Rita Wilson Dib, Ray Hachem, Anne-Marie Chaftari* and Issam Raad.
The University of Texas
M. D. Anderson Cancer Center, Department of Infectious Diseases,
Infection Control and Employee Health, 1515 Holcombe, Unite 1460,
Houston, Texas, 77030.
Corresponding
author: Dr. Anne-Marie Chaftari, Department of Infectious
Diseases, Infection Control and Employee Health, Unit 1460, The
University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd.,
Houston, TX 77030. Tel: (713) 792-3491; Fax: (713) 742-7943. E-mail:
achaftar@mdanderson.org
Published: May 1, 2018
Received: , 2018
Accepted: , 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018028 DOI
10.4084/MJHID.2018.028
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
In
this review, we have analyzed the available literature pertaining to
the total duration of intravenous (IV) therapy and the appropriate
timing of step down to oral therapy in the management of candidemia.
Overview of the guidelines and literature seem to indicate that a
minimum of 14 days of antifungal therapy is required in the treatment
of candidemia without deeply seated infection. However, this was never
based on evidence. Furthermore, step down to oral therapy seems to be
dependent on the clinical stability criteria of the patient with
candidemia after 4 to 7 days of IV therapy. Further studies are
required to evaluate the appropriate total duration of IV therapy,
appropriate timing of step down to oral therapy and to validate the
clinical criteria that would allow the switch to happen.
|
Introduction
Candidemia
is the most common form of invasive candidiasis and one of the leading
causes of bloodstream infections (BSI) in critically ill and
immunosuppressed patients.[1,2] It is widely recognized for its high morbidity and mortality rates ranging between 10 to 47%.[3,4]
Furthermore, candidemia has an added severity in immunosuppressed and
critically ill patients. Given the high risk it poses, appropriate
treatment and eradication of the organism remain crucial.
The
most recent guidelines for the management of Candidemia without deeply
seated complications (published by the Infectious Diseases Society of
America (IDSA) in 2016) recommended a minimum of 14 days of antifungal
therapy after documented blood culture clearance in a clinically stable
state.[5] In the case of neutropenia, the guidelines also entail recovery of the white cell count.[5]
However, these recommendations are based on limited clinical evidence
grounded on the results of a number of trials in which this practice
has been implied and routinely applied both in the non-neutropenic and
less often the neutropenic population.[6-9]
In a
milestone study by Rex et al. published in the New England Journal of
Medicine in 1994 showing the equivalence of fluconazole to amphotericin
B in the treatment of candidemia, the duration of therapy in both arms
was mandated to be 2 weeks after the last negative blood culture.[7]
Unfortunately, this practice has been carried through routinely as norm
through the literature and guidelines over the last three decades in
the absence of other studies to compare the impact of total duration of
therapy and appropriate time to step down to oral therapy.
Duration of Therapy
Early initiation of antifungal agents in this population has been associated with favorable survival outcome[10]
but the question remains as to when it should be stopped. To our
knowledge, there were no randomized studies in the literature comparing
different duration of treatment and a limited number looked at the
appropriate timing of the step down from intravenous (IV) to oral
antifungal therapy.
Hence, it is necessary to evaluate the
available data on the total duration of therapy of uncomplicated
candidemia given the importance of the subject matter.
One would
argue that a long duration of therapy is useful for prevention or
unintentional treatment of undiscovered foci of infection given the
fact that up to 16% of candidemic patients have some exhibition of
ocular involvement, with devastating consequences in inappropriately
treated patients.[11] In a study by Blennow et al.,
two to three weeks of antifungal treatment was found to be adequate to
treat undetected ocular infections in the setting of candidemia without
signs of metastatic infection at onset. In this cohort, 21 patients
received <=14 days of therapy. Among them, only one patient
developed proven endophthalmitis after having received only 2 days
(total) of therapy.[12] However, we cannot draw
conclusions from this study on the effect of duration of therapy since
the patients could be treated longer as a consequence to having a
possible or probable ocular candidiasis. In addition, the authors did
not distinguish the duration of IV versus oral therapy.
Step Down to Oral Therapy
A
suggested strategy to keep the balance between the need for aggressive
therapy and not overdoing it, is to do step down to oral therapy. It
was recommended by the IDSA to step down within 5 to 7 days once the
patient achieves symptom resolution and clearance of the blood cultures
(Figure 1).[5]
In 2012, the European Society for Clinical Microbiology and Infectious
Diseases (ESCMID) proposed stepping down to oral fluconazole after 10
days of therapy if the patient was stable and the isolated candida
species demonstrate appropriate minimal inhibitory concentrations
(MICs) to the drug.[13]
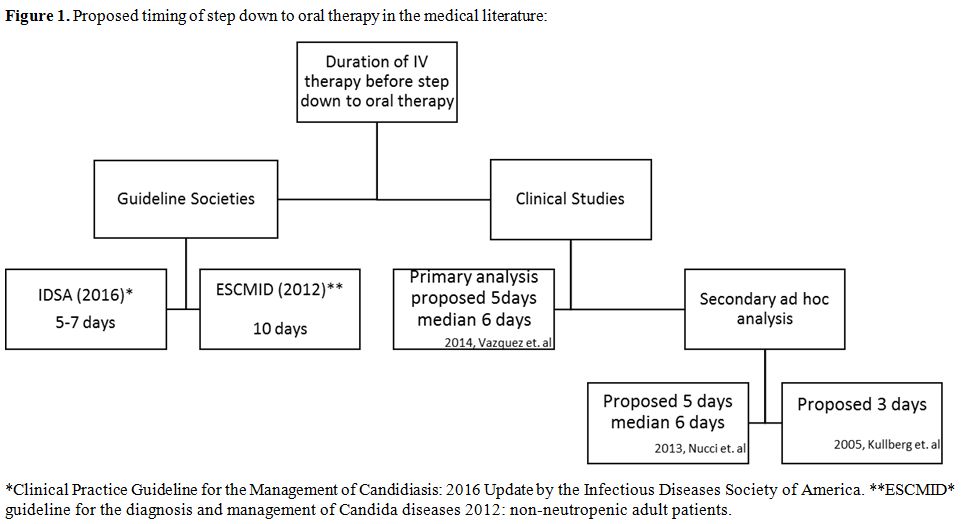 |
Figure 1. Proposed timing of step down to oral therapy in the medical literature |
In
the actual practice, physicians are applying the step down therapy as
per their clinical judgment. Setting clinical stability is not
uniformly defined. Some studies relied on the hemodynamic status and
microbiologic eradication, while others relied on the improvement in
clinical signs and symptoms (defervescence for 24 hours) along with
microbiological eradication.[14,15]
A trial
conducted in several centers in Latin America, patients were eligible
for step down after at least 5 days of anidulafungin if they had
“stable blood pressure “ and at least two negative blood cultures. Only
14 out of 44 qualified for step down to voriconazole with a median
duration of IV therapy of six days. They were all found to have lower
APACHE II score and lower incidence of solid tumors compared with
others making them less sick. Global response and overall mortality
were significantly lower in the step down group.[15]
Even though the number of enrolled patients is small, this study showed
the feasibility of the step down therapy but it did not give us an idea
on the efficacy of stepping down the therapy to oral formulation in
high risk patients especially that the two approved/used agents
(fluconazole and voriconazole) show >90% oral bioavailability.
An
open-label non-comparative trial evaluated global response rates,
defined by clinical improvement and microbiological eradication, of
patients with candidemia who were treated with anidulafungin followed
by oral fluconazole (if baseline cultures revealed C. albicans or C. parapsilosis)
or voriconazole (all other species) after a minimum of 5 days of
anidulafungin provided that the patients were clinically stable.[16]
The step down criteria consisted of 24 hours off fever, hemodynamic
stability and documentation of sterile blood cultures and resolved
neutropenia. A total of 150 patients underwent a step down to oral
therapy, 56% of them qualified for the switch to oral therapy within 6
days with a median of 5 days [range, 1-6]. On the other hand, 44% did
not meet the criteria within the first 6 days of therapy and the median
duration of their IV therapy was 10 days [range, 7-27]. The overall
response in the group of patients who underwent early step down versus
the modified intention to treat (MITT) population did not differ.[16]
Again it was noted that the patients who were switched to oral therapy
before 7 days from onset had lower APACHE scores. This study shows that
early step down to oral therapy within 6 days is dependent on certain
clinical stability criteria (Table 1).
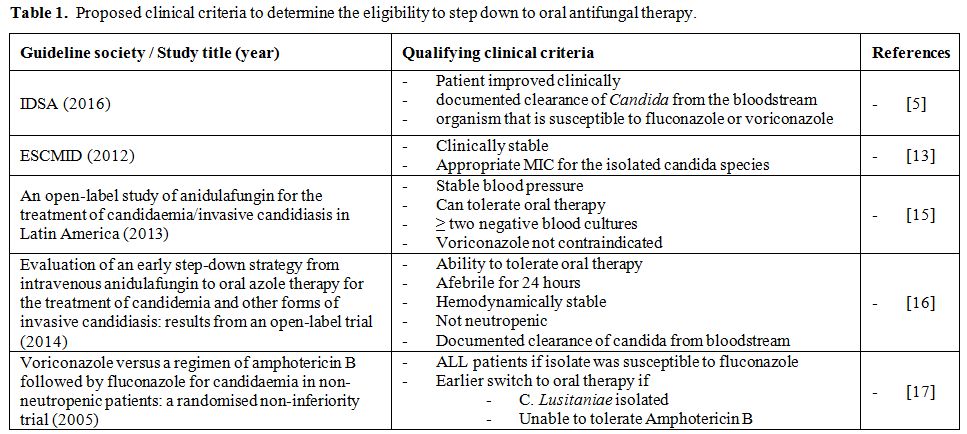 |
Table 1. Proposed clinical criteria to determine the eligibility to step down to oral antifungal therapy. |
Another
study compared voriconazole to amphotericin B therapy whereby the
protocol allowed switch from IV voriconazole to oral voriconazole and
from IV amphotericin to oral fluconazole. The median duration of
amphotericin B was 4 days.[17] Even though the
authors did not mention the median duration of IV voriconazole therapy
and the percentage of patients switched to the oral formulation, there
was no significant difference in overall response between the two
groups.
Another concern with the current proposed step down
strategies was raised by Glockner et.al which is the vague definition
of the timing of documented negative blood culture. This timing
may vary depending on how often the blood cultures are taken and the
fact that they are known to have slow turnaround times with median time
to positivity of 2–3 days reaching 7 days in some situations.[18,19]
Glockner at al., therefore, suggest to consider the timing of
collection of the first negative blood culture as a starting point to
initiate step down strategies.
What is notable in many of the
conducted studies is that microbiologic eradication ranged between 2
and 5 days regardless of the antimicrobial agent and the route of
administration.[16,17,20] This finding could be used to set an appropriate time to consider stepping down or de-escalating the treatment safely.
No
studies showed superiority of any agent, however, both the ESCMID and
the IDSA guidelines suggest the initiation of echinocandins with a
later step down to an appropriate agent based on the susceptibility
pattern and the patient’s clinical status.[5,13]
The reason that these agents have become the common practice is their
fungicidal activity whereby susceptibility studies have shown low MIC
for Candida species including C. glabrata and C. krusei.[21,22] Furthermore, echinocandins demonstrated a survival advantage in non-neutropenic patients.[23]
In addition, they are only available in intravascular formulation which
puts the patients in situations where they have to stay as inpatients
to receive their treatment or face the hurdles of home IV therapies. In
addition, they reportedly have minimal adverse effects with limited
drug interactions.[24]
However, recent case series have described treatment failure associated with growing resistance among strains comprising C. glabrata and C. tropicalis.[25,26]
Hence,
it is important to establish the feasibility of a step down therapy
from echinocandins especially to address the aforementioned increasing
risk of resistance. On the other hand, stepping down from IV therapy
when feasible might also positively affect the healthcare cost in this
subset of patients while maintaining the successful clinical outcome as
shown in previous des-escalation cost effective analysis from studies
from the UK and China.[27,28] Conclusions
In
conclusion, the current practice in the management and treatment of
candidemia and invasive candidiasis is based on inference rather than
evidence. This incites the need for more comprehensive studies
comparing the different management strategies and their outcomes. Such
strategies should ideally account for specific risk factors and
comorbidities which will help identify candidates for early step down.
However, based on the available data and the occurrence of clinical
response, step down to oral therapy between days 4-7 after initiation
of IV therapy seems to be reasonable in most cases. Additional studies
are needed to further validate and define the clinical criteria that
would allow early step down to oral therapy.
References
- Vincent JL, Rello J, Marshall J, Silva E, Anzueto
A, Martin CD, et al. International study of the prevalence and outcomes
of infection in intensive care units. JAMA. 2009;302(21):2323-9. PubMed
PMID: 19952319. https://doi.org/10.1001/jama.2009.1754

- Martin
GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the
United States from 1979 through 2000. New Engl J Med.
2003;348(16):1546-54. PubMed PMID: WOS:000182248900005. https://doi.org/10.1056/NEJMoa02213

- Gudlaugsson
O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, et al.
Attributable mortality of nosocomial candidemia, revisited. Clin Infect
Dis. 2003;37(9):1172-7. PubMed PMID: 14557960. https://doi.org/10.1086/378745

- Pappas
PG, Rex JH, Lee J, Hamill RJ, Larsen RA, Powderly W, et al. A
prospective observational study of candidemia: epidemiology, therapy,
and influences on mortality in hospitalized adult and pediatric
patients. Clin Infect Dis. 2003;37(5):634-43. PubMed PMID: 12942393. https://doi.org/10.1086/376906

- Pappas
PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et
al. Clinical Practice Guideline for the Management of Candidiasis: 2016
Update by the Infectious Diseases Society of America. Clin Infect Dis.
2016;62(4):e1-50. PubMed PMID: 26679628; PubMed Central PMCID:
PMCPMC4725385. https://doi.org/10.1093/cid/civ933

- Kuse
ER, Chetchotisakd P, da Cunha CA, Ruhnke M, Barrios C, Raghunadharao D,
et al. Micafungin versus liposomal amphotericin B for candidaemia and
invasive candidosis: a phase III randomised double-blind trial. Lancet.
2007;369(9572):1519-27. PubMed PMID: 17482982. https://doi.org/10.1016/S0140-6736(07)60605-9

- Rex
JH, Bennett JE, Sugar AM, Pappas PG, Vanderhorst CM, Edwards JE, et al.
A Randomized Trial Comparing Fluconazole with Amphotericin-B for the
Treatment of Candidemia in Patients without Neutropenia. New Engl J
Med. 1994;331(20):1325-30. doi: PubMed PMID: WOS:A1994PR21600001.

- Pappas
PG, Rotstein CM, Betts RF, Nucci M, Talwar D, De Waele JJ, et al.
Micafungin versus caspofungin for treatment of candidemia and other
forms of invasive candidiasis. Clin Infect Dis. 2007;45(7):883-93.
PubMed PMID: 17806055. https://doi.org/10.1086/520980

- Betts
RF, Nucci M, Talwar D, Gareca M, Queiroz-Telles F, Bedimo RJ, et al. A
Multicenter, double-blind trial of a high-dose caspofungin treatment
regimen versus a standard caspofungin treatment regimen for adult
patients with invasive candidiasis. Clin Infect Dis.
2009;48(12):1676-84. PubMed PMID: 19419331. https://doi.org/10.1086/598933

- Morrell
M, Fraser VJ, Kollef MH. Delaying the empiric treatment of Candida
bloodstream infection until positive blood culture results are
obtained: a potential risk factor for hospital mortality. Antimicrob
Agents Ch. 2005;49(9):3640-5. PubMed PMID: WOS: 000231542900006. https://doi.org/10.1128/AAC.49.9.3640-3645.2005

- Oude
Lashof AM, Rothova A, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, et al.
Ocular manifestations of candidemia. Clin Infect Dis. 2011;53(3):262-8.
PubMed PMID: 21765074. https://doi.org/10.1093/cid/cir355

- Blennow
O, Tallstedt L, Hedquist B, Gardlund B. Duration of treatment for
candidemia and risk for late-onset ocular candidiasis. Infection.
2013;41(1):129-34. PubMed PMID: 23212461. https://doi.org/10.1007/s15010-012-0369-8

- Cornely
OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, et
al. ESCMID* guideline for the diagnosis and management of Candida
diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect.
2012;18 Suppl 7:19-37. PubMed PMID: 23137135. https://doi.org/10.1111/1469-0691.12039

- Reboli
AC, Rotstein C, Pappas PG, Chapman SW, Kett DH, Kumar D, et al.
Anidulafungin versus fluconazole for invasive candidiasis. N Engl J
Med. 2007;356(24):2472-82. PubMed PMID: 17568028. https://doi.org/10.1056/NEJMoa066906

- Nucci
M, Colombo AL, Petti M, Magana M, Abreu P, Schlamm HT, et al. An
open-label study of anidulafungin for the treatment of
candidaemia/invasive candidiasis in Latin America. Mycoses.
2014;57(1):12-8. PubMed PMID: 23710653. https://doi.org/10.1111/myc.12094

- Vazquez
J, Reboli AC, Pappas PG, Patterson TF, Reinhardt J, Chin-Hong P, et al.
Evaluation of an early step-down strategy from intravenous
anidulafungin to oral azole therapy for the treatment of candidemia and
other forms of invasive candidiasis: results from an open-label trial.
BMC Infect Dis. 2014;14:97. PubMed PMID: 24559321; PubMed Central
PMCID: PMCPMC3944438. https://doi.org/10.1186/1471-2334-14-97

- Kullberg
BJ, Sobel JD, Ruhnke M, Pappas PG, Viscoli C, Rex JH, et al.
Voriconazole versus a regimen of amphotericin B followed by fluconazole
for candidaemia in non-neutropenic patients: a randomised
non-inferiority trial. Lancet. 2005;366(9495):1435-42. PubMed PMID:
16243088. https://doi.org/10.1016/S0140-6736(05)67490-9

- Glockner
A, Cornely OA. Practical considerations on current guidelines for the
management of non-neutropenic adult patients with candidaemia. Mycoses.
2013;56(1):11-20. PubMed PMID: 22574925. https://doi.org/10.1111/j.1439-0507.2012.02208.x

- Zaoutis
TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology
and attributable outcomes of candidemia in adults and children
hospitalized in the United States: a propensity analysis. Clin Infect
Dis. 2005;41(9):1232-9. PubMed PMID: 16206095. https://doi.org/10.1086/496922

- Reboli
AC, Shorr AF, Rotstein C, Pappas PG, Kett DH, Schlamm HT, et al.
Anidulafungin compared with fluconazole for treatment of candidemia and
other forms of invasive candidiasis caused by Candida albicans: a
multivariate analysis of factors associated with improved outcome. BMC
Infect Dis. 2011;11:261. PubMed PMID: 21961941; PubMed Central PMCID:
PMCPMC3203347. https://doi.org/10.1186/1471-2334-11-261

- Pfaller
MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, et al. In
vitro susceptibility of invasive isolates of Candida spp. to
anidulafungin, caspofungin, and micafungin: six years of global
surveillance. J Clin Microbiol. 2008;46(1):150-6. PubMed PMID:
18032613; PubMed Central PMCID: PMCPMC2224271. https://doi.org/10.1128/JCM.01901-07

- Pfaller
MA, Boyken L, Hollis RJ, Kroeger J, Messer SA, Tendolkar S, et al.
Wild-type MIC distributions and epidemiological cutoff values for the
echinocandins and Candida spp. J Clin Microbiol. 2010;48(1):52-6.
PubMed PMID: 19923478; PubMed Central PMCID: PMCPMC2812271. https://doi.org/10.1128/JCM.01590-09

- Mora-Duarte
J, Betts R, Rotstein C, Colombo AL, Thompson-Moya L, Smietana J, et al.
Comparison of caspofungin and amphotericin B for invasive candidiasis.
N Engl J Med. 2002;347(25):2020-9. PubMed PMID: 12490683. https://doi.org/10.1056/NEJMoa021585

- Bassetti
M, Righi E, Montravers P, Cornely OA. What has changed in the treatment
of invasive candidiasis? A look at the past 10 years and ahead. J
Antimicrob Chemother. 2018;73(suppl_1):i14-i25. PubMed PMID: 29304208. https://doi.org/10.1093/jac/dkx445

- Kontoyiannis
DP, Vaziri I, Hanna HA, Boktour M, Thornby J, Hachem R, et al. Risk
Factors for Candida tropicalis fungemia in patients with cancer. Clin
Infect Dis. 2001;33(10):1676-81. PubMed PMID: 11568858. https://doi.org/10.1086/323812

- Farmakiotis
D, Tarrand JJ, Kontoyiannis DP. Drug-resistant Candida glabrata
infection in cancer patients. Emerg Infect Dis. 2014;20(11):1833-40.
PubMed PMID: 25340258; PubMed Central PMCID: PMCPMC4214312. https://doi.org/10.3201/eid2011.140685

- Masterton
RG, Casamayor M, Musingarimi P, van Engen A, Zinck R, Odufowora-Sita O,
et al. De-escalation from micafungin is a cost-effective alternative to
traditional escalation from fluconazole in the treatment of patients
with systemic Candida infections. J Med Econ. 2013;16(11):1344-56.
PubMed PMID: 24003830. https://doi.org/10.3111/13696998.2013.839948

- Chen
D, Wan X, Kruger E, Chen C, Yue X, Wang L, et al. Cost-effectiveness of
de-escalation from micafungin versus escalation from fluconazole for
invasive candidiasis in China. J Med Econ. 2018;21(3):301-7. PubMed
PMID: 29303621. https://doi.org/10.1080/13696998.2017.1417312

[TOP]































