene deletions have higher levels of
parasitemia than one gene deletion, the single case of HbH (3 genes
deletion) had an unexpectedly lower rather than higher level of
parasitemia. We could not make a meaningful conclusion from this one
case as it could have occurred by chance. It was possible that this
patient was referred early, so parasitemia was still low. It is
hypothesized that people with α-thalassemia have more baseline
erythropoiesis, resulting in a high proportion of reticulocytes which
is the susceptible stage for P. vivax infection.[23] This hypothesis, however, is unlikely as there is no evidence of reticulocytosis in people with α+-thalassemia heterozygous.[32]
Our results showed increased parasitemia of P. falciparum
in heterozygous HbE, but also a decrease in one single case of
homozygote. Our finding is in line with a previous study in Myanmar
population.[33] In vitro studies reveal conflicting results. Nagel et al. demonstrated impairment of the growth of P. falciparum in homozygous HbE, but an average growth in heterozygous HbE.[34]
Whereas, Chotivanich et al. found in vitro a reduction in RBC invasion
in HbAE heterozygotes, associated with a 4-fold increase in the
selectivity index compared the other hemoglobin types studied and in
particular the EE homozygotes suggesting that in heterozygote
individuals with AE hemoglobin, only a quarter of the RBC population
can be invaded by P. falciparum, so parasitemia could remain low.[13] Parasitemia of P. vivax in HbE patients had been previously observed but did not reach significant difference.[28] The effect of HbE on P. vivax
parasitemia was not found in this study. Nevertheless, O'Donnell and
colleagues showed that HbE patients might be more susceptible for
malaria infection, especially P. vivax
because their malarial antibodies were significantly increased than
non-thalassemia children, which reflected in their clinical severity.[20]
Although limited by a small number of patients, one strength of our
study is that G6PD deficiency was excluded, which has been well known
to confer protection against vivax malaria.[7]
Acknowledgments
We
thank all study participants and staff at Hospital for Tropical
Diseases, Faculty of Tropical Medicine, Mahidol University. All authors
have no conflict of interest. This work was granted by the 90th
anniversary of Chulalongkorn University Fund (Ratchadaphiseksomphot
Endowment Fund) and Ratchadapiseksompotch Fund, Faculty of Medicine,
Chulalongkorn University (Grant No. RA57/006).
.
References
- WHO. World malaria report 2015. Geneva: WHO and UNICEF 2015.
- Thailand
malaria report 2016. Bangkok: Bureau of Vector Borne Diseases
Department of Disease Control, Ministry of Public Health. http://www.thaivbd.org. Accessed 26 Oct 2016.
- De
Sanctis V., Kattamis C., Canatan D., Soliman A. T., Elsedfy H., Karimi
M., Daar S., Wali Y., Yassin M., Soliman N., Sobti P., Al Jaouni S., El
Kholy M., Fiscina B., Angastiniotis M. β-thalassemia distribution in
the old world: an ancient disease seen from a historical standpoint.
Mediterr J Hematol Infect Dis 2017, 9(1): e2017018, DOI: http://dx.doi.org/10.4084/MJHID.2017.018

- Flint
J, Hill AV, Bowden DK, Oppenheimer SJ, Sill PR, Serjeantson SW,
Bana-Koiri J, Bhatia K, Alpers MP, Boyce AJ, Weatherall DJ, Clegg JB.
High frequencies of alpha-thalassaemia are the result of natural
selection by malaria. Nature. 1986;321:744-50 https://doi.org/10.1038/321744a0 PMid:3713863

- Kwiatkowski
DP. How malaria has affected the human genome and what human genetics
can teach us about malaria. Am J Hum Genet. 2005;77:171-92
https://doi.org/10.1086/432519 PMid:16001361 PMCid:PMC1224522

- Verra
F, Mangano VD, Modiano D. Genetics of susceptibility to Plasmodium
falciparum: from classical malaria resistance genes towards genome-wide
association studies. Parasite Immunol. 2009;31:234-53 https://doi.org/10.1111/j.1365-3024.2009.01106.x PMid:19388945

- Louicharoen
C, Patin E, Paul R, Nuchprayoon I, Witoonpanich B, Peerapittayamongkol
C, Casademont I, Sura T, Laird M. N, Singhasivanon P, Quintana-murci L,
Sakuntabhai A. Positively selected G6PD-Mahidol mutation reduces
Plasmodium vivax density in Southeast Asians. Science. 2009;326:1546-49
https://doi.org/10.1126/science.1178849 PMid:20007901

- Williams
TN, Wambua S, Uyoga S, Macharia A, Mwacharo JK, Newton CR, Maitland K.
Both heterozygous and homozygous alpha+ thalassemias protect against
severe and fatal Plasmodium falciparum malaria on the coast of Kenya.
Blood. 2005;106:368–71 https://doi.org/10.1182/blood-2005-01-0313 PMid:15769889

- Wambua
S, Mwangi TW, Kortok M, Uyoga SM, Macharia AW, Mwacharo JK, Weatherall
DJ, Snow RW, Marsh K, Williams TN. The effect of α+-thalassaemia on the
incidence of malaria and other diseases in children living on the coast
of Kenya. PLoS Med. 2006;3:158 https://doi.org/10.1371/journal.pmed.0030158 PMid:16605300 PMCid:PMC1435778

- Agarwal
A, Guindo A, Cissoko Y, Taylor JG, Coulibaly D, Koné A, Kayentao K,
Djimde A, Plowe CV, Doumbo O, Wellems TE, Diallo D. Hemoglobin C
associated with protection from severe malaria in the Dogon of Mali, a
West African population with a low prevalence of hemoglobin S. Blood.
2000;96:2358–63 PMid:11001883

- Modiano
D, Luoni G, Sirima BS, Simporé J, Verra F, Konaté A, Rastrelli E,
Olivieri A, Calissano C, Paganotti GM, D'Urbano L, Sanou I, Sawadogo A,
Modiano G, Coluzzi M. Haemoglobin C protects against clinical
Plasmodium falciparum malaria. Nature. 2001;414:305–8 https://doi.org/10.1038/35104556 PMid:11713529

- Cabrera
G, Cot M, Migot-Nabias F, Kremsner PG, Deloron P, Luty AJ. The sickle
cell trait is associated with enhanced immunoglobulin G antibody
responses to Plasmodium falciparum variant surface antigens. J Infect
Dis. 2005;191:1631–38 https://doi.org/10.1086/429832 PMid:15838789

- Chotivanich
K, Udomsangpetch R, Pattanapanyasat K, Chierakul W, Simpson J,
Looareesuwan S, White N. Hemoglobin E: a balanced polymorphism
protective against high parasitemias and thus severe P. falciparum
malaria. Blood. 2002;100:1172–76 PMid:12149194

- Taylor
SM, Parobek CM, Pairhurst RM. Haemoglobinopathies and the clinical
epidemiology of malaria: a systematic review and meta-analysis. Lancet
Infect Dis. 2012;12:457-68 https://doi.org/10.1016/S1473-3099(12)70055-5

- Chen
YL, Shih CJ, Ferrance J, Chang YS, Chang JG, Wu SM. Genotyping of
alpha-thalassemia deletions using multiplex polymerase chain reactions
and gold nanoparticle-filled capillary electrophoresis. J Chromatogr A.
2009;1216:1206-12 https://doi.org/10.1016/j.chroma.2008.12.027 PMid:19128803

- Fucharoen
S, Winichagoon P. Haemoglobinopathies in Southeast Asia. Indian J Med
Res. 2011;134:498-506 PMid:22089614 PMCid:PMC3237250

- Rosanas-Urgell
A, Senn N, Rarau P, Aponte JJ, Reeder JC, Siba PM, Michon P, Mueller I.
Lack of association of a+-thalassemia with the risk of Plasmodium
falciparum and Plasmodium vivax infection and disease in a cohort of
children aged 3-21 months from Papua New Guinea. Int J Parasitol.
2012;1:1107-13 https://doi.org/10.1016/j.ijpara.2012.10.001 PMid:23085147

- Mockenhaupt
FP, Ehrhardt S, Gellert S, Otchwemah RN, Dietz E, Anemana SD, Bienzie
U. Alpha(+)-thalassemia protects African children from severe malaria.
Blood. 2004;104:2003-06 https://doi.org/10.1182/blood-2003-11-4090 PMid:15198952

- Allen
SJ, O'Donnell A, Alexander ND, Alpers MP, Peto TE, Clegg JB, Weatherall
DJ. alpha+-Thalassemia protects children against disease caused by
other infections as well as malaria. Proc Natl Acad Sci USA.
1997;94:14736-41 https://doi.org/10.1073/pnas.94.26.14736

- May
J, Evans JA, Timmann C, Ehmen C, Busch W, Thye T, Agbenyega T,
Horstmann RD. Hemoglobin variants and disease manifestations in severe
falciparum malaria. JAMA. 2007;297:2220-26 https://doi.org/10.1001/jama.297.20.2220 PMid:17519411

- Opoku-Okrah
C, Gordge M, KwekuNakua E, Agbenyega T, Parry M, Robertson C, Smith CL.
An investigation of the protective effect of alpha+-thalassemia against
severe Plasmodium falciparum amongst children in Kumasi, Ghana. Int J
Lab Hematol. 2014;36:62-70 https://doi.org/10.1111/ijlh.12122 PMid:23837700

- Qiu
Q, Wu D, Yu L, Yan T, Zhang W, Li ZT, Liu YH, Zhang YP, Xu XM. Evidence
of recent natural selection on the Southeast Asian deletion (--SEA)
causing a-thalassemia in South China. BMC Evol Biol. 2013;13:63 https://doi.org/10.1186/1471-2148-13-63 PMid:23497175 PMCid:PMC3626844

- Williams
TN, Maitland K, Bennett S, Ganczakowski M, Peto TEA, Newbold CI, Bowden
DK, Weatherall DJ, Clegg JB. High incidence of malaria in
a-thalassaemic children. Nature. 1996;383:522-25 https://doi.org/10.1038/383522a0 PMid:8849722

- O'Donnell
A, Premawardhena A, Arambepola M, Samaranyake R, Allen SJ, Peto TE,
Fisher CA, Cook J, Corran PH, Olivieri NF, Weatherall DJ. Interaction
of malaria with a common form of severe thalassemia in an Asian
population. Proc Natl Acad Sci USA. 2009;106:18716-21 https://doi.org/10.1073/pnas.0910142106 PMid:19841268 PMCid:PMC2774006

- Hsia
YE, Miyakawa F, Baltazar J, Ching NS, Yuen J, Westwood B, Beutler E.
Frequency of glucose-6-phosphate dehydrogenase (G6PD) mutations in
Chinese, Filipinox, and Laotians from Hawaii. Hum Genet. 1993;92:470-6 https://doi.org/10.1007/BF00216453 PMid:8244337

- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Second ed. New York. Cold Spring Harbour. 1989
- Chong
SS, Boehm CD, Higgs DR, Cutting GR. Single-tube multiple-PCR screen for
common deletional determinants of alpha-thalassemia. Blood.
2000;95:360-62 PMid:10607725

- Makonkawkeyoon
L, Sanguansermsri T, Asato T, Nakashima Y, Takei H. Rapid detection of
chain termination mutations in the alpha 2 globin gene. Blood.
1993;82:3503-04 PMid:8241518

- Tachavanich
K, Viprakasit V, Chinchang W, Glomglao W, Pung-Amritt P, Tanphaichitr
VS. Clinical and hematological phenotype of homozygous hemoglobin E:
revisit of a benign condition with hidden reproductive risk. Southeast
Asian J Trop Med Public Health. 2009;40:306-16 PMid:19323016

- Kuesap
J, Chaijaroenkul W, Rungsihirunrat K, Pongjantharasatien K,
Na-Bangchang K. Coexistence of Malaria and Thalassemia in Malaria
Endemic Areas of Thailand. Korean J Parasitol. 2015;53: 265-70 https://doi.org/10.3347/kjp.2015.53.3.265 PMid:26174819 PMCid:PMC4510677

- Than
AM, Harano T, Harano K, Myint AA, Ogino T, Okada S. High incidence of
a-thalassemia, Hemoglobin E, and glucose-6-phosphate dehydrogenase
deficiency in population of malaria-endemic southern Shan state,
Myanmar. Int J Hematol. 2005;82:119-23 https://doi.org/10.1532/IJH97.05028 PMid:16146842

- Krügner
F, Zaccariotto TR, Rosim ET, Costa FF, Grotto HZ, Sonati MF.
Reticulocyte evaluation in alpha(+)-thalassemia. Am J Hematol.
2006;81:68-70 https://doi.org/10.1002/ajh.20487 PMid:16369954

- Oo
M, Shwe T, Than M, O'Sullivan WJ. Genetic red cell disorders and
severity of falciparum malaria in Myanmar. B World Health Organ.
1995;73:659-65 PMid:8846492 PMCid:PMC2486814

- Nagel
RL, Raventos-Suarez C, Fabry ME, Tanowitz H, Sicard D, Labie D.
Impairment of the growth of Plasmodium falciparum in HbEE erythrocytes.
J Clin Invest. 1981;68:303-05 https://doi.org/10.1172/JCI110248 PMid:7019245 PMCid:PMC370798

[TOP]


































