Aya Nakaya, Shinya Fujita,
Atsushi Satake, Takahisa Nakanishi, Yoshiko Azuma, Yukie Tsubokura,
Akiko Konishi, Masaaki Hotta, Hideaki Yoshimura, Kazuyoshi Ishii,
Tomoki Ito and Shosaku Nomura.
First Department of Internal Medicine, Kansai Medical University
Corresponding
author: Aya Nakaya. First Department of
Internal Medicine, Kansai Medical University, 2-5-1, Shin-machi,
Hirakata, Osaka 573-1010, JAPAN. Tel: +81-72-804-2503. E-mail:
nakaya1016@yahoo.co.jp
Published: April 20, 2018
Received: January 30, 2018
Accepted: March 21, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018024 DOI
10.4084/MJHID.2018.024
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
The
correlation between human T-cell leukemia virus type І (HTLV-І)
infection and malignant neoplasms other than adult T-cell lymphoma
(ATL) remains unknown. However, some previous papers have reported the
frequency of primary malignant tumors occurring with HTLV-І infection[1,2,3],
and it seems likely that HTLV-І infection may contribute to the
development of primary malignant neoplasms other than ATL. Thus, we
analyzed the frequency of primary malignant neoplasms other than ATL in
HTLV-І- seropositive patients.
From January 2006 to December
2016, 203 patients were diagnosed as HTLV-І-seropositive at Kansai
Medical University Hospital. Serological tests were performed to
identify patients with HTLV-І infection. The presence of serum
antibody against HTLV-І was determined by western blot analysis, and
the clonal integration of provirus DNA was confirmed by southern blot
analysis. Subtypes of ATL were defined based on the presence of
abnormal lymphocytes, serum lactate dehydrogenase, and calcium, using
the criteria described by Shimoyama et al.[4]
The
study included a total of 203 HTLV-І- seropositive patients with a
median age of 62 (range: 19–86) years old, and 45% of these subjects
were male. Of this population, 43% were diagnosed as HTLV-І carriers,
and 57% were identified as having ATL. The distribution of ATL subtypes
was: 21% smoldering type, 3% chronic type, 16% acute type, and 17%
lymphoma type (Table 1). Among
the 203 HTLV-І-seropositive patients, 32 developed a primary
malignant neoplasm. Their median age was 64 (range: 41–84) years old,
63% of them were male, and 69% of them were HTLV-I carriers. This group
had the following distribution of ATL subtypes: 31% smoldering type, 0%
chronic type, 3% acute type, and 3% lymphoma type (Table 1).
Additionally, 54% of them had a hematological malignancy other than
ATL, and 46% had a solid tumor. The most frequent type of hematological
malignancy in this group was T-cell lymphoma (23%) (17% anaplastic
large cell lymphoma (ALCL); 3% peripheral T-cell lymphoma, not
otherwise specified (PTCL, NOS); and 3% natural killer (NK)/T-cell
lymphoma), followed B-cell lymphoma (16%) (all diffuse large B-cell
lymphoma (DLBCL)), myeloproliferative
neoplasms (MPN) (9%), and myelodysplastic syndromes
(MDS) (6%). Patients with MDS were either a carrier or smoldering type
of ATL; thus, they have no history of chemotherapies.
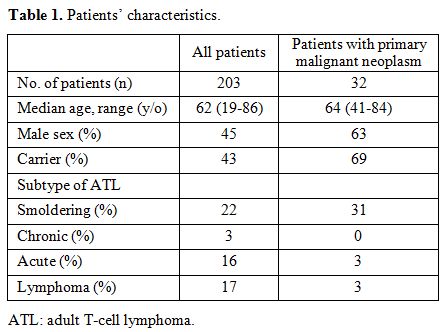 |
Table 1. Patients’ characteristics. |
The
most frequent primary extra-hematological tumor locations were the lung
(15%), followed by the colon (9%), prostate (6%), kidney (6%), cervix
(2%), breast (2%), liver (2%), pancreas (2%), and oral cavity (2%) (Table 2).
Three cases were overlapping more than two malignancies; colon and
cervix, colon and renal, T cell lymphoma and breast. They were two
carriers and one smoldering type. The median overall survival of
patients with acute type ATL was 9.6 months, and that of lymphoma type
ATL was 7.6 months, whereas, those of carrier, smoldering type, and
chronic type were not achieved.
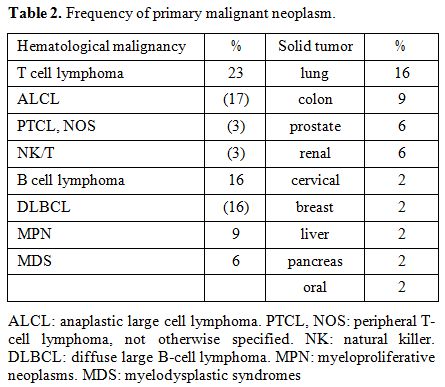 |
Table 2. Frequency of primary malignant neoplasm. |
Some
studies have reported a positive correlation between HTLV-І infection
and malignancies other than ATL. Asou et al. signaled that the
prevalence of HTLV-І among 394 patients with malignant neoplasm was
higher than that among healthy individuals in Kumamoto prefecture in
southwestern Japan (15.48% vs. 2.98%)[1]. In that
study, the most frequent neoplasm site was the lung (n=82), followed
the lymphatics (n=48), stomach (n=47), and liver (n=33). Notably, their
finding that the lung was the most common site for solid tumors is
consistent with the results of our study. Additionally, the frequency
of malignant lymphoma reported by the Asou et al.[1]
study is also compatible with our finding. The high incidence of
hepatocellular carcinoma appears to be regionally specific; the
prevalence of hepatitis virus infection is higher in western Japan.
Regarding
lymphoma, Suefuji et al. reported that B-cell lymphoma patients who
were positive for HTLV-І had a worse prognosis than HTLV-І- negative
patients (5-year overall survival: 49% vs. 78%, p=0.007).[5]
Furthermore, a study by Brady et al. described a positive relationship
between HTLV-І infection and Epstein-Barr virus (EBV) infection. In
their study, 3 of 7 HTLV-І carriers developed de novo DLBCL, and these
patients were also positive for EBV.[6] Although not
all cases of B-cell lymphoma in HTLV-I carriers involve patients who
are also positive for EBV infection, it is possible that when host
immunity is suppressed by HTLV-І, EBV may become activated,
subsequently leading to the development of B-cell malignant lymphoma.
In our institute, we do not routinely examine EBV because it is not
covered by insurance. Thus, it is not clear whether our cases are
positive for EBV or not.
The prevalence of T-cell lymphoma in our
study was high, but this may be the result of inaccurate diagnoses.
Morphologically, ATL is challenging to distinguish from PTCL-NOS in
pathological tissue unless the monoclonal proliferation of HTLV-І
provirus can be proven. Although flow cytometry to detect CC chemokine
receptor 4 (CCR4) often helps to distinguish between these diagnoses,
there are some cases in which the flow cytometry results are
inconclusive. Furthermore, the confirmation of provirus proliferation
cannot be performed without a block of residual tissue, and this
procedure is not covered by medical insurance. Therefore, it is
presumed that there might be some cases in which provirus proliferation
could not be confirmed, leading to these cases being diagnosed
clinically as PTCL-NOS.
This study has some limitations,
including its retrospective design, a small number of patients and a
single facility. Despite a small number of patients, our results
revealed that neoplasm prevalence rate with HTLV-І positive is
significantly high (about 15%), compared to total cancer prevalence
rate to the population in Osaka (about 0.6%).[7]
The
mechanism responsible for the high prevalence of primary malignant
neoplasm associated with HTLV-І infection is currently unknown. We
hypothesize that because the chronic HTLV-І infection is associated
with host immunosuppression, this condition leads to an increased risk
of developing other malignancies.
There are several reports
supporting this speculation. Kannagi et al. revealed that the cytotoxic
T-cell response was reduced in HTLV-І- infected patients.[8] Additionally, Ogura et al. reported that NK cells were suppressed in ATL.[9]
Notably, regulatory T-cells (Tregs) commonly contribute to suppressing
immune responses against tumors, and ATL is described as the malignant
proliferation of CD4- and CD25- positive cells such that Tregs become
tumor cells.[10,11] Thus, the primary mechanism
for the link between HTLV-I infection and malignancy might be host
immunosuppression, caused by Tregs becoming tumorigenic, allowing the
development of a malignant clone. Further studies are needed to
establish what triggers the onset and subsequent development of ATL or
other malignancies in HTLV-I-seropositive patients.
In conclusion,
our results suggest that HTLV-І infection is often associated with the
development of other malignant neoplasms. Therefore, HTLV- І-positive
patients, including HTLV-I carriers, should be made aware of their
increased risk for the onset of a malignant neoplasm and undergo
increased surveillance. Further investigations are required to
elucidate how HTLV-І affects antitumor immunity.
Acknowledgments
The
authors would like to thank Dr. Utsunomiya (Imamura General Hospital,
Kagoshima, Japan) for giving us great advice regarding this work.
.
References
- Asou N, Kumagai T, Uekihara S, Ishii M, Sato M,
Sakai K, Nishimura H, Yamaguchi K, Takatsuki K. HTLV-I seroprevalence
in patients with malignancy. Cancer. 1986;58(4):903-907. https://doi.org/10.1002/1097-0142(19860815)58:4<903::AID- CNCR2820580417>3.0.CO;2-J

- Imamura
N, Inada T, Tagaya Y, Yodoi J, Kuramoto A. Socinski MA, Bondarenko I,
Karaseva NA, et al. Association between ATL and non- hematopoietic
neoplasms. Hematol Oncol. 1993;11(3):127-137. https://doi.org/10.1002/hon.2900110303 PMid:8112727

- Kozuru
M, Uike N, Muta K, Goto T, Suehiro Y, Nagano M. High occurrence of
primary malignant neoplasms in patients with adult T- cell
leukemia/lymphoma, their siblings, and their mothers. Cancer.
1996;78(5):1119-1124. https://doi.org/10.1002/(SICI)1097- 0142(19960901)78:5<1119::AID-CNCR24>3.0.CO;2-4

- Shimoyama
M. Diagnostic criteria and classification of clinical subtypes of adult
T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group
(1984-87). Br J Haematol. 1991; 79(3):428- 437. https://doi.org/10.1111/j.1365-2141.1991.tb08051.x PMid:1751370

- Suefuji
H, Ohshima K, Hayabuchi N, Nakamura K, Kikuchi M. HTLV- 1 carriers with
B-cell lymphoma of localized stage head and neck: prognosis, clinical
and immunopathological features. Br J Haematol. 2003; 123(4):606-612. https://doi.org/10.1046/j.1365- 2141.2003.04653.x PMid:14616963

- Beltran
BE, Qui-ones P, Morales D, Revilla JC, Alva JC, Castillo JJ. Diffuse
large B-cell lymphoma in human T-lymphotropic virus type 1 carriers.
Leuk Res Treatment. 2012; 2012:262363. https://doi.org/10.1155/2012/262363

- http://www.mc.pref.osaka.jp/ocr/en/index.html [accessed Jan 24, 2018]
- Kannagi
M, Matsushita S, Shida H, Harada S. Cytotoxic T cell response and
expression of the target antigen in HTLV-I infection. Leukemia. 1994; 8
Suppl 1:S54-59. PMid:8152305

- Ogura
M, Ishida T, Tsukasaki K, Takahashi T, Utsunomiya A. Effects of
first-line chemotherapy on natural killer cells in adult T-cell
leukemia-lymphoma and peripheral T-cell lymphoma. Cancer Chemother
Pharmacol. 2016; 78(1):199- 207. https://doi.org/10.1007/s00280-016-3070-2 PMid:27289375 PMCid:PMC4921106

- Karube
K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, Utsunomiya
A, Harada M, Kikuchi M. Expression of FoxP3, a key molecule in CD4CD25
regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J
Haematol. 2004; 126(1):81-84. https://doi.org/10.1111/j.1365-2141.2004.04999.x PMid:15198736

- Matsubara
Y, Hori T, Morita R, Sakaguchi S, Uchiyama T. Phenotypic and functional
relationship between adult T-cell leukemia cells and regulatory T
cells. Leukemia. 2005; 19(3):482-483. https://doi.org/10.1038/sj.leu.2403628 PMid:15674359

[TOP]













