Priyanka Sehrawat1, Ashutosh Biswas1, Prabhat Kumar1, Paras Singla1, Naveet Wig1, Lalit Dar2 and Rita Sood1.
1 Department of Medicine, All India Institute of Medical Sciences, New Delhi, India.
2 Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
Corresponding
author: Dr. Prabhat Kumar. Assistant
Professor, Department of Medicine, Third floor, Teaching block. All
India Institute of Medical Sciences. Ansari Nagar. New Delhi, India.
110029. Tel: +911126593303. E_mail:
drkumar.prabhat@gmail.com
Published: April 20, 2018
Received: November 27, 2017
Accepted: March 21, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018023 DOI
10.4084/MJHID.2018.023
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Objective:
Dengue infection is a rapidly spreading vector-borne disease and is
endemic in the Indian subcontinent. It has varied manifestations
ranging from subclinical infection to severe fatal shock syndrome. This
study aimed to estimate cytokine level in dengue patients and correlate
them with dengue severity.
Methods:
Cases of dengue fever diagnosed in the department of medicine of our
institute from July 2015 to November 2016 were included in the study.
The clinical features, biochemical, hematological and radiological
parameters along with cytokine levels (Interferon-gamma, Interleukin-6,
and Tumour Necrosis Factor-alpha) were recorded in all patients.
Results:
Out of 80 confirmed cases of dengue included in the study, 50 had
nonsevere dengue (Group 1), and 30 patients had severe dengue (Group
2). The median level of serum TNF-α in group 2 (62.5 pg/mL) was
significantly higher than the median level in group 1 (20 pg/mL), (p=0.043).
Similarly, the median level of serum IFN-γ in group 2 (10.25 pg/mL) was
significantly higher than the median level in group 1 (8.5 pg/mL), (p=0.002).
The median level of IL-6 was also higher in group 2 (29 pg/ml) as
compared group 1(14.2 pg/ml), but this result was not significant
(p>0.05).
Conclusion: Some cytokines may play a role in the pathogenesis of severe manifestations of dengue.
|
Introduction
Dengue
infection is a common vector-borne disease which can cause a myriad of
features ranging from mild febrile episode to severe manifestations
like catastrophic bleeding and organ impairment. South East Asia Region
(SEAR) and western Pacific region bear nearly 75% of the current global
disease burden due to dengue.[1] The pathological
basis of dengue fever lies in a complex series of immunological
response resulting in a rapid increase in the levels of cytokine and
other chemical mediators that are central to the severe manifestations
of dengue hemorrhagic fever, such as plasma leakage, shock, and
bleeding.[2] However, at the onset of illness, it is
difficult to predict which dengue patients are going to progress to
severe dengue. By identifying the predictors of severity of dengue, we
can target those patients who are likely to proceed to severe dengue,
thereby reducing the morbidity and mortality related to dengue
infection.
Material and Methods
This
was a cross-sectional comparative study conducted in the department of
medicine at All India Institute of Medical Sciences, New Delhi between
July 2015 and November 2016; it was approved by the ethical committee
of the institute. During this period, 102 cases of suspected dengue
fever who attended our outpatient and emergency department were
screened for dengue fever. Six patients were excluded during screening
(three patients tested positive for malaria, one patient had
tuberculosis, and two had chronic kidney disease). Samples from
remaining 96 patients were subjected to a confirmatory test for dengue,
and 80 patients were confirmed positive. Patients presenting within
five days of onset of fever were tested for NS1 antigen in serum and
those after the fifth day were tested for IgM antibody in serum. Both
tests were done using enzyme-linked immunosorbent assay (ELISA) based
kits. The patients suffering from other acute febrile illness and
chronic diseases like tuberculosis, HIV, and hepatitis were excluded
from the study.
These 80 patients were further classified into
nonsevere dengue (Group 1) and severe dengue (Group 2) by WHO
classification of dengue 2009.[3] Therefore, patients
with and without warning signs were included in group 1, and those with
severe manifestations like severe plasma leakage, severe bleeding, and
severe organ impairment were included in group 2. There were 50
patients in group 1 and 30 in group 2. Blood samples were collected
from both groups for complete hemogram, liver function test, renal
function test and cytokine levels. Blood samples collected for
estimation of cytokine levels were centrifuged to separate the serum
and then stored at -80 degree Celsius. Serum levels of all three
cytokines (INF-γ, IL-6, and TNF-α) were measured using ELISA kits. All
admitted patients were monitored daily till discharge or death in the
hospital. Both the groups were compared on the basis of serum cytokine
levels along with clinical, biochemical and radiological parameters.
Statistical analysis.
Data were recorded using a structured proforma and managed on an excel
sheet. All qualitative variables were compared with Chi-square test or
Fisher's exact test. Quantitative variables with a normal distribution
were compared using Student's t-test for two groups and ANOVA test with
post hoc Bonferroni correction for the three-group analysis.
Quantitative variables not following a normal distribution such as
cytokine levels were compared using non-parametric test (Kruskal-Wallis
test). A p-value of less than 0.05 (<0.05) was considered as
significant. The analysis was done between Group 1 (nonsevere dengue,
n=50) and Group 2 (severe dengue, n=30).
Results
Out
of 80 positive patients, 37 patients were IgM antibody positive, and 43
patients were NS-1 antigen positive. The mean duration of fever in
group1 and group 2 were 5.09±1.7 days and 5.22±2 days respectively. The
most common symptoms apart from fever were headache (92%), followed by
chills (84.1%), myalgia (74.6%), nausea (55%), vomiting (49%),
retro-orbital pain (58%), arthralgia (39%) and rash (28%). Also,
patients with severe dengue had more headache, myalgia, retro-orbital
pain, abdominal pain, diarrhea, respiratory distress, oliguria and
altered sensorium as compared to patients with nonsevere dengue (Figure 1, 2). The mean systolic BP (SBP) in group 2 (94±11.0 mm Hg) was lower than the mean SBP in patients with group 1 (113±09 mm Hg) (p-value
<0.001). Similarly, mean diastolic pressure (DBP) in group 2
(50±10.0 mm Hg) was significantly lower than mean DBP in group 1
(70±11.0 mmHg) (p-value <0.001) (Table 1).
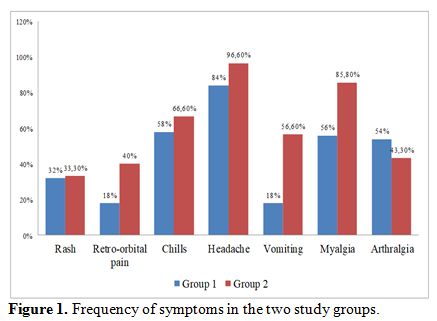 |
Figure 1.
Frequency of symptoms in the two study groups. |
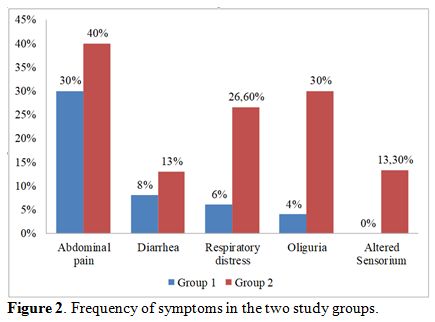 |
Figure 2.
Frequency of symptoms in the two study groups. |
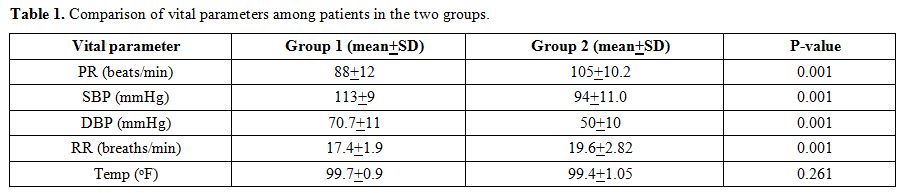 |
Table 1. Comparison of vital parameters among patients in the two groups. |
The
most common clinical finding amongst all patients was positive
tourniquet test (76%), followed by a rash (55.5%). The mean platelet
count in group 2 (70364±50431/ul) was lower than in group 1
(93460±65000/uL), but this difference was not statistically
significant. Mean AST levels in group 2 (451±633.2 IU) were
significantly higher than in group 1 (96.5±157.4 IU, p=0.01). The mean serum ALT levels in group 2 (270.3±334.25 I.U.) were also higher than in group 1 (60.16±73.22 IU, p=0.01).
Bleeding manifestations were more in group 2 (83.3%) as compared to
group 1 (46%). The most common form of bleeding manifestation was skin
petechiae, seen in 36% of group 1 patients and 63.3% of group 2
patients. Gastrointestinal bleeding (hematemesis, melena, or
hematochezia) was present in 8% of group 1 and 56.6% of group 2. One
patient had iliopsoas bleed and required surgical intervention (Figure 3).
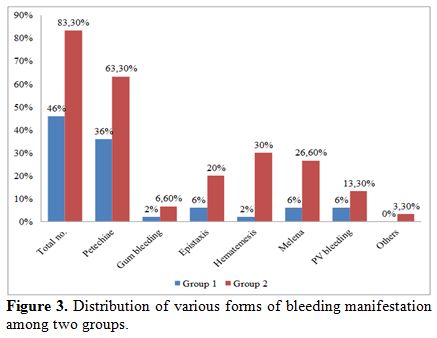 |
Figure 3. Distribution of various forms of bleeding manifestation among two groups. |
Tumor
Necrosis Factor-α, Interleukin-6 and Interferon-γ levels were estimated
in the serum of 80 patients from the sample collected on the day of the
presentation. The median level of serum TNF-α in group 2 (62.5 pg/mL)
was significantly higher than the median level in group 1 (20 pg/mL), (p=0.043).
Similarly, the median level of serum IFN-γ in group 2 (10.25 pg/mL) was
significantly higher than the median level in group 1 (8.5 pg/mL),
(p=0.002). The median level of IL-6 was also higher in group 2 (29
pg/ml) as compared group 1 (14.2 pg/ml), but this result was not
significant (p>0.05) (Table 2).
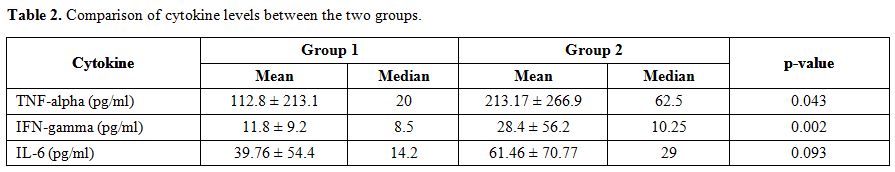 |
Table 2. Comparison of cytokine levels between the two groups. |
Further,
the median levels of both TNF-α and IFN-γ were calculated for each day
of fever according to the day of fever on presentation and sample
collection. The serum levels of TNF-α were significantly higher on day
2-3 of illness, followed by a fall on day 4-5 and a late upsurge in
group 2 as compared to group 1. Similarly, IFN-γ levels were also
plotted across the day of fever and significant increase was noticed on
initial days of presentation followed by a fall and a second rise in
the levels in group 2 (Figure 4, 5).
The decline in the mid-phase is not expected but may be attributed to
hemodilution due to intensive intravenous fluid therapy.
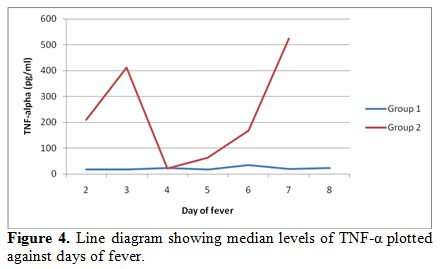 |
Figure 4.
Line diagram showing median levels of TNF-α plotted against days of fever. |
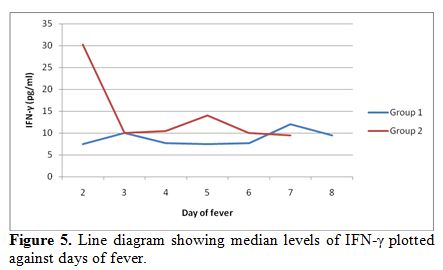 |
Figure 5. Line diagram showing median levels of IFN-γ plotted against days of fever. |
Discussion
The
most common presenting symptoms in our study were a headache, myalgia,
nausea, and vomiting. The observed frequencies of symptoms in our study
are similar to those previously reported in the literature but with
some notable differences.[4,5] Retro-orbital pain and
arthralgias were infrequent in previous reviews, but in the present
study, almost half of the patients had these symptoms. In our study,
the mean platelet count in group 2 was significantly lower than the
mean platelet count in group 1. Few studies have shown that low
platelet counts are a predictor of dengue severity.[6,7] However, our study did not demonstrate any statistical significance between mean platelet count in these two groups.
Deranged
liver function in dengue infection can be a result of the direct effect
of the virus on hepatocytes or unregulated host immune response against
the virus.[8] Mahmuduzzaman and colleagues showed that
both AST and ALT were significantly raised in patients with DHF as
compared to those with dengue fever and increase in AST was much higher
than the increase in ALT.[9] Similarly, Pancharoen and
coworkers also reported that levels of AST and ALT were significantly
higher among patients with more severe disease.[10] In present study too, the difference in both AST and ALT levels in the two groups were significant.
Studies
in the recent past have highlighted the role of cytokines and other
biomarkers in the pathogenesis of severe dengue and have studied the
utility of these biomarkers as risk factors.[11,12]
It has been clearly demonstrated that the inflammatory response
associated with deregulated cytokine production perform critical roles
in the development of severe dengue.[13] The higher
levels of cytokines like TNF- α, IL-1β, IFN-γ, IL-4, IL-6, IL-13, IL-7,
and GM-CSF were associated with severe dengue fever in various studies.[14,15,16]
In present study too, the levels of TNF-α and IFN-γ were significantly
higher in severe dengue group (group 2). The elevated levels of
cytokine in severe dengue make them good predictors of severity of
dengue fever. Cytokine estimation at presentation can provide us a clue
whether a patient is likely to develop severe manifestations of dengue
or not. However, our study has certain limitations, like small sample
size and the serotype of dengue virus was not studied. Further large
prospective studies are warranted for better comprehension of the
balance between circulating cytokines and their effect on the
development of severe dengue.
Conclusions
Some
cytokines like TNF-α and INF-γ may play a role in pathogenesis of
severe dengue fever and can act as predictors of dengue severity.
.
References
- World Health Organization. Regional Office for
South-East Asia. Comprehensive guidelines for prevention and control of
dengue and dengue hemorrhagic fever. New Delhi, India: World Health
Organization Regional Office for South-East Asia; 2011.

- Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev. 2009;22:564-581. https://doi.org/10.1128/CMR.00035-09 PMid:19822889 PMCid:PMC2772360

- World
Health Organization and the Special Programme for Research and Training
in Tropical Diseases (TDR). Dengue guidelines for diagnosis, treatment,
prevention and control: new edition. http://www.who.int/rpc/guidelines/9789241547871/en/ (published 2009)
- Kumar
A, Rao CR, Pandit V, Shetty S, Bammigatti C, Samarasinghe CM. Clinical
Manifestations and Trend of Dengue Cases Admitted in a Tertiary Care
Hospital, Udupi District, Karnataka. Indian J Community Med
2010;35:386-390. https://doi.org/10.4103/0970-0218.69253 PMid:21031102 PMCid:PMC2963875

- Agarwal
R, Kapoor S, Nagar R, Misra A, Tandon R, Mathur A, Misra AK, Srivastava
KL, Chaturvedi UC. A clinical study of the patients with dengue
hemorrhagic fever during the epidemic of 1996 at Lucknow, India.
Southeast Asian J Trop Med Public Health. 1999;30:735-740.
PMid:10928368

- Brasier
AR, Ju H, Garcia J, Spratt HM, Victor SS, Forshey BM, Halsey ES, Comach
G, Sierra G, Blair PJ, Rocha C, Morrison AC, Scott TW, Bazan I, Kochel
TJ; Venezuelan Dengue Fever Working Group. A Three-Component Biomarker
Panel for Prediction of Dengue Hemorrhagic Fever. Am J Trop Med Hyg.
2012;86:341-348. https://doi.org/10.4269/ajtmh.2012.11-0469 PMid:22302872 PMCid:PMC3269290

- Jayashree
K, Manasa GC, Pallavi P, Manjunath GV. Evaluation of Platelets as
Predictive Parameters in Dengue Fever. Indian J Hematol Blood Transfus.
2011;27:127-130. https://doi.org/10.1007/s12288-011-0075-1 PMid:22942561 PMCid:PMC3155720

- Rachman
A, Rinaldi I. Coagulopathy in dengue infection and the role of
interleukin-6. Acta Medica Indones. 2006;38:105-108. PMid:16799214

- Mahmuduzzaman
M, Chowdhury AS, Ghosh DK, Kabir IM, Rahman MA, Ali MS. Serum
transaminase level changes in dengue fever and its correlation with
disease severity. Mymensingh Med J. 2011;20:349-355. PMid:21804492

- Pancharoen
C, Rungsarannont A, Thisyakorn U. Hepatic dysfunction in dengue
patients with various severity. J Med Assoc Thai. 2002;85 Suppl
1:S298-301. PMid:12188427

- Bethell
DB, Flobbe K, Cao XT, Day NP, Pham TP, Buurman WA, Cardosa MJ, White
NJ, Kwiatkowski D. Pathophysiologic and prognostic role of cytokines in
dengue hemorrhagic fever. J Infect Dis. 1998;177:778-782. https://doi.org/10.1086/517807 PMid:9498463

- Green
S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Suntayakorn S, Nisalak A,
Lew R, Innis BL, Kurane I, Rothman AL, Ennis FA. Early immune
activation in acute dengue illness is related to development of plasma
leakage and disease severity. J Infect Dis. 1999;179:755-762. https://doi.org/10.1086/314680 PMid:10068569

- Pang
T, Cardosa MJ, Guzman MG. Of cascades and perfect storms: the
immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome
(DHF/DSS). Immunol Cell Biol. 2007;8:43-45. https://doi.org/10.1038/sj.icb.7100008 PMid:17130899

- Bozza
FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT,
Kubelka CF.Multiplex cytokine profile from dengue patients: MIP-1beta
and IFN-gamm as predictive factors for severity. BMC Infect Dis.
2008;8:86. https://doi.org/10.1186/1471-2334-8-86 PMid:18578883 PMCid:PMC2474613

- Azeredo
EL, Zagne SM, Santiago MA, Gouvea AS, Santana AA, Neves-Souza PC,
Nogueira RM, Miagostovich MP, Kubelka CF. Characterisation of
lymphocyte response and cytokine patterns in patients with dengue
fever. Immunobiology. 2001;204:494-507. https://doi.org/10.1078/0171-2985-00058 PMid:11776403

- Priyadarshini
D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S, Mokashi
N, Vaidya D, Shah PS, Cecilia D. Clinical Findings and Pro-Inflammatory
Cytokines in Dengue Patients in Western India: A Facility-Based Study.
PLoS ONE. 2010;5:e8709 https://doi.org/10.1371/journal.pone.0008709 PMid:20090849 PMCid:PMC2806829

[TOP]























