Igor Stoma1, Igor Karpov1, Igor Iskrov2, Svetlana Krivenko2, Anatoly Uss2, Svetlana Vlasenkova2, Irina Lendina2, Veronika Cherniak2 and Dmitrii Suvorov2.
1 Belarusian state medical university, Minsk, 220116, Dzerzhinski ave., 83, Belarus.
2 City clinical hospital №9, Minsk, 220045, Semashko str., 8, Belarus.
Corresponding
author: Dr. Igor Stoma, M.D., Ph.D. Tel. +375 296178488, Fax. +375 173341462. E-mail:
igor.stoma@gmail.com
Published: May 1, 2018
Received: March 8, 2018
Accepted: April 4, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018030 DOI
10.4084/MJHID.2018.030
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background: Intestinal
colonization by MDR/XDR gram-negative bacteria leads to an increased
risk of subsequent bloodstream infections (BSI) in patients receiving
chemotherapy as a treatment for hematologic malignancies.
Objectives:
The objective of this study was to evaluate the efficacy of oral
colistin in eradicating the intestinal carriage of MDR/XDR
Gram-negative bacteria in patients with hematological malignancies.
Methods:
In a tertiary hematology center, adult patients with intestinal
colonization by MDR/XDR Gram-negative bacteria were included in a
randomized controlled trial (RCT) during a period from November 2016 to
October 2017. Patients were treated with oral colistin for 14 days or
observed with the primary outcome set as decolonization on day 21
post-treatment. Secondary outcomes included treatment safety and
changes in MICs of isolated microorganisms. ClinicalTrials.gov
Identifier: NCT02966457.
Results:
Short-time positive effect (61.3% vs 32.3%; OR 3.32; 95% CI 1.17–9.44;
p=0.0241) was demonstrated on the day 14 of colistin treatment, without
any statistical difference on day 21 post-treatment. The incidence of
BSI in decolonization group was lower in the first 30 days after the
intervention (3.2% vs. 12.9%), but overall in the 90-day observation
period, it did not show any advantages comparing to control group
(log-rank test; p=0.4721). No serious adverse effects or increase in
resistance to colistin was observed.
Conclusions:
This study suggests that in hematological patients the strategy of
selective intestinal decolonization by colistin may be beneficial to
decrease the rate of MDR/XDR Gram-negative intestinal colonization and
the risk of BSI in the short-term period, having no long-term
sustainable effects.
|
Introduction
MDR/XDR
(multidrug-resistant/extensively drug-resistant) gram-negative bacteria
have emerged as the most dangerous cause of bloodstream infection (BSI)
in hospitalized patients, especially in immunocompromised hosts. It was
shown earlier, that intestinal colonization with extended-spectrum
β-lactamases (ESBL)-producing or carbapenem-resistant Enterobacteriaceae spp., carbapenem-resistant A. baumannii and P. aeruginosa might be a prolonged condition in certain populations of patients.[1,2]
It is especially dangerous in patients with hematological malignancies
and HSCT, during the chemotherapy-induced neutropenia, when mucosal
colonization by MDR/XDR pathogens is considered as a risk factor for
subsequent infectious complication.[3–6] It was also
demonstrated previously, that the inadequacy of empirical antibacterial
therapy and the isolation of carbapenem-resistant A. baumannii or P. aeruginosa
were among the most significant risk factors for mortality in adult
patients with BSI in the pre-engraftment period after hematopoietic
stem cells transplantation (HSCT).[7]
There were numerous studies published on decolonization strategies in patients with different primary conditions,[8-12]
but due to the broad preventive use of antibiotics and profound
neutropenia, the problem of choice of strategy of intestinal
decolonization of MDR/XDR Gram-negative bacteria is primarily important
in hematology. Earlier decolonization regimens have been studied for Staphylococcus aureus, but there is a noticeable lack of data on the regimens to decolonize Gram-negative carriage nowadays.[13,14]
To the investigator's knowledge, no randomized clinical trial has been
performed to study the efficacy and safety of selective intestinal
decolonization by Colistimethate sodium (colistin) in high-risk adult
patients with hematological malignancies. It is important to mention
that in a condition of high incidence of carbapenem-resistant
Gram-negative bacteria colistin remains a single therapeutic option in
a number of cases. Colistin, being a non-absorbable antibiotic may have
certain importance as a decolonizing agent, especially in case of
Gram-negative carbapenem-resistant colonization. Gram-negative We have
estimated that possible decolonization of MDR/XDR gram-negative
bacteria in hematological patients could be beneficial for the patients
by reducing the risk of infection and for the community by reducing the
risk of transmission. The aim of the proposed study is to assess the
efficacy and safety of selective intestinal decolonization of MDR/XDR
gram-negative bacteria with oral administration of Colistimethate
sodium in adult patients with hematological malignancies.
Methods
Trial design and setting.
This was a non-blind parallel assignment controlled trial with balanced
(1:1) randomization. The primary purpose of this Phase 4 trial was the
prevention of BSI caused by XDR/MDR Gram-negative bacteria in patients
with hematological malignancies by decreasing the intestinal
colonization level through selective intestinal decolonization. The
trial protocol was approved by the local institutional review board
(IRB) and Ethical Committee (Protocol №11) of the Republican center for
hematology and bone marrow transplantation (Minsk, Belarus) and has
been registered with the US National Institute of Health (NIH) and the
National Library of Medicine (NLM): A Study of Decolonization in
Patients with Haematological Malignancies (DEHAM); ClinicalTrials.gov
Identifier: NCT02966457.
Republican center for hematology and bone
marrow transplantation is a tertiary national clinical and research
center for adult patients situated in Minsk, Republic of Belarus.
Clinical departments are based in the 9th
clinical hospital of Minsk, which is one of the largest teaching
hospitals in Belarus performing more than a hundred HSCT every
year. This center has 150 beds including intensive care unit for
patients with various hematological diseases and patients undergoing
HSCT, as well as an out-patient clinic. Center also includes:
microbiology laboratory, laboratory of bone marrow separation and
freezing, laboratory of cellular biotechnology, HLA-typing laboratory
and clinical diagnostics laboratory.
Participants.
Participants were enrolled in the study during the period from November
2016 to October 2017. Patients with hematological malignancies aged ≥18
years with a positive rectal swab for MDR/XDR Gram-negative
microorganism and the ability to provide informed consent were
eligible. MDR/XDR classification of Gram-negative bacteria was
performed according to Magiorakos et al. was used in the study.[15]
Active
screening of patients admitted to hospital with hematological
malignancies, primarily to receive a course of chemotherapy, was
performed by way of rectal swabs during the study period. Patients with
signs or symptoms of active bacterial, viral, fungal or protozoal
infection were excluded from the study. Among other exclusion criteria
used: pregnant or nursing women, use of antibacterial therapy in
previous 10 days; contraindication to the use of the study drug
(including known hypersensitivity); enrollment in another study, or in
the present study for a previous episode; psychiatric disorder or no
ability to understand or to follow the protocol directions; resistance
of the primarily isolated colonizing microorganism to polymyxin
antibiotics (MIC ≥ 0.5 mg/L). No standard antibacterial prophylaxis was
used during the study period in the included patients. In all cases
measures of contact, precautions were established to prevent the spread
of XDR/MDR microorganisms. Prophylaxis against Pneumocystis jirovecii
with trimethoprim-sulfamethoxazole was administered to all patients in
the study with absolute neutrophil count (ANC) < 100 cells/mm3.
Prophylaxis of infections caused by herpes simplex viruses (HSV) was
performed by acyclovir only in patients with high clinical risk of HSV
reactivation. Real-time quantitative polymerase chain reaction (PCR)
was used for monitoring CMV and EBV DNA levels in high risk patients
weekly.
Interventions.
Patients randomized to the treatment arm received selective intestinal
decolonization with colistin in a dose of 2 mln I.U. 4 times per day PO
for 14 days. Patients in the control group were observed during the
study period without any interventions while they received their
standard treatment for hematological malignancies (“watch and wait”
strategy).
Outcomes.
Patients were assessed at baseline, on the last day of treatment (day
14) and on day 21 after the end of treatment. At each visit, rectal
swabs were performed by inserting the swab immediately in culture
media. The pre-defined primary outcome of the study was the detection
of intestinal MDR/XDR Gram-negative bacteria carriage by a rectal swab
during day 21 post-treatment (rate of eradication of MDR/XDR
Gram-negative bacteria at day 21 post-treatment). Safety of the study
regimen (incidence and intensity of possible adverse effects) and
change in colistin MICs between baseline and the final visit were taken
as secondary outcomes.
Microbiological procedures.
Microbial cultures were isolated and grown on different manufactured
culture media. Identification and antimicrobial susceptibility testing
were performed using a bioMerieux
VITEK 2 automatic system and commercial panels, and the ESBL-phenotype
was determined using a VITEK 2 ESBL Test System. Additional
antimicrobial susceptibility in carbapenem-resistant isolates
(resistance to imipenem, meropenem, and doripenem) was confirmed by
E-tests and disc-diffusion assays. The minimum inhibitory concentration
(MIC) breakpoints used for susceptibility testing were based on current
EUCAST guidelines,[16] with commercially available Mueller–Hinton agar and antimicrobial discs used in disc-diffusion method (bioMerieux).
According
to previously published studies, colistin resistance of Gram-negative
bacteria may be underestimated by Phoenix100/Vitek2 systems,
potentially leading to inappropriate colistin administration. It is
also recommended to retest the isolates with MIC to colistin at
susceptibility breakpoint (2 mg/L).[17] Keeping these
arguments in mind, we have decided to estimate as a susceptible to
colistin only isolates with MIC < 0.5 mg/l.
Sample size and power calculation.
Based on our clinical experience, we assumed that 25% of patients would
clear the MDR/XDR Gram-negative intestinal colonization spontaneously
within the period of study and hypothesized, that a decolonization
regimen would be clinically effective if able to clear colonization in
a 60% of patients. Using a two-sided Alpha of 0.05 and a power of the
study of 80%, with an enrollment ratio of 1 and a dichotomous endpoint,
we calculated a minimal sample size of 60 patients.
Randomization.
Randomization was performed by computerized randomization program
(ALEA) in the proof assistant Coq v. 8.3., which is validated for use
in randomized clinical trials. The block size randomly varies between
4, 6 and 8.
Blinding.
Due to the decision of IRB and Ethical Committee, blinding in the
planned study was not considered appropriate from the ethical
positions, so the study protocol did not include it.
Statistical methods.
Based on the study design, the intention-to-treat analysis was
performed, while none of the patients were excluded in the process of
the trial and the study characteristics were analyzed according to the
randomization scheme. Due to the ongoing chemotherapy treatment for a
primary hematological disease, there were no cases of data missing or
exclusion of the patients in the process of the trial due to loss to
follow-up. All of the patients in the study were included in the
monitoring of adverse effects of the decolonization regimen. The
distribution of the variables was determined by the Shapiro-Wilk test.
Differences in MDR/XDR Gram-negatives carriage between the study groups
were analyzed by methods of non-parametric statistics for categorical
variables (Chi-squared or Fisher’s exact tests). Univariate logistic
regression was used to determine the odds ratio for the presence of
MDR/XDR Gram-negative intestinal colonization in the treatment and
control group. The probability of development of BSI after the
decolonization was estimated using the Kaplan–Meier method and compared
with log-rank test. Day count in Kaplan-Meier probability test started
from day 21 post-treatment and included 90 days of observation. Data
processing and analysis were performed using MedCalc Statistical
Software v. 18 (MedCalc Software bvba, Ostend, Belgium) and SPSS v.
21.0 (IBM Co., Armonk, NY, USA), and results were regarded
statistically significant when p<0.05. .
Results
Participant flow and recruitment. The study flowchart according to CONSORT Statement[18] is shown in Figure 1. Therefore,
among the main causes of exclusion from the study before a
randomization procedure were: absence of MDR/XDR Gram-negative
intestinal colonization on baseline screening and the use of
antibacterial therapy in previous ten days. After the baseline
assessment, there were 62 patients included in the parallel allocated
groups in a balance of 1:1.
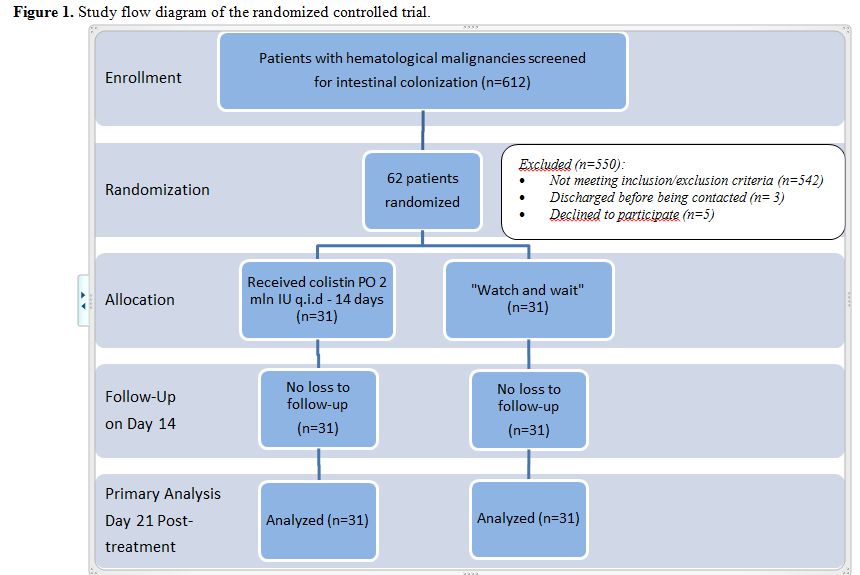 |
Figure 1. Study flow diagram of the randomized controlled trial. |
Baseline data. After the randomization procedure, two study groups were showing similar baseline clinical and demographic characteristics (Table 1).
The
median age of all of the participants in the study was 49 years
(interquartile interval 36-63 years); 31/62 (50%) were female. More
patients in the control group were colonized by MDR/XDR E. coli
(8/31 versus 4/31), while the decolonization group had more patients
with MDR/XDR K. pneumoniae colonization at baseline (16/31 vs. 13/31).
Overall, K. pneumoniae was
the most frequent intestinal colonizer in adult hematological patients
in the study, detected in 29/62 (46.8%) patients. All of the selected
microorganisms at baseline showed susceptibility to colistin (with
MIC<0.5 mg/l). Among the patients included, in the absence of
chemotherapy, recovery of the peripheral neutrophil count over 500
cells/mm3 estimated on the last day
of decolonization regimen (day 14) was observed in 12 (38.7%) in
decolonization group and in 15 (48.4%) in a control group.
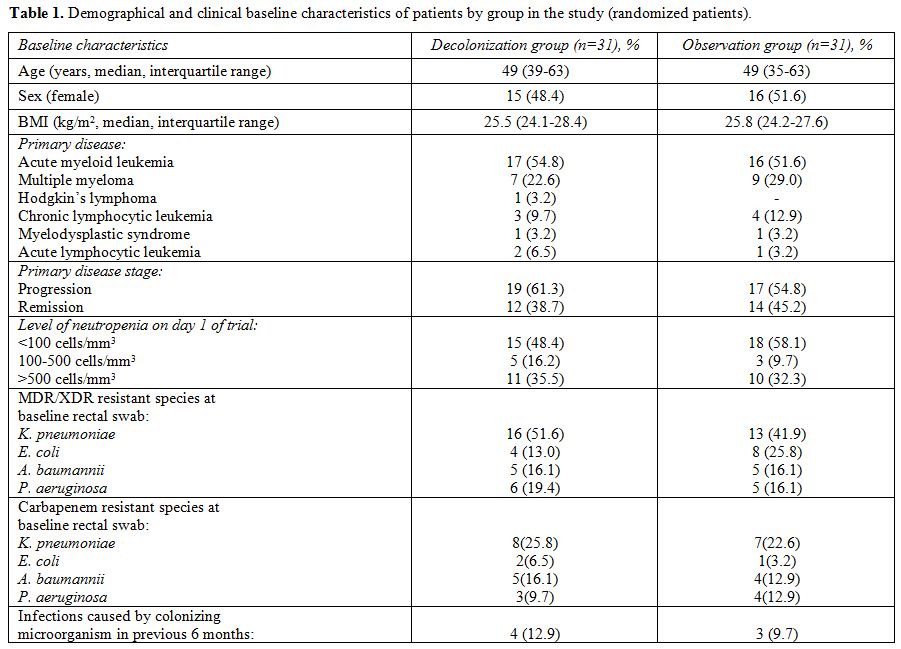 |
Table 1. Demographical and clinical baseline characteristics of patients by group in the study (randomized patients). |
Outcomes and estimation.
In the primary outcome analysis, 19 of 31 patients (61.3%) in the
treatment group and 10 of 31 (32.3%) in the control group have shown
negative rectal swab for MDR/XDR Gram-negative bacteria on the last day
of oral decolonization regimen (day 14). Although, later on day 21
post-treatment the numbers of intestinal colonization by the same
pathogens remained to some extent similar, with 13 of 31 patients
(41.9%) showing decolonization effect in the treatment group and 12 of
31 (38.7%) – in the control group. The observed changes may indicate
that this procedure of selective oral decolonization by colistin had
only a short-time effect, with no long-lasting microbiological benefits.
Based
on the results of univariate statistical analysis using Chi-squared
test and logistic regression, there was a favourable microbiological
effect of oral decolonization by colistin on intestinal MDR/XDR
Gram-negative bacteria in the conducted study (OR 3.32; 95% CI
1.17–9.44; p=0.0241) on the last day of treatment (day 14). Although,
on day 21 post-treatment there was already no statistical significance
shown in the treatment and control groups (OR 1.14; 95% CI 0.41–3.16;
p=0.7958). As an additional characteristic of the efficacy of oral
decolonization of MDR/XDR Gram-negative bacteria in patients with
hematological malignancies, the number needed to treat (NNT) was
analyzed for the last day of treatment (NNT 3.44; 95% CI 1.89–18.99;
p=0.0241), showing the short-time effect of treatment. Figure 2
displays the evolution of MDR/XDR carriage over time in the colistin
oral decolonization group and observation control and shows the
short-time effect of decolonization in the study.
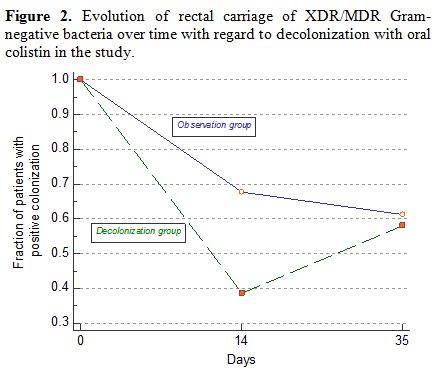 |
Figure 2. Evolution of rectal carriage of
XDR/MDR Gram-negative bacteria over time with regard to decolonization
with oral colistin in the study. |
Estimation of risk for development of bloodstream infection.
Additionally, to get an understanding of the possible clinical effect
of decolonization in MDR/XDR colonized hematological patients the
incidence of bloodstream infections (BSI) was monitored for 90 days in
both decolonized and control groups. All of the patients included in
the study were continuing to receive chemotherapy and have follow-up
visits for their primary hematological disease while being monitored
clinically and microbiologically for possible infectious complications.
Totally, there were 5/31 (16.13%) cases of BSI observed in
decolonization group and 7/31 (22.58%) cases in the control group. Due
to adequately prescribed empiric antibiotic treatment, no adverse
clinical outcomes in the study groups was reported up-to-date. The
incidence of BSI in decolonization group was lower in the first 30 days
after the intervention compared to control group (3.2% vs. 12.9%), but
overall in the 90-day observation period, it did not show any
advantages comparing to control group (log-rank test; p=0.4721). It is
important to add, that during the first 14 days after the intervention
none of the decolonized patients had a documented BSI, while during the
later period BSIs occurred in both groups.
Probability graph for
subsequent bloodstream infections in patients with intestinal
colonization by MDR/XDR Gram-negative bacteria with regard to
decolonization by oral colistin is shown in Figure 3.
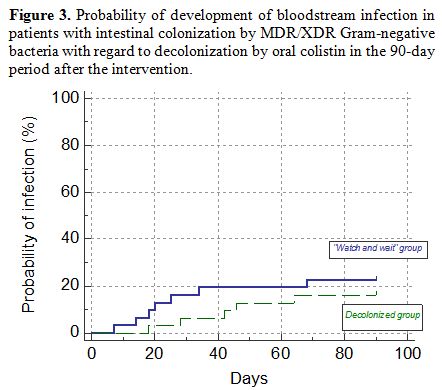 |
Figure 3. Probability of development of
bloodstream infection in patients with intestinal colonization by
MDR/XDR Gram-negative bacteria with regard to decolonization by oral
colistin in the 90-day period after the intervention. |
Adverse effects.
No increase in resistance to colistin above MIC
of 0.5 mg/l was observed in any of isolates during the
study and follow-up period. Among the registered events, there were
only 6 cases of liquid stool without any systemic effects or signs of
infection occurring in 4 patients in decolonization arm and 2 patients
in control arm of the study (Fisher's exact test; 12.9% vs. 6.45%;
P =0 .06713). None of the patients in the study had to stop treatment
prematurely due to serious adverse effects of the treatment. This may
be explained by low intestinal absorption of colistin, leading to
potentially minimal numbers of systemic effects of the drug.
Discussion
This
randomized, controlled trial of an oral colistin decolonization regimen
of MDR/XDR Gram-negative bacteria in adult patients with hematological
malignancies demonstrated a significant temporary suppression of rectal
colonization rate on the last day of treatment (day 14), with no
sustained effect at 21 days after the treatment. Observation of the
incidence of BSI in the studied groups during a 90-days period has
additionally shown the short-time protective effect of decolonization
on the risk of BSI up to first 30 days after the treatment. It may be
explained in a quantitative decrease of MDR/XDR colonizing bacteria in
the gut, what may have some protective effect during
chemotherapy-induced mucositis. To our knowledge, we report the first
randomized, controlled trial examining a decolonization strategy with
colistin for carriers of MDR/XDR Gram-negative bacteria in a group of
adult patients with hematological malignancies, including patients with
chemotherapy-induced neutropenia.
The possibilities for
eradicating the colonizing microorganisms in various groups of patients
were studied earlier in different settings. For example, Huttner et al.
have shown the temporary decolonizing effect of oral colistin on
ESBL-producing Enterobacteriaceae spp. rectal carriage in patients with various comorbidities, what may correspond with results of our study.[9]
Additionally, Saidel-Odes et al. have demonstrated in the study, that
colistin-based regimen could be a suitable decolonization therapy for
selected patients colonized with carbapenem-resistant K. pneumoniae, such as transplant recipients or immunocompromised patients pending chemotherapy.[8] Oral gentamicin was also reported as a possible decolonizing agent in an HSCT setting.[12]
It is important to mention, that one of the most effective directions
of research in the studied area should be based on investigation of
changes in intestinal microbiota composition, leading to expansion and
domination of certain bacteria, with a future possibilities to
establish the risk factors for domination of MDR/XDR microorganisms and
potential preventive strategies, including decolonization regimens.[19,20]
In future studies it may be suggested, that not only rectal swabs
should be studied in hematological and HSCT patients populations, but a
pharyngeal carriage and skin colonization by MDR/XDR Gram-negatives,
what may lead to important practical recommendations.[21]
Limitations and generalizability.
One of the most important limitations of the conducted study is an
absence of blinding procedure due to ethical reasons, especially in
high-risk patients with hematological malignancies. The other important
issue is that rectal swabs may be inadequate to detect resistant
pathogens present in small amounts and stool cultures may be an
inappropriate way to monitor gut colonization.[22] In
some of the cases, we were not able to differentiate the exogenous and
endogenous rebound of colonization, what may have been controlled by
genotyping techniques.
Finally, this study was conducted in one clinical center, meaning that the external validity of this trial may be limited.
Recommendations.
Due to the fact, that intestinal colonization by MDR/XDR Gram-negatives
is an independent risk factor for adverse clinical outcome in
hematological patients with neutropenia, even a temporary suppression
of MDR/XDR Gram-negatives intestinal carriage may result in a clinical
benefit during the period of profound chemotherapy-induced neutropenia.
Thus, a strategy of early detection and selective suppression of
highly-resistant microorganisms in such patients during prolonged
periods of immunosuppression could result in a reduction in the
incidence of subsequent bloodstream infections in a short period. A
large multicentre trial would be needed to test this hypothesis.
Conclusions
We
observed a temporary suppression of MDR/XDR Gram-negative bacteria
carriage during oral antibiotic treatment by colistin at the end of
decolonization regimen. The study, though, did not demonstrate an
effect of the used decolonization regimen on rectal MDR/XDR
Gram-negative bacteria carriage 21 days after the end of treatment.
Therefore, in high risk hematological patients with
chemotherapy-induced neutropenia, the strategy of selective intestinal
decolonization with colistin may be beneficial to decrease the
short-term probability of developing bloodstream infections up to 30
days from the end of treatment with low incidence of the adverse
effects and minimal risk of increase in colistin drug resistance in
Gram-negative colonizing bacteria.
References
- Birgand G, Armand-Lefevre L, Lolom I, Ruppe E,
Andremont A, Lucet JC. Duration of colonization by
extended-spectrum ß-lactamase-producing
Enterobacteriaceaeafter hospital discharge. Am J Infect Control. 2013;
41(5):443-7. https://doi.org/10.1016/j.ajic.2012.05.015

- Löhr
IH, Rettedal S, Natås OB, Naseer U, Oymar K, Sundsfjord A.
Long-termfaecal carriage in infants and intra-household transmission of
CTX-M-15-producingKlebsiella pneumoniae following a nosocomial
outbreak. J Antimicrob Chemother.2013 May;68(5):1043-8. https://doi.org/10.1093/jac/dks502

- Schwaber MJ, Carmeli Y. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA. 2008; 24;300(24):2911-3. https://doi.org/10.1001/jama.2008.896

- Yu
VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens
H, Wagener MM, Benedi VJ; International Klebseilla Study Group.
Virulence characteristics of Klebsiella and clinical manifestations of
K. pneumoniae bloodstream infections. Emerg Infect Dis. 2007;
13(7):986-93. https://doi.org/10.3201/eid1307.070187

- Denis
B, Lafaurie M, Donay JL, Fontaine JP, Oksenhendler E, Raffoux E,
Hennequin C, Allez M, Socie G, Maziers N, Porcher R, Molina JM.
Prevalence, risk factors, and impact on clinical outcome of
extended-spectrum beta-lactamase-producing Escherichia coli
bacteraemia: a five-year study. Int J Infect Dis. 2015; 39:1-6. https://doi.org/10.1016/j.ijid.2015.07.010

- Freifeld
AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II,
Rolston KV, Young JA, Wingard JR; Infectious Diseases Society of
America. Clinical practice guideline for the use of antimicrobial
agents in neutropenic patients with cancer: 2010 update by the
infectious diseases society of America. Clin Infect Dis. 2011; 52(4):
e56-93. https://doi.org/10.1093/cid/cir073

- Stoma
I, Karpov I, Milanovich N, Uss A, Iskrov I. Risk factors for mortality
in patients with bloodstream infections during the pre-engraftment
period after hematopoietic stem cell transplantation. Blood Res. 2016
;51(2):102-6. https://doi.org/10.5045/br.2016.51.2.102

- Saidel-Odes
L, Polachek H, Peled N, Riesenberg K, Schlaeffer F, Trabelsi Y, Eskira
S, Yousef B, Smolykov R, Codish S, Borer A. A randomized, double-blind,
placebo-controlled trial of selective digestive decontamination using
oral gentamicin and oral polymyxin E for eradication of
carbapenem-resistant Klebsiella pneumoniae carriage. Infect Control
Hosp Epidemiol. 2012 Jan;33(1):14-9. https://doi.org/10.1086/663206

- Huttner
B, Haustein T, Uçkay I, Renzi G, Stewardson A, Schaerrer D, Agostinho
A, Andremont A, Schrenzel J, Pittet D, Harbarth S. Decolonization of
intestinal carriage of extended-spectrum ß-lactamase-producing
Enterobacteriaceae with oral colistin and neomycin: a randomized,
double-blind, placebo-controlled trial. J Antimicrob Chemother. 2013;
68(10):2375-82. https://doi.org/10.1093/jac/dkt174

- de
Jonge E, Schultz MJ, Spanjaard L, Bossuyt PM, Vroom MB, Dankert J,
Kesecioglu J. Effects of selective decontamination of digestive tract
on mortality and acquisition of resistant bacteria in intensive care: a
randomised controlled trial. Lancet. 2003 Sep 27;362(9389):1011-6. https://doi.org/10.1016/S0140-6736(03)14409-1

- Rieg
S, Küpper MF, de With K, Serr A, Bohnert JA, Kern WV. Intestinal
decolonization of Enterobacteriaceae producing extended-spectrum
ß-lactamases (ESBL): a retrospective observational study in patients at
risk for infection and a brief review of the literature. BMC Infect
Dis. 2015 Oct 28;15:475. https://doi.org/10.1186/s12879-015-1225-0

- Zuckerman
T, Benyamini N, Sprecher H, Fineman R, Finkelstein R, Rowe JM, Oren I.
SCT in patients with carbapenem resistant Klebsiella pneumoniae: a
single center experience with oral gentamicin for the eradication of
carrier state. Bone Marrow Transplant. 2011 Sep;46(9):1226-30. doi:
10.1038/bmt.2010.279. https://doi.org/10.1038/bmt.2010.279

- Mody
L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. Mupirocin-based
decolonization of Staphylococcus aureus carriers in residents of 2
long-term care facilities: a randomized, double-blind,
placebo-controlled trial. Clin Infect Dis. 2003 Dec 1;37(11):1467-74. https://doi.org/10.1086/379325 PMid:14614669 PMCid:PMC3319403

- Weintrob
A, Bebu I, Agan B, Diem A, Johnson E, Lalani T, Wang X, Bavaro M, Ellis
M, Mende K, Crum-Cianflone N. Randomized, Double-Blind,
Placebo-Controlled Study on Decolonization Procedures for
Methicillin-Resistant Staphylococcus aureus (MRSA) among HIV-Infected
Adults. PLoS One. 2015;10(5): e0128071. eCollection 2015. https://doi.org/10.1371/journal.pone.0128071

- Magiorakos
AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth
S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB,
Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL.
Multidrug-resistant, extensively drug-resistant and pandrug-resistant
bacteria: an international expert proposal for interim standard
definitions for acquired resistance. Clin Microbiol Infect. 2012
Mar;18(3):268-81. https://doi.org/10.1111/j.1469-0691.2011.03570.x

- The
European Committee on Antimicrobial Susceptibility Testing. Breakpoint
tables for interpretation of MICs and zone diameters, version 7.1,
2017, http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.1_Breakpoint_Tables.pdf.
- Chew
KL, La MV, Lin RTP, Teo JWP. Colistin and Polymyxin B Susceptibility
Testing for Carbapenem-Resistant and mcr-Positive Enterobacteriaceae:
Comparison of Sensititre, MicroScan, Vitek 2, and Etest with Broth
Microdilution. J Clin Microbiol. 2017 Sep;55(9):2609-2616. https://doi.org/10.1128/JCM.00268-17

- Schulz
KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 Statement: updated
guidelines for reporting parallel group randomised trials. BMC Med.
2010; 8:18. https://doi.org/10.1186/1741-7015-8-18

- Taur
Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin
KA, Socci ND, Viale A, Perales MA, Jenq RR, van den Brink MR, Pamer EG.
Intestinal domination and the risk of bacteremia in patients undergoing
allogeneic hematopoietic stem cell transplantation. Clin Infect Dis.
2012; 55(7):905-14. https://doi.org/10.1093/cid/cis580

- Taur
Y, Pamer EG. The intestinal microbiota and susceptibility to infection
in immunocompromised patients. Curr Opin Infect Dis. 2013; 26(4):332-7.
Review. https://doi.org/10.1097/QCO.0b013e3283630dd3

- Tschudin-Sutter
S, Frei R, Dangel M, Stranden A, Widmer AF. Sites of colonization with
extended-spectrum ß-lactamases (ESBL)-producing enterobacteriaceae: the
rationale for screening. Infect Control Hosp Epidemiol. 2012
Nov;33(11):1170-1. https://doi.org/10.1086/668027

- D'Agata
EM, Gautam S, Green WK, Tang YW. High rate of false-negative results of
the rectal swab culture method in detection of gastrointestinal
colonization with vancomycin-resistant enterococci. Clin Infect Dis.
2002 Jan 15;34(2):167-72. https://doi.org/10.1086/338234 PMid:11740703

[TOP]

























