Ekaterina Chelysheva1, Sergey Aleshin2, Evgenia Polushkina3, Roman Shmakov3, Igor Shokhin2 ,Ghermes Chilov4 and Anna Turkina1.
1 National Research Center for Hematology, Moscow, Russian Federation.
2 Center of Pharmaceutical Analytics Ltd, Moscow, Russian Federation.
3
FSBI National Research Center of Obstetrics, Gynecology and
Perinatology of the Healthcare Ministry named after V.I. Kulakov,
Moscow, Russian Federation.
4 FSBI N.D. Zelinsky Institute of Organic Chemistry of Russian Academy of Sciences, Moscow, Russian Federation.
Corresponding
author: Ekaterina Chelysheva. Scientific and Advisory Department of
Chemotherapy of Myeloproliferative Neoplasms, National Research
Centre for Haematology, Moscow, Russian Federation, 125167, Novy
Zykovsky pr, 4, Moscow, Russian Federation. Tel: +7 495 6124860, fax:
+7 495 6122123. E-mail:
denve@bk.ru,
Published: May 1, 2018
Received: February 10, 2017
Accepted: March 22, 2017
Mediterr J Hematol Infect Dis 2018, 10(1): e2018027 DOI
10.4084/MJHID.2018.027
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Breastfeeding
in patients with chronic myeloid leukaemia (CML) during tyrosine kinase
inhibitors (TKIs) therapy is not recommended but interruption of TKI
treatment may cause the loss of remission. We studied the 3 cases of
pregnancy and breastfeeding in women with CML and observed that
stopping treatment without major molecular response may end in
haematological relapse. The concentrations of nilotinib and imatinib in
maternal milk were measured and nilotinib distribution in human breast
milk was demonstrated for the first time. The estimated maximal doses
of imatinib and nilotinib which an infant may ingest with the maternal
milk were less than the therapeutical doses. However, the unknown
impact of the low dose chronic exposure to these TKIs in infants
imposes the limitations on their use during breastfeeding.
Breastfeeding without TKI treatment may be safe with molecular
monitoring, but preferably in those patients with CML who have durable
deep molecular response.
|
Introduction
Currently,
patients with chronic myeloid leukaemia (CML) who achieve an optimal
response being treated by tyrosine kinase inhibitors (TKIs) have a high
life expectancy, and therefore planning a family is a significant issue
for them.[1,2] However, the TKIs used for CML treatment have been
classified as Category D by the US Food and Drug Administration (FDA)
due to their potential teratogenicity and the use during pregnancy is
not recommended unless treatment benefits overweigh potential
risks.[3-5]
It has been proved that the first-generation TKI
imatinib is distributed into breast milk.[6-10] It is reasonable to
suggest that the second and third generation of TKIs used for CML
treatment (nilotinib, dasatinib, bosutinib, ponatinib and radotinib)
also distribute into maternal milk, but at the present time it has
never been demonstrated in humans, as of our knowledge. According to
the calculations made from the experimental data, the dose of imatinib
which a child may ingest with the maternal milk is considerably lower
than the therapeutic drug dose, since it corresponds to the plasmatic
level.[7] However, the effects that even low doses of TKIs may cause on
infants in the first months of life are unknown. Therefore,
breastfeeding for women who use these drugs is not recommended. On the
other hand, if a woman insists on breastfeeding, a delay in resuming
TKI after labour may lead to loss of response to treatment. We aimed to
describe the course of the disease in women with CML who were
off-treatment during the breastfeeding period and to measure the
concentrations of TKIs in breast milk when available.
Materials and Methods
Three
women with Ph+ positive CML in chronic phase (CP) were observed during
the years 2014 to 2017. Two patients interrupted imatinib in order to
conceive without TKI, one of them had an in vitro
fertilization. One woman conceived while taking nilotinib and stopped
the drug immediately after pregnancy confirmation. The haematological
and the molecular response of the patients were assessed every 4-6
weeks during the off-treatment period or more often if required. The
definitions of the haematological and molecular response were in
accordance with the European LeukaemiaNet (ELN) recommendations.[11]
One patient resumed imatinib in the second trimester due to the loss of
complete haematological response (CHR) and 2 patients were
off-treatment until labour (Table 1).
The pregnancy ended in childbirth in all 3 patients, all 3 babies were
healthy. The women insisted on breastfeeding their children and were
observed without treatment during the breastfeeding period.
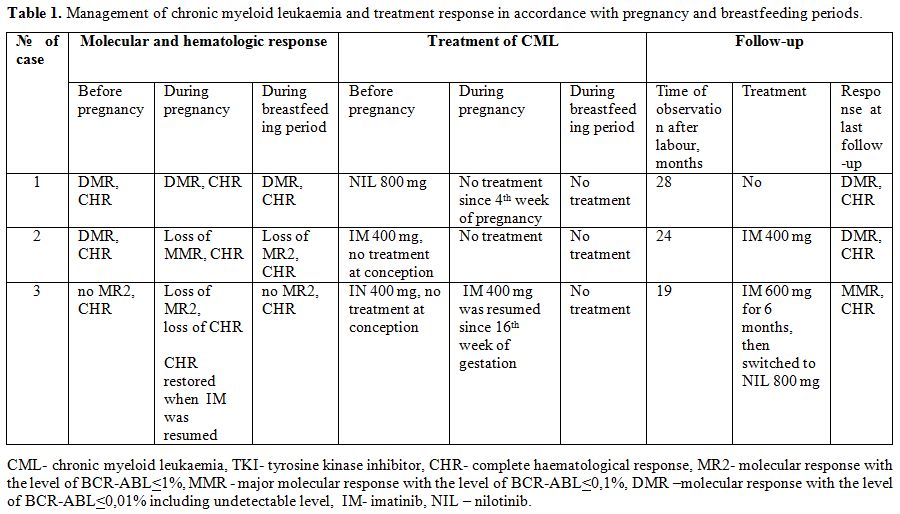 |
Table 1. Management of chronic myeloid leukaemia and treatment response in accordance with pregnancy and breastfeeding periods. |
When the
breastfeeding period came to an end, the patients were asked to collect
breast milk samples after TKI intake. The patients took the same TKI
they had before pregnancy and avoided breastfeeding during the sampling
day. The time points for the milk sample collection were established as
1, 2, 4, 6, 8, 12 and 24 hours after the drug intake. The samples were
stored at -20°C until evaluation. Quantitative detection of drug
concentrations was done by high-performance liquid
chromatography-tandem mass spectrometry (HPLC-MS/MS). All patients
signed an appropriate informed consent for analysis of their biological
samples and clinical data.
Results
Molecular monitoring of BCR-ABL levels during pregnancy and breastfeeding. The molecular response and management of CML differed in each case (Table 1). In order to provide the details, we present a brief description of these cases.
Case 1.
A 32-year old woman with CML CP and low Sokal score had achieved a CHR
but no cytogenetic response after 6 months on imatinib 400 mg and
was switched to nilotinib at a dose of 800 mg. The patient conceived
after 3.8 years of nilotinib therapy and stopped the drug from the
fourth week of gestation. The patient had a stable DMR for 2 years
before pregnancy and during whole pregnancy with BCR-ABL levels less
than 0.0032%. Median (Me) time interval between subsequent molecular
tests during pregnancy was 7 weeks (from 5 to 9 weeks). The
treatment-free period was prolonged in order to breastfeed and it
lasted for 19 months with no loss of DMR. Me time interval of molecular
monitoring during breastfeeding period was 12 weeks (from 3 to 33
weeks). On the day when the breastfeeding was ended, the patient took
400 mg nilotinib and samples of breast milk were collected. After that,
the patient did not restart nilotinib and continued treatment-free
observation with molecular monitoring. Molecular tests were done every
3-6 months. The DMR was maintained (Figure 1a).
Her total treatment-free period at the last follow-up was 37 months.
The follow-up of the child for more than 2 years showed no
developmental delay.
Case 2.
A 30-year-old woman with CML CP and low Sokal score had been receiving
treatment with imatinib at a dose of 400 mg for 7 years. A DMR was
achieved which was stable for more than 6 years, and the BCR-ABL level
was undetectable with the sensitivity of the PCR method of > 4.5 lg.
The patient wished to become pregnant and stopped the drug intake. A
pregnancy occurred after 5 months. At the onset of the pregnancy, the
major molecular response (MMR) was lost and the level of BCR-ABL was
0.11%. Further tests during pregnancy showed fluctuations of BCR-ABL
levels between 0.1% and 0.35%. Me time interval between molecular tests
during pregnancy was 6 weeks (from 3 to 9 weeks). The patient insisted
on breastfeeding. The treatment-free period was extended. Two molecular
tests were done during breastfeeding period with time interval of 10
and 5 weeks. The last test showed the BCR-ABL level was 1.65% after
nearly 3 months of breastfeeding. The breastfeeding was terminated,
treatment with imatinib at a dose of 400 mg was resumed. The total
duration of the treatment-free period for conception, pregnancy and
breastfeeding was 18 months. The DMR was restored 4 months after
restarting imatinib and remained stable for the following 2 years of
follow-up. Molecular monitoring was done every 6 months after treatment
resuming (Figure 1b). The child met the milestones of development during 2.5 years of follow-up.
Case 3.
A 33-year old woman with CML CP and low Sokal score had received
imatinib treatment before pregnancy for nearly 9 years. A first attempt
to conceive was made after 1 year of imatinib 400 mg, when no MMR was
achieved and only BCR-ABL level<1% was observed. The patient stopped
taking imatinib and was switched to interferon alpha (IFN). No
pregnancy took place, the BCR-ABL level increased to 35%, and the
patient restarted treatment with imatinib. The dose of imatinib was
increased to 600 mg and the patient continued this treatment for 6
years. A DMR was reached but it was not stable and long-lasting. Two
more attempts to conceive with imatinib interruption for 3-7 months
were made by the patient. The DMR was lost, the BCR-ABL level raised to
3%, and again no pregnancy occurred. The patient restarted treatment
with imatinib at a dose of 400 mg (Figure 1c).
The last attempt to stop taking imatinib and to conceive with the help of in vitro
fertilization was successful. The off-treatment period for conception
lasted for 1 month and it was prolonged after pregnancy
confirmation. The molecular test which was done at the 10th
week of gestation (2.5 months after treatment was stopped) showed a
BCR-ABL level of 65%. The haematological relapse of CML which was
reflected by the loss of CHR was observed after 1 month. The whole
treatment-free period during conception/pregnancy lasted for 5 months.
Imatinib at 400 mg was resumed in the second trimester after the 16th
week of gestation as imatinib was a drug with a high efficacy in this
patient and has a low placental transfer.[12] The CHR was restored in 3
weeks. The next molecular test during pregnancy was done 3 months after
the administration of imatinib. The level of BCR-ABL was 5,16%. It was
strongly recommended to the patient that she should continue imatinib
after labour. However, the patient interrupted treatment to
breastfeed and resumed imatinib at a dose of 600 mg after 1 month. She
maintained CHR, but nearly 3 months after delivery the level of BCR-ABL
increased to 10%. No BCR-ABL mutations were found. The patient was
switched to nilotinib at a dose of 800 mg and the MMR was achieved in 3
months. (Figure 1d). The MMR
remained stable during further observation. The recommended frequency
of molecular monitoring every 3 months was not followed properly by the
patent. The follow-up of the child for nearly 3 years showed no
developmental delay and no growth retardation.
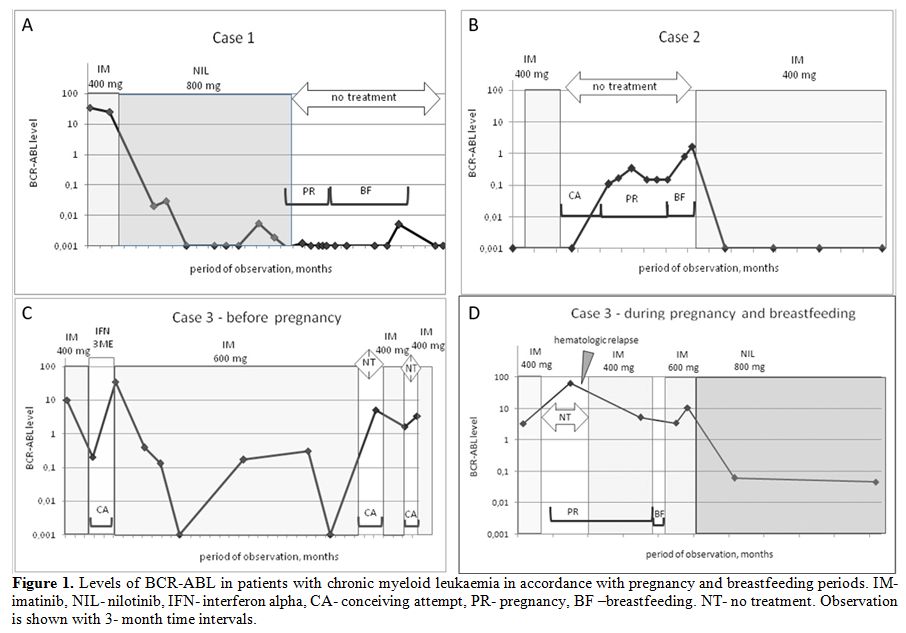 |
Figure 1. Levels of BCR-ABL in patients
with chronic myeloid leukaemia in accordance with pregnancy and
breastfeeding periods. IM- imatinib, NIL- nilotinib, IFN- interferon
alpha, CA- conceiving attempt, PR- pregnancy, BF –breastfeeding. NT- no
treatment. Observation is shown with 3- month time intervals. |
Concentration of imatinib and nilotinib in maternal breast milk. Four series of samples were analysed (Figure 2).
In case 1, the patient received nilotinib at 400 mg; in case 2, the
patient received imatinib at 400 mg; and in case 3, the patient
received imatinib at 400 mg on day 1 and imatinib at 600 mg on the
second day of milk-sample collection. One sample after 24 hours of
nilotinib intake was missed, and other samples were collected according
to the schedule.
The maximum concentration (Cmax) of nilotinib
in breast milk was 129 ng/ml after 4 hours of the drug intake in case
1. The Cmax of imatinib in breast milk at a dose of 400 mg was 1402
ng/ml after 4 hours of the drug intake and 420 ng/ml after 8 hours in
cases 2 and case 3, respectively. The Cmax of imatinib after a
dose of 600 mg was 1411 ng/ml after 6 hours of the drug intake in case
3.
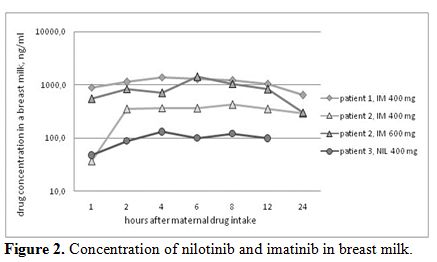 |
Figure 2. Concentration of nilotinib and imatinib in breast milk. |
Discussion
Lactation
and breastfeeding are biological mechanisms that have been established
in mammals, including humans, during years of evolution. Besides
nutrition, the benefits of breastfeeding for the child include
supporting the immune system and protection from infectious, autoimmune
and other diseases.[13] The emotional perception of women regarding
breastfeeding may be connected with psychological, social and cultural
factors.[14] Mothers with CML may also ask whether they are permitted
to breastfeed their children
It has been found that imatinib
distributes to maternal milk as well as its active metabolite
N-desmethyl derivative (or CGP74588) (Table 2).
The milk/plasma ratio for CGP74588 was higher than for imatinib: 0.9-3
vs 0,5.[7,9] The calculated maximal dose of imatinib plus CGP74588 that a
child could take daily with the maternal milk was less than 3 mg. This
dose corresponds to 0.75% of the standard maternal dose of 400 mg
and it is much lower than the lowest paediatric dose of imatinib of 260
mg/m² recommended for children with CML.[15] However, experience of
imatinib use in the first year of an infant's life is very rare as the
median age of paediatric CML patients is nearly 12 years.[16] Some
studies have reported impaired bone growth, growth hormone synthesis
and vitamin D metabolism resulting in growth retardation in children
with CML who received imatinib.[17,18] Nilotinib has been just recently
approved for use in children with CML and no extensive data can be
taken from the pediatric population today.
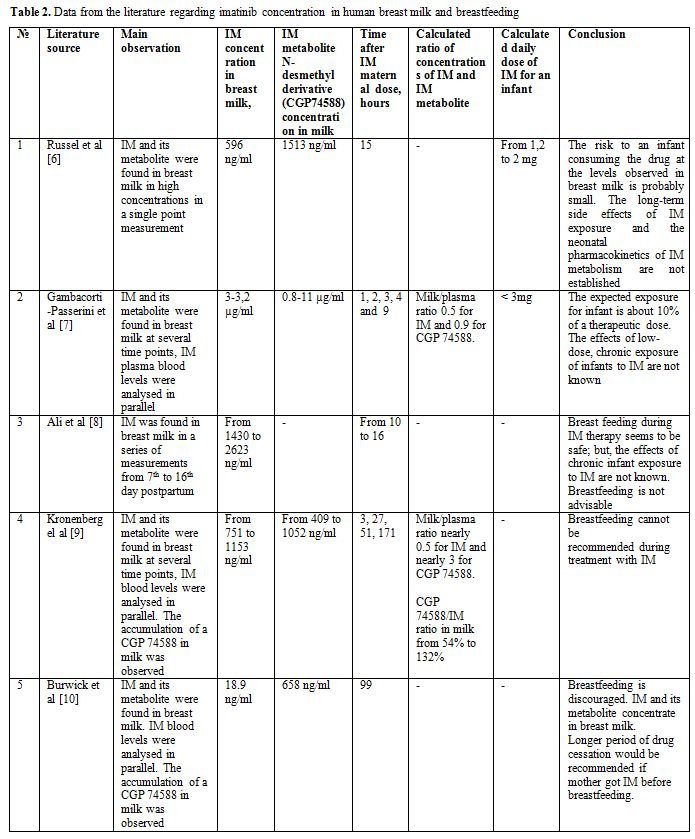 |
Table 2. Data from the literature regarding imatinib concentration in human breast milk and breastfeeding |
Our concentration measurements of imatinib in maternal breast milk correspond with the drug levels described earlier (Table 2) and demonstrate the inter-individual and dose-depending variations (Figure 2).
The concentration measurements of nilotinib in maternal milk described
here are, to the best of our knowledge, the first in a woman with a
single dose of nilotinib 400 mg once a day. Nilotinib penetration
into human breast milk is evident. Based on our data, the estimated
maximum daily dose which an infant may take is nearly 1 mg for imatinib
and 0,1 mg for nilotinib since the maximum daily milk intake is
considered as being 1000 ml. Therefore, we deduce that the calculated
doses of these TKIs which an infant may ingest with the maternal milk
are less than the therapeutical doses. However, the unknown effects of
the low-dose chronic exposure to imatinib in infants in the first year
of life and no data of nilotinib durable impact on infants' development
are the main concerns limiting the use of these TKIs during
breastfeeding.
The key issue for treatment interruption
during pregnancy or breastfeeding in patients with CML is the
risk of disease progression. It has been demonstrated that
treatment-free remission is safe in CML patients with stable and
long-lasting DMR with a 40%-60% probability of maintaining an MMR
without treatment.[19,20] Our case series represents different
situations of the leukaemic cells kinetics in CML patients without
treatment ranging from stable DMR to haematological relapse. Stopping
treatment during breastfeeding may be dangerous in patients without
DMR/MMR and lead to further insufficient treatment response. A close
molecular monitoring is needed for the patients who extend the
off-treatment period for the breastfeeding. If the MMR loss after
treatment cessation is confirmed breastfeeding needs to be terminated
and TKI treatment should be restarted. We consider that recommendation
to use a bottle feeding is the safe choice. The recommendation to avoid
TKIs and to give breastfeeding for the short period of the first 2-5
days after labour to give the child colostrum5 may be acceptable as
well.
The women with CML who plan pregnancy should be aware of the
risks of taking TKIs during breastfeeding as well as the risks of
remission loss if the treatment is discontinued.
.
References
- Sasaki K, Kantarjian HM, Jain P, et al. Conditional
survival in patients with chronic myeloid leukemia in chronic phase in
the era of tyrosine kinase inhibitors. Cancer 2016; 122(2): 238-248. https://doi.org/10.1002/cncr.29745

- Law AD, Dong Hwan Kim D, Lipton JH. Pregnancy: part of life in chronic myelogenous leukemia. Leuk Lymphoma 2017; 58(2):280-287. https://doi.org/10.1080/10428194.2016.1201571

- Cortes
JE, Abruzzese E, Chelysheva E, Guha M, Wallis N, Apperley JF. The
impact of dasatinib on pregnancy outcomes. Am J Hematol
2015;90(12):1111-1115. https://doi.org/10.1002/ajh.24186

- Palani R, Milojkovic D, Apperley JF. Managing pregnancy in chronic myeloid leukemia. Ann Hematol 2015;94(2):S167-S176. https://doi.org/10.1007/s00277-015-2317-z

- Abruzzese
E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine Kinase
Inhibitors and Pregnancy. Mediterr J Hematol Infect Dis. 2014 Apr
7;6(1):e2014028. https://doi.org/10.4084/mjhid.2014.028

- Russel
MA, Carpenter MW, Akhtar MS, Lagattuta TF, Egorin MJ. Imatinib mesylate
and metabolite concentration in maternal blood, umbilical cord blood,
placenta and breast milk. J Perinatol 2007; 27(4):241-243. https://doi.org/10.1038/sj.jp.7211665

- Gambacorti-Passerini
CB, Tornaghi L, Marangon E, Franceschino A, Enrico M. Pogliani EM,
D'Incalci M, and Zucchetti M. Imatinib concentrations in human milk.
Blood. (2007) 109: 1790. https://doi.org/10.1182/blood-2006-08-039545

- Ali
R, Ozkalemkas F,Kimya Y,Koksal N, Ozkocaman V, Gulten T, Yorulmaz H,
Tunali A. Imatinib use during pregnancy and breast feeding: a case
report and review of the literature. Arch Gynecol Obstet 2009;
280:169-175. https://doi.org/10.1007/s00404-008-0861-7

- Kronenberger
R, Schleyer E, Bornhäuser M, Ehninger G, Gattermann N, Blum S. Imatinib
in breast milk. Ann Hematol 2009; 88:1265-1266. https://doi.org/10.1007/s00277-009-0754-2

- Burwick
RM, Kuo K, Brewer D, Druker BJ. Maternal, fetal, and neonatal imatinib
levels with treatment of chronic myeloid leukemia in pregnancy. Obstet
Gynecol 2017;129:831-4. https://doi.org/10.1097/AOG.0000000000001972

- Baccarani
M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations
for the management of chronic myeloid leukemia: 2013. Blood.
2013;122(6):872-84. https://doi.org/10.1182/blood-2013-05-501569

- Chelysheva
E, Turkina A, Polushkina E et al, Placental transfer of tyrosine kinase
inhibitors used for chronic myeloid leukemia treatment. Leuk Lymphoma.
2018; Mar;59(3):733-738. https://doi.org/10.1080/10428194.2017.1347929

- Brahm
P, Valdés V. The benefits of breastfeeding and risks associated with
not breastfeeding. Rev Chil Pediatr. 2017;88(1): 15-21. https://doi.org/10.4067/S0370-41062017000100001

- Shepherd
L, Walbey C, Lovell B. The role of social-cognitive and emotional
factors on exclusive breastfeeding duration. Journal of Human Lactation
2017, Vol. 33(3) 606-613. https://doi.org/10.1177/0890334417708187

- De
la Fuente J, Baruchel A, Biondi A, et al. International BFM Group
(iBFM] Study Group Chronic Myeloid Leukaemia Committee. Managing
children with chronic myeloid leukaemia (CML): recommendations for the
management of CML in children and young people up to the age of 18
years. Br J Haematol. 2014;167:33-47. https://doi.org/10.1111/bjh.12977

- Millot
F, Guilhot J, Suttorp M et al, Prognostic discrimination based on the
EUTOS long-term survival score within the International for Chronic
Myeloid Leukemia in children and adolescents Registry. Haematologica.
2017 Oct;102(10): 1704-1708. https://doi.org/10.3324/haematol.2017.170035

- Millot
F, Guilhot J, Baruchel A et al. Growth deceleration in children treated
with imatinib for chronic myeloid leukaemia. Eur J Cancer. 2014 Dec;
50(18):3206-11. https://doi.org/10.1016/j.ejca.2014.10.007

- Rastogi
MV, Stork L, Druker B et al. Imatinib mesylate causes growth
deceleration in pediatric patients with chronic myelogenous leukemia.
Pediatr Blood Cancer. 2012 Nov;59(5):840-5. https://doi.org/10.1002/pbc.24121 PMid:22378641

- Saussele
S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free
remission in chronic myeloid leukemia. Leukemia 2016; 30(8):1638-1647. https://doi.org/10.1038/leu.2016.115

- Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice of CML. Blood 2016; 128: 17-23. https://doi.org/10.1182/blood-2016-01-694265

[TOP]
























