Roberto Antonucci1, Nadia Vacca1, Giulia Boz1, Cristian Locci1, Rosanna Mannazzu1, Claudio Cherchi2, Giacomo Lai1 and Claudio Fozza3.
1 Pediatric Clinic, Department of Medical, Surgical and Experimental Sciences, University of Sassari, Sassari, Italy.
2 Respiratory Unit, Academic Department of Pediatrics, Bambino Gesù Children's Hospital, IRCCS, Rome, Italy.
3 Hematology, Department of Medical, Surgical and Experimental Sciences, University of Sassari, Sassari, Italy.
Corresponding
author: Prof. Roberto Antonucci, MD, Associate Professor of Pediatrics.
Pediatric Clinic, Department of Medical, Surgical and Experimental
Sciences, University of Sassari, Sassari, Italy. Tel. +39 079 228239.
E-mail:
rantonucci@uniss.it
Published: May 1, 2018
Received: February 21, 2018
Accepted: April 19, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018034 DOI
10.4084/MJHID.2018.034
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Severe
hypereosinophilia (HE) in children is rare, and its etiological
diagnosis is challenging. We describe a case of a 30-month-old boy,
living in a rural area, who was admitted to our Clinic with a 7-day
history of fever and severe hypereosinophilia. A comprehensive
diagnostic workup could not identify the cause of this condition. On
day 6, the rapidly increasing eosinophil count (maximum value of
56,000/mm3), the risk of developing
hypereosinophilic syndrome, and the patient’s history prompted us to
undertake an empiric treatment with albendazole. The eosinophil count
progressively decreased following treatment. On day 13, clinical
condition and hematological data were satisfactory, therefore the
treatment was discontinued, and the patient was discharged. Three
months later, anti-nematode IgG antibodies were detected in patient
serum, thus establishing the etiological diagnosis. In conclusion, an
empiric anthelmintic treatment seems to be justified when parasitic
hypereosinophilia is strongly suspected, and other causes have been
excluded.
|
Introduction
Hypereosinophilia (HE) is defined as an eosinophil count in peripheral blood >1,500/mm3. The World Health Organisation categorizes eosinophilia into mild (600-1,500/mm3), moderate (1,500-5,000/mm3), and severe (>5,000/mm3). An increased number of eosinophils can be potentially associated with organ damage.[1]
HE can be distinguished into primary, secondary, familial and
idiopathic. The primary form is a clonal disease classified in the
context of hematologic malignancies.[2] Secondary (or
reactive) HE is caused by underlying conditions, such as parasitosis,
allergic or autoimmune diseases, or drug reactions, and results from a
non-clonal increase in blood eosinophil levels, often driven by the
overproduction of IL-5.[3] Hereditary (familial) HE
(HEFA) is a rare autosomal dominant condition, characterized by HE
associated with end-organ damage. Idiopathic hypereosinophilia (or
hypereosinophilia of undetermined significance) (HEUS) is a diagnosis
of exclusion which can be considered when all causes of a reactive HE
have been ruled out. Rarely, a severe HE can be associated with organ
damage or dysfunction, in the context of the so-called
hypereosinophilic syndrome (HES). In such cases, the tissue
infiltration by eosinophils can result in cell damage, due to the
release of eosinophil granule contents, thus leading to significant
morbidity.[4] Severe hypereosinophilia (HE) is a rare
condition in children. The etiological diagnosis of this condition is
often challenging, with a possible delay in treatment.
Case Report
We
describe a case of a 30-month-old boy who was admitted to the Pediatric
Clinic, University of Sassari, Italy, with a 7-day history of fever.
Two weeks before hospitalization, the patient had had a transient
episode of diarrhea. Just before admission, laboratory tests revealed a
raised white blood cell (WBC) count of 49,920/mm3 with an eosinophil count of 26,310/mm3,
mild microcytic anemia (11.1 g/dL) and high serum total IgE levels (968
UI/mL). The child lived in a rural area of the Mediterranean island of
Sardinia, in close contact with animals, and had never experienced any
medical problem. Moreover, the history revealed that the patient was
not atopic, had been regularly vaccinated, and had not received any
pharmacological treatment before hospital admission. Moreover, he had
no history of recent travels.
On admission, the patient was
apparently well, afebrile, with no other clinical manifestations. At
physical examination, there was no evidence of hepato-splenomegaly and
lymphadenopathy. Laboratory tests showed an increase in both WBC count
(WBC, 58,020/mm3), and eosinophil count (eosinophils, 33,400/mm3;
57.5%); in addition, a moderate elevation of CRP levels (2.85 mg/dL)
was found, while serum electrolytes, hepatic and renal markers were
normal. Anamnestic, clinical and laboratory findings were considered
suggestive for neoplastic or parasitic etiology. In order to exclude a
neoplastic HE, a peripheral blood smear and a bone marrow aspirate were
performed. The former was normal except for the raised eosinophil
percentage, while the bone marrow smear showed an expansion of the
eosinophilic lineage (90%) in the absence of blasts. RT-PCR excluded
the presence of leukemia-associated genetic abnormalities, and
lymphocyte subpopulations, when analyzed by flow cytometry, were
normal. In order to exclude a parasitic etiology, the major types of
helminths and protozoa responsible for infections associated with
hypereosinophilia were investigated by examination of fresh stool,
“scotch tape test” (specific for Enterobius Vermicularis),
examination of urine, and serologic testing (Echinococcus, Toxocara
canis, Cysticercus and Trichinella sp.). All tests were negative for
parasitic infection. Chest X-Ray, Doppler echocardiography, abdominal
and pelvic ultrasound as well as an eye examination excluded thoracic,
abdominal or pelvic lesions and eosinophilic organ infiltration.
During hospitalization, blood eosinophil count further increased, reaching the maximum value of 56,000/mm3
after six days. Under suspicion of parasitic etiology, mainly driven by
the patient's living conditions, and to prevent possible organ damage
due to eosinophil infiltration, empiric treatment with albendazole (15
mg/kg/day) was started. After only 24 hours, the eosinophil count was
21,000/mm3 and continued to decrease in the following days (Figure 1). On day 13, blood eosinophils were 6,700/mm3,
the clinical condition was satisfactory, and therefore the treatment
was discontinued, and the patient was discharged home. The eosinophil
count was found to be within the normal range one month after the end
of therapy.
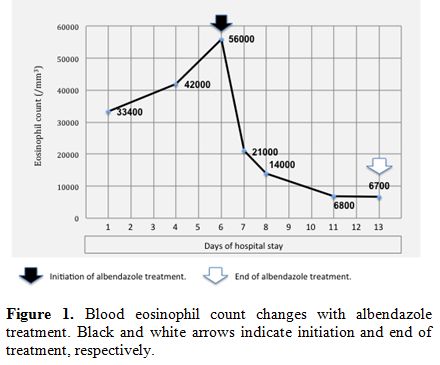 |
Figure 1. Blood eosinophil count changes
with albendazole treatment. Black and white arrows indicate initiation
and end of treatment, respectively. |
At three months
after discharge, IgG anti-nematode antibodies were detected in serum
samples from the patient and his father by ELISA test (using raw
antigen), which was performed by the Italian Higher Institute of Health.
Discussion
Hypereosinophilia
can be associated with many infectious, neoplastic, immunological and
genetic diseases, or to drug reactions. Therefore, a careful family and
personal history is mandatory to investigate conditions related to
eosinophilia, such as atopy, medications, diet, travels and
environmental exposure to parasites.[5] In this case,
the specific living environment of the child, a country farm with close
contact with animals, played a central role in generating our
diagnostic hypothesis. In details, an atopic condition was excluded
based on the dramatic increase in eosinophils and the history of fever.
On the other hand, a neoplastic etiology was ruled out by performing
peripheral and medullary smears, RT-PCR and lymphocyte subpopulations
studies. Eosinophilic Granulomatosis with Polyangiitis (EGPA), a rare
systemic necrotizing vasculitis, was also taken into consideration in
the differential diagnosis, but it was ruled out because of the age of
the patient, the absence of history of atopy, asthma or rhinitis, as
well as the lack of signs of vasculitis and extra-vascular granulomas.
Finally,
a parasitic etiology, which was supported by the patient's history,
remained the most likely option. In this regard, it is known that the
specific diagnostic characterization of a parasitic intestinal
infection is difficult. A wide variety of parasites can elicit
eosinophilia,[6] even if only relatively few of them can be responsible for such a marked increase in eosinophil levels.[7]
The pattern and degree of eosinophilia in parasitic infections result
from the development, migration, and distribution of the parasite
within the host, as well as from the host's immune response. Parasites
tend to elicit marked eosinophilia when they or their products come
into contact with immune effector cells in tissues, particularly during
migration. When mechanical barriers separate the parasite from the
host, or when parasites no longer invade tissues, the stimulus to
eosinophilia is usually absent. Therefore, eosinophilia is highest in
infections with a phase of parasite development that involves migration
through tissues (eg, trichinosis, ascariasis, gnathostomiasis,
strongyloidiasis, schistosomiasis, and filariasis).[8]
Detection of eggs, larvae or adult worms in feces is necessary to make
a diagnosis. However, being very difficult to obtain, a negative
examination does not allow to exclude a parasitic infection with
certainty. The rapid increase in eosinophil count, the potential risk
of evolution to the hypereosinophilic syndrome or to organ damage,[9]
and the history suggestive of a parasite infection prompted us to
undertake an albendazole-based empiric therapy. Albendazole is a safe
medication, and its use is promoted by WHO to control the infection in
high endemic areas, even when an exact diagnosis is lacking.[10]
Also, empiric albendazole therapy is recommended by the British
Infection Society in returning travelers and migrants from the tropics
to cover the possibility of geohelminth infection as the cause of
transient eosinophilia with negative stool microscopy.[11]
In our case, the treatment was readily effective, leading to a steep
decrease in the eosinophil count in just 24 hours. Therefore, the
diagnosis of parasitic hypereosinophilia was challenging and required
an ‘ex-iuvantibus’ approach, while the diagnosis of nematode infection
was established by serologic testing only three months later.
The
picture of extreme hypereosinophilia is rare in childhood. The
etiological diagnosis of this condition is often challenging for the
clinician, and this may lead to difficulties in deciding upon the
specific choice of treatment for individual patients. The case
described here can be considered emblematic of this hematological
condition and offers the example of a diagnostic-therapeutic approach
that might be applicable in similar contexts. Based on this case report
and the literature data, we can conclude that an empiric anthelmintic
treatment is justified and potentially decisive when hypereosinophilia
of parasitic origin is strongly suspected. References
- Valent P, Klion AD, Horny H-P, Roufosse F, Gotlib
J, Weller PF, Hellmann A, Metzgeroth G, Leiferman KM, Arock M,
Butterfield JH, Sperr WR, Sotlar K, Vandenberghe P, Haferlach T, Simon
H-U, Reiter A, Gleich GJ. Contemporary consensus proposal on criteria
and classification of eosinophilic disorders and related syndromes. J
Allergy Clin Immunol 2012;130:607-12. https://doi.org/10.1016/j.jaci.2012.02.019

- Bain
BJ, Fletcher SH. Chronic eosinophilic leukemias and the
myeloproliferative variant of the hypereosinophilic syndrome. Immunol
Allergy Clin North Am. 2007;27:377-88. https://doi.org/10.1016/j.iac.2007.06.001

- Ackerman
SJ, Bochner BS. Mechanisms of eosinophilia in the pathogenesis of
hypereosinophilic disorders. Immunol Allergy Clin North Am.
2007;27:357-75. https://doi.org/10.1016/j.iac.2007.07.004

- Crane
MM, Chang CM, Kobayashi MG, Weller PF. Incidence of myeloproliferative
hypereosinophilic syndrome in the United States and an estimate of all
hypereosinophilic syndrome incidence. J Allergy Clin Immunol.
2010;126:179-81. https://doi.org/10.1016/j.jaci.2010.03.035

- Curtis
C, Ogbogu PU. Evaluation and Differential Diagnosis of Persistent
Marked Eosinophilia. Immunol Allergy Clin North Am. 2015;35:387-402. https://doi.org/10.1016/j.iac.2015.04.001

- Weller PF. Eosinophilia in travelers. Med Clin North Am. 1992;76:1413-32. https://doi.org/10.1016/S0025-7125(16)30294-2

- Nutman
TB. Evaluation and differential diagnosis of marked, persistent
eosinophilia. Immunol Allergy Clin North Am. 2007;27:529-49. https://doi.org/10.1016/j.iac.2007.07.008

- Moore TA, Nutman TB. Eosinophilia in the returning traveler. Infect Dis Clin North Am. 1998;12:503-21. https://doi.org/10.1016/S0891-5520(05)70016-7

- Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010;126:39-44. https://doi.org/10.1016/j.jaci.2010.04.011

- Crompton
DWT. Preventive chemotherapy in human helminthiasis: coordinated use of
anthelminthic drugs in control interventions: a manual for health
professionals and programme managers. Geneva: World Health Organization
2006 http://apps.who.int/iris/bitstream/10665/43545/1/9241547103_eng.pdf

- Checkley
AM, Chiodini PL, Dockrell DH, Bates I, Thwaites GE, Booth HL, Brown M,
Wright SG, Grant AD, Mabey DC, Whitty CJM, Sanderson F, British
Infection Society and Hospital for Tropical Diseases. Eosinophilia in
returning travellers and migrants from the tropics: UK recommendations
for investigation and initial management. J Infect. 2010; 60:1-20. https://doi.org/10.1016/j.jinf.2009.11.003














