Karin P.S. Langenberg-Ververgaert1, Ronald M. Laxer2. Angela S. Punnett1, L. Lee Dupuis3-5, Yaron Finkelstein6 and Oussama Abla1.
1 Division of
Hematology/Oncology, Department of Paediatrics, The Hospital for Sick
Children, University of Toronto, Toronto, Ontario, Canada.
2
Division of Rheumatology, Department of Paediatrics, The Hospital for
Sick Children, University of Toronto, Toronto, Ontario, Canada.
3 Department of Pharmacy, The Hospital for Sick Children, Toronto, Canada.
4 Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, Canada.
5 Research Institute, The Hospital for Sick Children, Toronto, Canada.
6 The Hospital for Sick Children, University of Toronto, Toronto, Canada.
Corresponding
author: Karin P.S. Langenberg-Ververgaert. Division of
Hematology/Oncology, Department of Paediatrics, The Hospital for Sick
Children, 555 University Avenue, Toronto, Ontario, M5G 1X8, Canada.
Tel: 416-813-7742. Fax: 416-813-5327. E-mail:
karin.langenberg@sickkids.ca
Published: March 1, 2018
Received: December 6, 2017
Accepted: January 15, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018019 DOI
10.4084/MJHID.2018.019
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Familial
Mediterranean fever (FMF) has been associated with hematological
malignancies but has not been reported in association with Hodgkin
lymphoma (HL). We hereby describe the first pediatric patient with FMF
and stage IIA nodular sclerosis HL. She was treated with prednisone,
doxorubicin, vincristine and etoposide (OEPA) being on therapy with
colchicine. However, she suffered more than expected treatment-related
toxicity attributed either to chemotherapy (severe neutropenia) or
colchicine (Abdominal pains and diarrhoea). Colchicine had to be
discontinued. In the absence of colchicine, she tolerated very well the
second cycle of chemotherapy. Currently, she is in remission at 17
months after her HL diagnosis, and her FMF is under control with
colchicine without any signs of toxicity.
|
Introduction
Hodgkin lymphoma (HL) is a lymphoid malignancy that accounts for approximately 7% of childhood cancers in the United States.[1]
Five-year survival rates are 98% and 97% for the <15 years and 15–19
years age cohorts, respectively. Several risk factors have been
associated with developing HL, such as EBV infection,[2] primary immune deficiencies[3] and autoimmune lymphoproliferative syndrome (ALPS).[4]
Familial
Mediterranean fever (FMF) (OMIM 249100) is an autoinflammatory syndrome
that can be adequately treated in the majority of cases with
colchicine.[5] Clinically, FMF is characterized by
recurrent episodes of fever, serositis, arthritis, dermal
manifestations and long-term renal complications.[6]
The goals of FMF treatment are to prevent acute attacks, decrease the
subclinical inflammation between the attacks and to prevent the
development of amyloidosis. Colchicine suppresses pyrin oligomerization
and interferes with neutrophil migration. It also prevents cytoskeletal
changes that lead to pyrin inflammasome assembly. Alternative treatment
options, particularly for colchicine-resistant or intolerant patients
include interleukin-1 inhibitors such as anakinra and canakinumab.[7]
FMF has been reported in association with adult hematological
malignancies such as multiple myeloma, acute lymphoblastic and myeloid
leukemias and myelodysplastic syndromes.[7-12]
However, this entity has not been reported in association with HL in
adults or children. We describe the first case of HL in a pediatric
patient with FMF, who developed myelotoxicity due to the combination of
colchicine with chemotherapy.
Case Presentation
A
6-year-old girl, of Turkish descent, presented with a 6-week history of
right posterior cervical lymphadenopathy. She had no recent history of
fever, night sweats or weight loss. Past medical history was
significant for FMF with a known M694V mutation in the MEFV gene (and
an r40w variant of unknown significance in the MVK gene), diagnosed at
age one year and well managed with colchicine 0.3 mg orally twice/day.
Complete blood count (CBC) showed white blood cell count 8.3 × 103/µl, hemoglobin 130 g/L, and platelet count 298,000/µl.
Lactate dehydrogenase was 850 IU/L, uric acid 174 umol/L and ESR 2
mm/h. An excisional biopsy of a cervical lymph node showed effacement
of the normal nodal architecture with a smaller population of large
atypical cells with large prominent eosinophilic nucleoli and
occasional classical diagnostic Reed-Sternberg cells.
Immunohistochemical stains revealed positivity for CD30, CD15 and PAX5.
The tumour cells were focally positive for CD3 and negative for ALK-1,
CD45 and CD20. In situ hybridization for EBV-EBER (where EBER is
Epstein–Barr encoding region) was positive in the majority of large
atypical cells. Staging included PET computerized tomography which
demonstrated localized hypermetabolic right cervical lymph nodes with
the absence of bulk disease. Therefore, the final diagnosis was stage
IIA classical Hodgkin lymphoma, nodular sclerosis subtype. The patient (Figure 1) was treated according to the local standard of care for low risk HL using 2 cycles of OEPA (vincristine 1.5 mg/m2/dose IV days 1, 8 and 15, etoposide 125 mg/m2/dose IV days 1-5, prednisone 60 mg/m2/day PO days 1–15, and doxorubicin 40 mg/m2 IV days 1 and 15) chemotherapy only.[13]
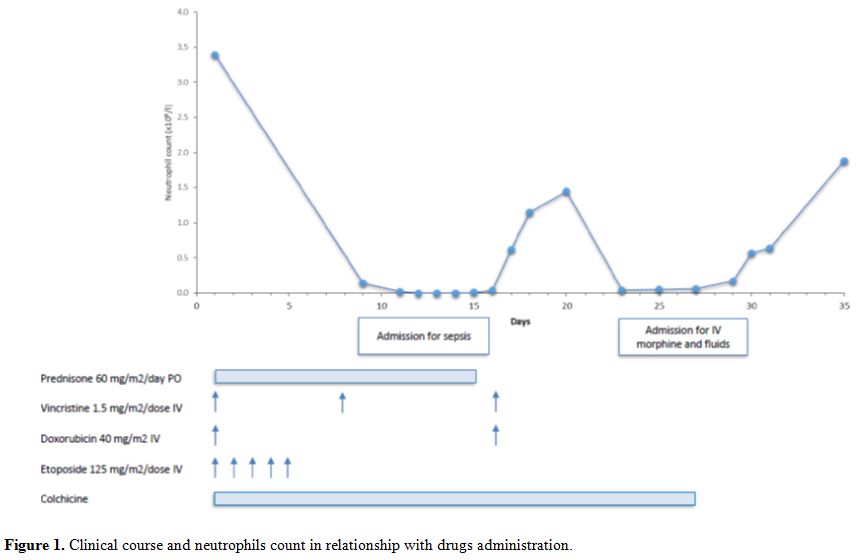 |
Figure 1. Clinical course and neutrophils count in relationship with drugs administration. |
Her
first treatment course was complicated by prolonged myelosuppression
including CTCAE grade 4 neutropenia, fever and severe abdominal pain.
She needed to be admitted on day 8 for clinical sepsis, requiring three
intravenous fluid boluses and antibiotics. Furthermore the second
infusion of Vincristine and Doxorubicin was postponed to Day 16. No
other signs of colchicine toxicity, such as bloody stool, renal failure
or disseminated intravascular coagulation were noted. Blood cultures
remained negative. She was discharged home after twelve days but needed
readmission on day 22 due to severe abdominal pain and loose stools,
treated with intravenous hydration and morphine for nine days. After
consultation with the Rheumatology service, colchicine was discontinued
from day 27 of cycle 1 onwards, to prevent possible interactions with
chemotherapy. Cycle 2 was started 36 days after start of cycle 1. She
tolerated this much better, without any complications and count
recovery within the expected timeframe. Clinical and radiographic
evaluations showed no evidence of disease at the end of therapy. The
patient currently remains in remission 17 months after completion of
therapy. Subsequently, she developed similar fever attacks and
colchicine was resumed at the same dose 3 months after completion of
chemotherapy. Her FMF has been well controlled since, without any signs
of colchicine toxicity. Discussion
Familial
Mediterranean fever (FMF) is an autosomal recessive disorder, although
autosomal dominant and heterozygous patients have been reported as
well. It is characterized by recurrent self-limiting acute painful
attacks of fever and inflammation in the peritoneum or pleura,
arthritis, splenomegaly and skin rashes, lasting 12–72 hours.
Amyloidosis with renal failure is a complication and may develop
without overt crises.[6] Several sets of criteria have
been developed in order to classify the disease, such as the Tel
Hashomer criteria in adults and the modified criteria in childhood.[14-15]
The disease is associated with mutations in the MEFV gene, localized on
chromosome 16p13, encoding for the protein pyrin, which is involved in
the regulation of inflammation and apoptosis.[16] It
has an important role in the innate immune system and interacts with
caspase-1 and other inflammasome components to regulate interleukin
IL-1β production. Different genotypes lead to distinct phenotypes. One
of the most common mutations, including among the Turkish population,
is the 2080A-G transition in the pyrin gene, resulting in a
met694-to-val (M694V) substitution.[17] In adults, a
high frequency of carriers of the MEFV gene in patients with
hematological neoplasms has been reported, none of whom had a diagnosis
of Hodgkin lymphoma. However, it remains unclear how inherited variants
in the MEFV gene are associated with tumor susceptibility or promotion
in hematologic neoplasms.[10]
Management of FMF with colchicine has benefitted patients by reducing painful attacks and preventing amyloidosis.[18]
Therefore, this drug was continued during initiation of anti-cancer
treatment. However, our patient did not tolerate the combination with
the low-intensity chemotherapy regimen.
Colchicine has a narrow
therapeutic index. It is readily absorbed after oral administration,
but has a variable bioavailability (ranging from 25% to 88%) and
undergoes extensive first-pass metabolism. It has a wide volume of
distribution (Vd) and binds to intracellular elements. Colchicine is
primarily metabolized by the liver, undergoes significant enterohepatic
re-circulation, and is also excreted by the kidneys. CYP3A4 and
P-glycoprotein inhibitors can increase serum colchicine concentrations.
Etoposide, prednisone, vincristine and doxorubicin are substrates of
CYP3A4, and therefore the clearance of these drugs may decrease with
concurrent administration of colchicine, and their side effects may
increase. Since colchicine is also a substrate of CYP3A4, its clearance
may be decreased by these drugs due to competitive inhibition. In
addition, the target for both colchicine and vincristine is tubulin
beta chain which may add to toxicity when administered together.[19]
Thus, our patient may have experienced gastrointestinal adverse effects
and prolonged bone marrow suppression, as described in patients with
colchicine toxicity, due to CYP3A4-mediated impairment of colchicine or
antineoplastic agent metabolism or an additive effect on tubulin
inhibition.[20]
Colchicine was discontinued in
our patient and the second cycle of chemotherapy was well tolerated,
without fever or abdominal pain. She is currently disease-free 17
months after completing therapy.
In summary, to the best of our
knowledge, we describe the first case of Hodgkin lymphoma in a patient
with familial Mediterranean fever. Colchicine should be given with
caution in patients treated with drugs substrate of CYP3A4 and/ or
effecting on tubulin polymerization, like vincristine. To this end, a
strict collaboration between oncology and rheumatology is warranted
when managing these patients.
.
References
- Ward E, DeSantis C, Robbins A, Kohler B, Jemal A.
Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin.
2014 Mar-Apr;64(2):83-103. https://doi.org/10.3322/caac.21219 PMid:24488779

- Lee
JH, Kim Y, Choi JW, Kim YS. Prevalence and prognostic significance of
Epstein-Barr virus infection in classical Hodgkin's lymphoma: a
meta-analysis. Arch Med Res. 2014 Jul;45(5):417-31 https://doi.org/10.1016/j.arcmed.2014.06.001 PMid:24937173

- Tran
H, Nourse J, Hall S, Green M, Griffiths L, Gandhi MK.
Immunodeficiency-associated lymphomas. Blood Rev. 2008
Sep;22(5):261-81. https://doi.org/10.1016/j.blre.2008.03.009 PMid:18456377

- Straus
SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rösen-Wolff A, et al. The
development of lymphomas in families with autoimmune
lymphoproliferative syndrome with germline Fas mutations and defective
lymphocyte apoptosis. Blood. 2001 Jul 1;98(1):194-200 https://doi.org/10.1182/blood.V98.1.194 PMid:11418480

- Goldfinger SE. Colchicine for familial Mediterranean fever. N Engl J Med. 1972. Dec 21;287(25):1302.

- Alghamdi M. Familial Mediterranean fever, review of the literature. Clin Rheumatol. 2017 Aug;36(8):1707-1713. https://doi.org/10.1007/s10067-017-3715-5 PMid:28624931

- Soriano
A, Verecchia E, Afeltra A, Landolfi R, Manna R. (2013) IL-1ß biological
treatment of familial Mediterranean fever. Clin Rev Allergy Immunol.
2013 Aug;45(1):117-30. https://doi.org/10.1007/s12016-013-8358-y PMid:23322405

- Celik
S, Tangi F, Oktenli C. Increased frequency of Mediterranean fever gene
variants in multiple myeloma. Oncol Lett. 2014 Oct;8(4):1735-1738. Epub
2014 Aug 4. https://doi.org/10.3892/ol.2014.2407 PMid:25202401 PMCid:PMC4156200

- Sayan
O, Kilicaslan E, Celik S, Tangi F, Erikci AA, Ipcioglu O, et al. High
Frequency of Inherited Variants in the MEFV Gene in Acute Lymphocytic
Leukemia. Indian J Hematol Blood Transfus. 2011 Sep;27(3):164-8. https://doi.org/10.1007/s12288-011-0095-x PMid:22942567 PMCid:PMC3155719

- Oktenli
C, Celik S. High frequency of inherited variants in the MEFV gene in
patients with hematologic neoplasms: a genetic susceptibility? Int J
Hematol. 2012 Apr;95(4):380-5. https://doi.org/10.1007/s12185-012-1061-6 PMid:22453916

- Celik
S, Oktenli C, Kilicaslan E, Tangi F, Sayan O, Ozari HO, et al.
Frequency of inherited variants in the MEFV gene in myelodysplastic
syndrome and acute myeloid leukemia. Int J Hematol. 2012
Mar;95(3):285-90. https://doi.org/10.1007/s12185-012-1022-0 PMid:22351163

- Celik
S, Erikci AA, Tunca Y, Sayan O, Terekeci HM, Umur EE, et al. The rate
of MEFV gene mutations in hematolymphoid neoplasms. Int J Immunogenet.
2010 Oct;37(5):387-91. https://doi.org/10.1111/j.1744-313X.2010.00938.x PMid:20518828

- Dorffel
W, Luders H, Ruhl U: Preliminary results of the multicenter trial
GPOH-HD 95 for the treatment of Hodgkin's disease in children and
adolescents: Analysis and outlook. Klin Padiatr 215:139-145, 2003 https://doi.org/10.1055/s-2003-39372 PMid:12838937

- Livneh
A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for
the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997
Oct;40(10):1879-85. https://doi.org/10.1002/art.1780401023 PMid:9336425

- Yalçinkaya
F, Ozen S, Ozçakar ZB, Aktay N, Cakar N, Düzova A, et al. A new set of
criteria for the diagnosis of familial Mediterranean fever in
childhood. Rheumatology (Oxford). 2009 Apr;48(4):395-8. https://doi.org/10.1093/rheumatology/ken509 PMid:19193696

- Masters
SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the
molecular pathophysiology of autoinflammatory disease (*). Annu Rev
Immunol. 2009; 27:621-68. https://doi.org/10.1146/annurev.immunol.25.022106.141627 PMid:19302049 PMCid:PMC2996236

- Yilmaz,
E., Ozen, S., Balci, B., Duzova, A., Topaloglu, R., Besbas, et al.
Mutation frequency of familial Mediterranean fever and evidence for a
high carrier rate in the Turkish population. Europ. J. Hum. Genet. 9:
553-555, 2001. https://doi.org/10.1038/sj.ejhg.5200674 PMid:11464248

- Zemer
D, Pras M, Sohar E, Modan M, Cabili S, Gafni J. Colchicine in the
prevention and treatment of the amyloidosis of familial Mediterranean
fever. N Engl J Med. 1986 Apr 17;314(16):1001-5. https://doi.org/10.1056/NEJM198604173141601 PMid:3515182

- Lu
Y, Chen J, Xiao M, Li W, Miller DD. An overview of tubulin inhibitors
that interact with the colchicine binding site. Pharm Res. 2012
Nov;29(11):2943-71. https://doi.org/10.1007/s11095-012-0828-z PMid:22814904 PMCid:PMC3667160

- Finkelstein
Y, Aks SE, Hutson JR, Juurlink DN, Nguyen P, Dubnov-Raz G, et al.
Colchicine poisoning: the dark side of an ancient drug. Clin Toxicol
(Phila). 2010 Jun;48(5):407-14. https://doi.org/10.3109/15563650.2010.495348 PMid:20586571

[TOP]






















