Paola Zanotti1*, Claudia Chirico1*, Maurizio Gulletta1, Laura Ardighieri2, Salvatore Casari3, Eugenia Quiros Roldan1, Ilaria Izzo1, Gabriele Pinsi4, Giovanni Lorenzin4,5, Fabio Facchetti2, Francesco Castelli1 and Emanuele Focà1.
1 Department of Infectious and Tropical Diseases, University of Brescia and ASST Spedali Civili General Hospital, Brescia, Italy.
2 Pathology Unit, University of Brescia and ASST Spedali Civili General Hospital, Brescia, Italy.
3 Unit of Infectious Diseases, Carlo Poma Hospital, Mantova.
4 Microbiology and Virology Unit, University of Brescia and ASST Spedali Civili General Hospital, Brescia, Italy.
5 Institute of Microbiology and Virology, Department of Biomedical, Surgical and Dental Sciences, University of Milan, Italy.
*These Authors equally contributed to this work.
Corresponding
author: Emanuele Focà MD, PhD. Department of Infectious and Tropical
Diseases, Department of Clinical Experimental Sciences. The University
of Brescia and Brescia Spedali Civili General Hospital. Piazzale
Spedali Civili, 1, 25123 Brescia (Italy). E-mail:
emanuele.foca@unibs.it
Published: July 1, 2018
Received: March 23, 2018
Accepted: May 14, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018040 DOI
10.4084/MJHID.2018.040
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Progressive
disseminated histoplasmosis (PDH) is an AIDS-defining illness with a
high lethality rate if not promptly treated. The wide range of its
possible clinical manifestations represents the main barrier to
diagnosis in non-endemic countries. Here we present a case of PDH with
haemophagocytic syndrome in a newly diagnosed HIV patient and a
comprehensive review of disseminated histoplasmosis focused on
epidemiology, clinical features, diagnostic tools and treatment options
in HIV-infected patients.
|
Introduction
Histoplasmosis is a mycosis resulting from the inhalation of the spores of the dimorphic fungus Histoplasma spp, which is a member of the family Ajellomycetaceae, a fungal group whose members may all cause systemic disease in otherwise healthy humans (Blastomyces, Coccidioides, Paracoccidioides).[1]
Although only occasionally reported in the pre-HIV-infection era, it
became a public health issue after the AIDS pandemic, being listed by
Center for Diseases Control and Prevention (CDC) among the
AIDS-defining illnesses in 1987.[2]
In the
immunocompetent host, exposure to Histoplasma microconidia is usually
responsible for a symptomless presentation or flu-like syndrome, as the
spores are contained by alveolar macrophages and subsequently cleared
by the activation of the adaptive immunity, especially the Th1
response. In the immunocompromised host, due to Th1 to Th2 shift, the
pathogen can invade the bloodstream and spread to other organs and
tissues, leading to progressive disseminated histoplasmosis (PDH), a
fatal disease in untreated patients.[3,4]
The PDH
incidence in HIV patients peaked in 1992, and then gradually declined
following the introduction of combined antiretroviral therapy (cART).
It is currently a health issue especially in countries where cART is
not widely available. In Europe, it is only sporadically reported,[5] mainly in migrants from endemic areas.[6]
In
the last years, in Italy, we observed an increase in the proportion of
AIDS cases in migrants: they account now for more than 1/3 of the new
HIV-infection diagnosis and, among them, the 68% comes from Africa and
Latin America.[7]
We believe that it is important
to raise the awareness of histoplasmosis also at our latitude, given
the recent increase of the migratory fluxes from endemic countries and
the high risk of fatal outcome associated with late diagnosis and
treatment.
Here we will present a case of a newly diagnosed AIDS
patient, previously suspected to have lymphoma with haemophagocytic
syndrome. Both microbiological and histological examinations revealed
disseminated histoplasmosis. Furthermore, we provide a comprehensive
review of the current literature on histoplasmosis in HIV-infected
patients focusing on epidemiological, clinical, diagnostic features and
treatment options.
Case-Report
In
February 2016, a 19-year-old woman coming from Ivory Coast was admitted
to a peripheral hospital of our city for persistent fever. After she
resulted positive for a screening HIV-Ab test, she was referred to the
Infectious and Tropical Diseases Department. At admission she had deep
asthenia, chills, fever (max 38.8°C), and tachycardia (110 bpm);
examination revealed mono-lateral tonsillar hypertrophy and
submandibular lymphadenopathy. Laboratory investigations showed
pancytopenia (haemoglobin 8.7 g/dL - normal value 12.0-16.0 g/dL, white
blood cells 3,050/µL -n.v. 4.00-10.80x103/µL, platelets 80,000/µL - n.v. 130-400x103/µL),
increased lactate dehydrogenase (5,201 U/L - n.v. 136-234 U/L),
increased levels of C Reactive Protein (63.3 mg/L - n.v. < 5.0
mg/L), and high ferritin and triglycerides serum levels (> 40,000
ng/mL and 440 mg/dL - n.v. 20-120 ng/mL and <150 mg/dL
respectively). Baseline lymphocytes CD4+ T-cell count was 19 cell/µL
and HIV-RNA was 1,787,000 cp/mL. In addition, the patient resulted
positive for EBV-DNA (1,297 cp/mL), CMV-DNA (266 cp/mL), and HBV-DNA
(> 700,000,000 UI/mL), and for blood stool test. Suspecting a
lymphoproliferative disorder evolving in haemophagocytic syndrome, a
bone marrow biopsy was performed and steroid treatment was started.
Consequently, we performed biopsies of the gut, lateral-cervical lymph
node, and tonsil. Microscopical evaluation of hematoxylin and eosin
(H&E) sections of the bone marrow showed a prominent
lymphohistiocytic infiltrate with budding yeast cells inside
macrophages, suggestive of histoplasmosis, which was confirmed by
Grocott Methenamine Silver stain (GMS) and periodic acid–Schiff (PAS)
stain. Antifungal treatment with liposomal Amphotericin B was started
while steroid therapy was gradually reduced. She rapidly became
afebrile and, in the following weeks, we observed a slow regression of
symptoms. Histological examination of submandibular lymph node, tonsil
and colic mucosa also confirmed the diagnosis (Figures 1, 2 and 3). Histoplasma spp serology was negative, while culture of bone marrow aspirate showed H. capsulatum
growth. After three weeks of induction therapy, she continued on
maintenance therapy with itraconazole, and cART with
tenofovir/emtricitabine + dolutegravir was prescribed. The patient was
referred to the Outpatient Clinic. She attended at the first follow-up
visit ten days after the hospital discharge with improved clinical
conditions and blood exams.
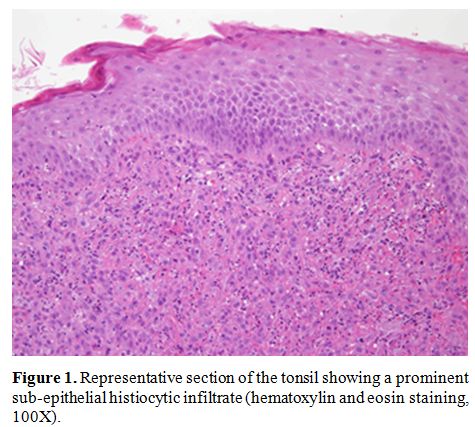 |
Figure 1.
Representative section of the tonsil showing a prominent
sub-epithelial histiocytic infiltrate (hematoxylin and eosin staining,
100X). |
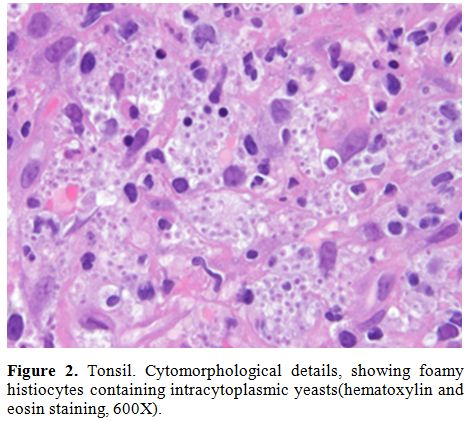 |
Figure 2. Tonsil.
Cytomorphological details, showing foamy histiocytes containing
intracytoplasmic yeasts(hematoxylin and eosin staining, 600X). |
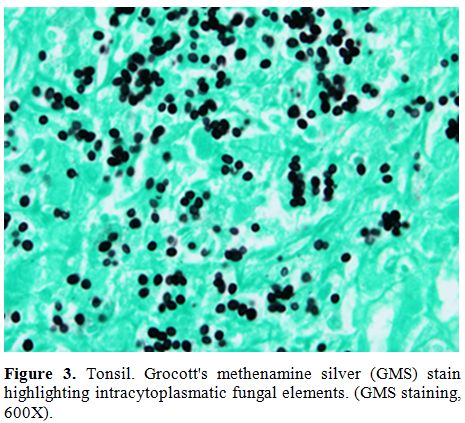 |
Figure 3. Tonsil. Grocott's methenamine
silver (GMS) stain highlighting intracytoplasmatic fungal elements.
(GMS staining, 600X). |
However
in April 2016 she returned to Ivory Coast and was re-linked to care in
October 2016. At the time of the visit lymphocytes CD4+ T count was 29
cell/µL, HIV-RNA was 153,300 cp/mL and HBV DNA was 212,600,000 UI/mL.
During her stay in Ivory Coast, she interrupted both antiretroviral
therapy and histoplasmosis maintenance therapy; moreover, at the moment
of the new visit she was pregnant and after proper counseling, she
decided to carry on the pregnancy. Only antiretroviral therapy with
tenofovir/emtricitabine + atazanavir/ritonavir was re-started and a
close follow-up was scheduled. After three weeks she was admitted to
the Intensive Care Unit (ICU) for internal abortion, complicated by
septic shock. She was treated with wide spectrum antibiotic therapy
(meropenem and vancomycin) without any microbiological isolation and
she went through several transfusions and mechanical ventilation. Once
stabilized, she was transferred to our Department, she was febrile,
pancytopenic and with a skin lesions on her chest, suggestive of
histoplasmosis reactivation so that antifungal treatment with liposomal
Amphotericin B was reintroduced. Bone marrow biopsy confirmed the
suspect. The same day antiretroviral therapy with
tenofovir/emtricitabine + dolutegravir was reintroduced. In the
following weeks, a slow progressive improvement of the conditions was
observed, therefore she was transferred to a facility with proper
social support and health assistance. At the time of the last visit in
December 2017, the patient was asymptomatic and fully adherent with
cART and histoplasmosis prophylaxis. Last CD4+ T-cell count was 150
cell/µL and a low-level viral load was detected for HIV and HBV (33
copies/mL and 95 UI/mL respectively).
Epidemiology
Histoplasma capsulatum
is recognized as a pathogen with worldwide distribution, with many
cases registered outside the historically known endemic areas of the
Americas.[8,9] Many countries of the American
continent are considered highly endemic as revealed by outbreak reports
and skin reactivity studies. In the United States, histoplasmin
sensitivity ranges from 60% to 90% in Ohio and in the Mississippi river
valley, while in Latin America the prevalence of H. capsulatum
infection is reported as 50% in some Panama’s areas, reaching 93% in
specific places of Brazil as in Ilha do Governador, Rio de Janeiro.[10] H. capsulatum is also endemic in Asia, where autochthonous cases have been reported since the seventies.[11] H. capsulatum var. duboisii has been isolated in Central and West areas of Africa and in Madagascar.[12]
Sporadic cases of histoplasmosis have been reported in Europe, mainly
in immigrants or travelers returning from endemic areas. Reports of
autochthonous cases in Europe suggest the possible endemic presence in
some European areas, such as the south of France and the Po river
valley in Italy.[13,14] Since the HIV/AIDS pandemic
spread out, many cases of histoplasmosis have been reported in
HIV-infected patients in locations with few previously reported cases.
In endemic areas, such as Latin America, PDH is one of the most common
causes of AIDS-related deaths, however, it remains underestimated due
to misdiagnosis and lack of first choice antifungal therapy.[15] Fungal infections were estimated to be responsible of 47% of over 1,500,000 AIDS-related death in 2013, and, among them, Histoplasma was the third responsible pathogen, after Pneumocystis jiroveci and Cryptococcus neoformans.[16] Some authors recently reported an increase in the diagnosis of histoplasmosis in AIDS-patients in Latin America.[17] In
some areas, the situation appears dramatic: histoplasmosis is one of
the first causes of death in HIV-infected patients in French Guiana,
with a lethality of more than 50%; in Fortaleza, Brazil, almost a half
(43%) of hospitalized HIV-infected patients had PDH.[6]
In other known endemic areas, such as Africa, there is a lack of data
about the incidence of PDH. A recent study estimated that targeting
opportunistic fungal infections through diagnostic and therapeutic
tools access could be a crucial factor in reducing AIDS-related deaths,
saving more than a million lives over five years.[16]
In
non-endemic areas as in Europe, most PDH cases are reported in
HIV-infected migrants. From 1984 to 2004, 72 patients with
HIV-associated histoplasmosis were reported in Europe, mostly observed
in Italy; among them, 7 cases were autochthonous.[18]
 |
|
Pathogenesis
H. capsulatum is
a dimorphic fungus that displays different morphologies depending on
environmental conditions: the mold in the soil and the budding yeast in
the mammalian host. H. capsulatum
infection is usually acquired through inhalation of microconidia
aerosolized from environmental sites hosting the fungus. The
saprophytic form seems to grow best in soils with a high nitrogen
content associated with the presence of birds and bats guano. Some
activities, such as demolition and construction works, archaeological
and speleological works or poultry farming, have been associated with
an increased risk of infection because they can lead to microconidia
exposure. Infection with the yeast form via tissue transplant,
laboratory accident or needle sharing has also been described. After
inhalation, the fungus reaches the alveolar space where it finds
favorable temperature; there it turns into the pathogenic yeast form
and begins his intracellular life in alveolar macrophages.[19]In
the absence of immune-compromising conditions, acute infection resolves
with the development of cell-mediated immunity. An antigen-specific
CD4+ T lymphocyte-mediated response leads to the formation of
granulomas; this immune activation can contain the fungus and protect
against reinfection, but it is not able to eradicate the pathogen. The
development of specific cell-mediated immune response results in a
delayed-type hypersensitivity reaction that can be induced by
intradermal injection of fungal antigens (histoplasmin skin test).[20]
In healthy individuals, the primary infection is usually asymptomatic
or mild-symptomatic, resulting in a self-limiting and non-specific
febrile syndrome with respiratory involvement. H.capsulatum
establishes a long-lasting quiescent infection that can reactivate in
case of immune system weakening, such as in advanced HIV infection,
chemotherapy, immunosuppressive therapy for transplants or autoimmune
diseases. Host-pathogen balance plays a crucial role in infection
course; when cell-mediated immunity is compromised the fungus moves
from the primary site to the whole body through the blood and lymph
stream or within cells as macrophages but also dendritic cells and
neutrophils, leading to disseminated disease.[21] The
main affected organs are liver, spleen, gastrointestinal tract, and
bone marrow. In addition to the immune status of the host, other
factors potentially involved in the evolution of Histoplasma infection are the number of organisms inhaled and the strains virulence.[22]
 |
|
Clinical Features
Histoplasmosis
has a very heterogeneous clinical presentation, ranging from mild and
self-limiting respiratory syndromes to disseminated forms with high
lethality rate, depending on immune system conditions.[19]
In most cases, the infection is completely asymptomatic or may be
associated with a non-specific syndrome characterized by fever, chills,
cough and chest pain. Rheumatologic manifestations including arthritis,
arthralgia, and erythema nodosum have also been described in a small percentage of cases.[23,24]
In endemic areas, peculiar forms of histoplasmosis have been described,
such as ocular histoplasmosis with chorioretinal involvement and
“histoplasmoma,” a slow enlarging lung nodule.[25,26] In
immunocompromised host, a deficit in cellular immunity can lead to
fungus dissemination, massive organ involvement and severe systemic
disease (PDH). PDH is usually diagnosed in the late stage of HIV
infection; however, some rare cases have been reported in other
conditions, such as hematologic patients[27,28] and even in immune-competent individuals living in developing countries.[29] Mortality rate reaches 39% in endemic areas, such as Latin America[10] but is even higher in non-endemic areas like Europe, where the disease is often misdiagnosed.[8]
PDH usually presents with persistent fever, deep asthenia, and weight
loss; diarrhea and other digestive symptoms are often described since
gastrointestinal tract is a frequent site of fungus dissemination.
Reticuloendothelial system is the main organ affected in PDH with
severity ranging from isolated lymphadenopathy, hepato- and
spleno-megaly to hemophagocytic lymphohistiocytosis (HLH). HLH is a
life-threatening disease in which a massive immune stimulation results
in macrophages activation and hemophagocytosis; it is a rapidly
progressive syndrome with non-specific symptoms so that it can mimic
many different etiologies: malaria (if patient has a recent history of
travel in endemic areas),[30] sepsis, hepatic failure, hematologic disorders and malignancies among others.[31]
Fever, splenomegaly, deep pancytopenia, altered liver function with
hypertriglyceridemia, hypofibrinogenemia and increased ferritin level
are the main clinical diagnostic criteria.[32] According to a recent review, 27 cases of HLH secondary to PDH were reported so far, with a mortality rate of 38%.[33]
Up to 10% of patients with PDH may develop central nervous system (CNS)
involvement as a primary manifestation or relapsing disease.[34]
Neurologic dissemination of the pathogen may occur as subacute or
chronic meningitis, focal neurologic deficit and encephalitis.[35,36] Skin involvement in PDH has been reported in up to 25% of AIDS patients.[37]
Dermatologic findings are not specific: papules, plaques, and nodules
are commonly described, usually affecting trunk and face. Mucosal
involvement is often reported, especially as painful and infiltrated
ulcers in oral mucosa.[38] Other less common manifestations of PDH include endocrine syndromes, such as chronic adrenal insufficiency,[39] and myositis, of which only 4 cases were reported so far.[40]
In AIDS patients from endemic areas starting cART, a few cases of
immune reconstitution inflammatory syndrome (IRIS) revealing PDH have
been described: fever, weight loss, and lymphadenitis were the main
symptoms in these reports.[41] The
recognition of PDH in AIDS may be challenging since most patients have
other concomitant opportunistic infections, especially those with CD4+
counts <150 cells/mL: pneumocystosis, cryptococcosis, and
mycobacteriosis are the main reported co-infections.[42]
Diagnostic Tools
There
are multiple methods for the diagnosis of histoplasmosis including
histopathology or cytology, direct examination using specific fungal
stains, cultures, Matrix-Assisted Laser Desorption/Ionization – Time Of
Flight (MALDI-TOF), antigen detection, immunization determination,
serological tests and molecular biology-based tests. Only hospitals
with a dedicated mycology sector in their laboratory allow the most
rapid and accurate diagnosis. It is worth noting that managing cultures
of Histoplasma spp, as for
other dimorphic fungi, requires a biosafety level 3 or 4 with class II
or III biological safety cabinets, and laboratory personnel involved
with Histoplasma cultures should use appropriate procedures to prevent exposure.Each
of the available tests is designed and performed for specific
histoplasmosis syndromes depending on the patient condition or level of
immunosuppression, but all can be used to complete the diagnostic path.
The diagnostic methods not only differ in sensibility and specificity
but also in time-to-response, total cost, quality of the information,
and have certain limitations that must be recognized if they are to be
used correctly.Histopathology. The diagnosis of histoplasmosis can be obtained through examination of histological or cytological specimens.[43]
Histological specimens from tissue biopsies of different anatomical
sites stained with H&E and special stains such as PAS and GMS can
be used. Cytological specimens of bone marrow aspirates stained with
Giemsa,[44] fluids [45] (ex. bronchoalveolar lavage) or tissues[46] (lung, lymph nodes, spleen, cutaneous lesions, etc.) can also be utilized. There are two different forms of H. capsulatum causing human histoplasmosis; H. capsulatum var. capsulatum and H. capsulatum var. duboisii but the two are difficult to distinguish. In H&E stains H. capsulatum
is characterized by the presence of a clear space or artefactual halo,
due to the retraction of basophilic cell cytoplasm from the cell wall.
Budding yeasts, usually difficult to identify, are connected to a
narrow base, a feature that helps the distinction of H. capsulatum from other fungi.[47,48]In
specimen, it can be highlighted with different staining methods:
Romanowsky-type stains, Giemsa, Wright-Giemsa stains, Grocott-Gömöri
methenamine–silver (GMS), mucicarmine, periodic acid–Schiff (PAS)
stains and Gram stains. GMS, PAS and mucicarmine are the most commonly
used. GMS and PAS provide contrast to yeast cells showing black-colored
and magenta-colored intracellular or extracellular yeasts respectively;
at mucicarmine stain, the yeast forms are barely visible. H. capsulatum must be distinguished, in particular, from other fungi such as a small variant of Blastomyces dermatitidis, capsule-deficient Cryptococci, endospores of Coccidioides spp., Pneumocystis jirovecii, and Candida glabrata.[49,50]H. capsulatum should also be distinguished from protozoa, like Leishmania spp (amastigotes), Toxoplasma gondii (bradyzoites) and Trypanosoma cruzi (amastigotes). The patient histological reaction to H. capsulatum
infection varies according to the severity and phase of the infection
and the host's immune system. In the acute phase, subsequent to
pulmonary infection, H. capsulatum
may be seen within alveolar space and in the interstitium, inside
macrophages. Usually, there is an associated lymphohistiocytic
infiltrate with necrosis and vasculitis. The histopathologic picture
resembles lymphomatoid granulomatosis, but scattered small granulomas
with small yeasts in the parenchyma should suggest the diagnosis of
histoplasmosis.[51]In
chronic pulmonary histoplasmosis, the most common host's reaction is a
necrotizing granulomatous inflammation with a low number of organisms,
resulting in nonviable (culture-negative) yeast. Special stains are
needed to detect Histoplasma in this setting since it cannot usually be visualized on H&E.In
disseminated histoplasmosis, there is extensive tissue infiltration by
organisms, and usually, the host's tissue reaction is poor, and it can
be represented by a subtle inflammatory associated with extensive tissue
necrosis.It
is important to remember that histoplasmosis can induce a variety of
organ-site responses with unfamiliar or unusual histological patterns,
and the diagnosis of histoplasmosis can be missed if the clinicians do
not provide adequate clinical data.[52] In
particular, when evaluating tissue lesions from patients with profound
immunodepression, histological tissues examination should include
special histochemical stains for infectious agents.[53]Microbiology. Microscopy:
The diagnosis of invasive histoplasmosis can be obtained through
various direct examination methods. The major part of collected
specimens can be freshly prepared on a wet mount and examined.[54,55,56]Direct
microscopic examination of the clinical specimen is a simple but useful
method to provide a rapid hint on the possible presence of fungal
infection. The limit of microscopy is the low specificity due to the
similarity between the different fungal species.[57,58] H. capsulatum can be easily misidentified with B. dermatitidis, various Candida species, Cryptococcus gattii, Cryptococcus neoformans, Talaromyces marneffei, and endospores of Coccidioides species.[59]Culture methods: H. capsulatum
is a dimorphic fungus: at temperatures above 30°C, there is the yeast
phase while in the cultures incubated at lower temperatures (25°C)
there is the development of mold phase. The yeast phase allows a fast
growth of the isolate, with an average growth time of 5-7 days; the
mold phase takes from 4 weeks to up to 12 weeks to grow.The
gold standard for the identification of the pathogen is the culture
demonstrating the thermal dimorphism of the fungus from yeast to mold
and vice versa.Isolation
from samples from the lower respiratory tract with bronchial and
bronchoalveolar lavage (BAL) in cases of chronic pulmonary
histoplasmosis has a sensitivity of 60-75%. In the case of disseminated
histoplasmosis, isolation through blood culture is the most sensitive
method while the sensitivity of bone marrow culture is 75%.[59,60]The
use of MALDI-TOF technology is a method of direct identification of the
colony of both the yeast phase and the mold phase, capable of providing
rapid detection of H. capsulatum with excellent sensitivity, greatly reducing diagnosis times, but until now only a scarce data have been published so far.[61]Non–culture methods:
Many non-culture methods were developed in order to make a correct and
rapid diagnosis of histoplasmosis. Other tests such as the research of
beta-3-D-glucan or the Platelia test (Bio-Rad Laboratories, Redmond,
WA) for Aspergillus can cross-react in case of histoplasmosis and are
not to be considered specific.[62]Antibody
detection methods: Most of these assays are based on the ability to
search for antibodies to histoplasmin (HMIN). HMIN has three antigens.
It is an extremely specific test (100%), but sensitivity is reduced
(70-100%) depending on the type of infection and the patient’s level of
immune depression. The
complement fixation test is a more sensitive method (94.3%) but less
specific (70%) than immunodiffusion; however, use of this test is of
little help in immunocompromised or AIDS patients. A Semi-Quantitative
Indirect Enzyme Immunoassay (EIA) testing for IgM and IgG against the
Histoplasma polysaccharide antigen is available by Mira-Vista
Diagnostics (Indianapolis, Indiana). It is validated on both serum and
CSF; false negative results may result in some progressive or chronic
cases, especially in immunocompromised patients. A western-blot test
has recently been validated in Brazil, providing sensitive, specific,
and faster results.[63]Antigen detection: Enzyme immunoassay is a quantitative test which detects the presence of Histoplasma
polysaccharide with a reported sensibility of up to 95%. The antigen is
present in larger quantities in the urine than in the serum and the
sensitivity of the test increases in immunocompromised patients and in
disseminated histoplasmosis. An indication of response to therapy is
the decrease over time of the urinary antigen.[64,65]An antigen-capture enzyme-linked immunosorbent assay (ELISA) to detect H. capsulatum antigenuria in immunocompromised has been validated and distributed by the CDC. Some other antigen-based tests for Aspergillus spp. have been proposed and showed promising accuracy on bronchoalveolar lavage fluid .[66,67,68]Molecular tools:
The absence of commercially available FDA-approved molecular tests has
led to the development of multiple homemade solutions from conventional
PCR to semi-nested, nested, real-time, LAMP, and RCA.[69,70,71,72]
Therapeutic and Preventive Approach
Guidelines
from the CDC, the National Institutes of Health (NIH), and the HIV
Medicine Association of the Infectious Diseases Society of America
(IDSA) recommend treatment with intravenous (IV) liposomal amphotericin
B (3 mg/kg daily) as induction treatment for at least 2 weeks, or up to
a clinical improvement and a possibility of oral treatment.[73]Amphotericin
B lipid complex and Amphotericin B deoxycholate may represent a less
expensive alternative in patients with a low risk of nephropathy;[74]
given the risk of nephrotoxicity and the high number of interaction
with other compounds, the patient should be strictly monitored during
induction therapy particularly for the renal function and electrolytes
balance. After
induction therapy, the treatment should be prolonged with oral
itraconazole (200 mg 3 times daily for three days and then 200 mg twice
daily for at least 12 months).Additionally,
long-term suppressive therapy with itraconazole (200 mg daily) may be
considered in permanently immunosuppressed patients and in patients
with recurrent symptoms of PDH. Even though the timing of
secondary prophylaxis is still unclear, few data suggest it should be
prolonged for one year and until the CD4+ T-cell count reaches 150
cells/mm3 and the patient is on effective cART for at least six months.Itraconazole
200 mg daily is also recommended as primary prophylaxis for
HIV-infected patients with CD4+ T-cell counts <150 cells/mm3 living in highly endemic areas or with potential occupational exposure to the fungus.[75]Among
other azoles, fluconazole at a dosage of 800 mg daily may be used as an
alternative regimen in patients who can't be treated with itraconazole,
but showed a lower effectiveness and a higher risk of developing
resistance. In addition, the administration of posaconazole and
voriconazole seems to be effective but current literature offers only a
few experiences in the usage of these antimycotic agents. Echinocandins
are not active against Histoplasma spp and should not be used. In
case of CNS involvement liposomal amphotericin B at a dosage of 5 mg/kg
daily for 4 to 6 weeks should be used as initial therapy, followed by
itraconazole at a dose of 200 mg 2 or 3 times daily for at least one
year and until cerebrospinal fluid normalization.The
cART should also be started as soon as possible, but in severe forms,
it can be delayed since the resolution of the acute phase to prevent
the potential development of IRIS. There
is a lack of data regarding the better antiretroviral regimen to
administer in these patients; however, a high genetic barrier drugs, as
well as unboosted regimens, should be preferred according to possible
drug-drug interactions between antiretroviral and antifungal treatment.
Protease inhibitor-based regimens may be chosen for their potency and
high genetic barrier, but they are not free from interactions because
of their boosting need (with ritonavir or cobicistat). The
administration of the new class of integrase inhibitors guarantees a
low risk of drug-drug interactions and a rapid viral load decline.
Nonetheless, among them, raltegravir does not have a high genetic
barrier, elvitegravir is boosted by cobicistat, and only a few data are
available on dolutegravir, and all these drugs are less available in
resource-limited countries.[76]Lastly, possible drug-drug interactions and adherence to cART should be strictly monitored by physicians.[77]
Conclusions
PDH
carries a poor prognosis, especially when the diagnosis is delayed;
giving its rarity in non-endemic areas and the variety of its clinical
presentation, along with the restricted range of sensitive tests in
non-endemic countries it poses a real challenge to clinicians.
We
are nowadays experiencing an increase in migratory flows from tropical
areas, especially those where cART is not widely available. This
phenomenon could raise the probability to encounter AIDS-related
diseases previously only anecdotal at our latitude in the daily
clinical practice.
In our case, the prompt diagnosis was possible
only thanks to direct microscopy identification since serology was
negative, due to the deep immunosuppression of the patient.
Prognosis may be favorable when antifungal therapy and cART are
promptly administered; the co-administration of steroid treatment may
be necessary when associated with HLH or immune-reconstitution
syndrome. In our case, the adequate combined treatment (antifungal,
antiretroviral and steroidal) allowed a good clinical outcome, also if
the relapse after an early interruption of the secondary prophylaxis
highlights the need of a prolonged course of antifungal maintenance
therapy.
Early diagnosis remains crucial to guarantee the survival
of the patients, and we believe that the improvement of surveillance on
this disease represents the best tool to reach this endpoint also in
non-endemic countries.
 |
|
References
- Köhler JR, Hube B, Puccia R, Casadevall A, Perfect JR. Fungi that Infect Humans. Microbiol Spectr. 2017 Jun;5(3). https://doi.org/10.1128/microbiolspec.FUNK-0014-2016 PMid:28597822

- Subedee
A, Van Sickels N. Hemophagocytic Syndrome in the Setting of AIDS and
Disseminated Histoplasmosis: Case Report and a Review of Literature. J
Int Assoc Provid AIDS Care. 2015 Sep-Oct;14(5):391-7. https://doi.org/10.1177/2325957415570740 PMid:25670709

- Horwath MC, Fecher RA, Deepe GS Jr. Histoplasma capsulatum, lung infection and immunity. Future Microbiol. 2015;10(6):967-75. https://doi.org/10.2217/fmb.15.25 PMid:26059620 PMCid:PMC4478585

- Jones
JL, Hanson DL, Dworkin MS, Alderton DL, Fleming PL, Kaplan JE, Ward J
Surveillance for AIDS-defining opportunistic illnesses, 1992-1997. MMWR
CDC Surveill Summ. 1999 Apr 16;48(2):1-22. https://doi.org/10.1001/archderm.135.8.897

- Martin-Iguacel
R, Kurtzhals J, Jouvion G, Nielsen SD, Llibre JM. Progressive
disseminated histoplasmosis in the HIV population in Europe in the
HAART era. Case report and literature review. Infection. 2014
Aug;42(4):611-20. https://doi.org/10.1007/s15010-014-0611-7 PMid:24627267

- Daher
EF, Silva GB Jr, Barros FA, Takeda CF, Mota RM, Ferreira MT, Oliveira
SA, Martins JC, Araújo SM, Gutiérrez-Adrianzén OA. Clinical and
laboratory features of disseminated histoplasmosis in HIV patients from
Brazil. Trop Med Int Health. 2007 Sep;12(9):1108-15. https://doi.org/10.1111/j.1365-3156.2007.01894.x PMid:17875020

- Istituto
Superiore di Sanità. Aggiornamento delle nuove diagnosi di infezione da
HIV e dei casi di AIDS in Italia al 31 Dicembre 2016.
- Bahr
NC, Antinori S, Wheat LJ, Sarosi GA. Histoplasmosis infections
worldwide: thinking outside of the Ohio River valley. Curr Trop Med
Rep. 2015 Jun 1;2(2):70-80. https://doi.org/10.1007/s40475-015-0044-0 PMCid:PMC4535725

- Benedict
K, Mody RK. Epidemiology of Histoplasmosis Outbreaks, United States,
1938-2013. Emerg Infect Dis. 2016 Mar;22(3):370-8. https://doi.org/10.3201/eid2203.151117 PMid:26890817 PMCid:PMC4766901

- Colombo
AL, Tobón A, Restrepo A, Queiroz-Telles F, Nucci M. Epidemiology of
endemic systemic fungal infections in Latin America. Med Mycol. 2011
Nov;49(8):785-98. https://doi.org/10.3109/13693786.2011.577821

- Wang
TL1, Cheah JS, Holmberg K. Case report and review of disseminated
histoplasmosis in South-East Asia: clinical and epidemiological
implications. Trop Med Int Health. 1996 Feb;1(1):35-42. https://doi.org/10.1046/j.1365-3156.1996.d01-10.x PMid:8673821

- Gugnani HC. Histoplasmosis in Africa: a review. Indian J Chest Dis Allied Sci. 2000 Oct-Dec;42(4):271-7. PMid:15597674

- Confalonieri
M1, Nanetti A, Gandola L, Colavecchio A, Aiolfi S, Cannatelli G, Parigi
P, Scartabellati A, Della Porta R, Mazzoni A. Histoplasmosis capsulati
in Italy: autochthonous or imported? Eur J Epidemiol. 1994
Aug;10(4):435-9. PMid:7843347

- Ashbee
HR, Evans EGV, Viviani MA, Dupont B, Chryssanthou E, Surmont I, et al.
Histoplasmosis in Europe: report on an epidemiological survey from the
European Confederation of Medical Mycology Working Group. Med Mycol.
2008;46:57–65. https://doi.org/10.1080/13693780701591481 PMid:17885939

- Centers
for Disease Control. Revision of the CDC Surveillance Case Definition
of Acquired Immunodeficiency Syndrome. MMWR 1987; 36 (Suppl. No 36).
- Denning
DW. Minimizing fungal disease deaths will allow the UNAIDS target of
reducing annual AIDS deaths below 500 000 by 2020 to be realized.
Philos Trans R Soc Lond B Biol Sci. 2016 Dec 5;371(1709)
 .
.
- López
Daneri GA, Arechavala A, Iovannitti CA, Mujica MT. Histoplasmosis
diseminada en pacientes HIV/SIDA: Buenos Aires, 2009-2014. Medicina.
76. 2016; 332-337.

- Antinori
S1, Magni C, Nebuloni M, Parravicini C, Corbellino M, Sollima S,
Galimberti L, Ridolfo AL, Wheat LJ. Histoplasmosis among human
immunodeficiency virus-infected people in Europe: report of 4 cases and
review of the literature. Medicine (Baltimore). 2006 Jan;85(1):22-36 https://doi.org/10.1097/01.md.0000199934.38120.d4 PMid:16523050

- Woods
JP. Revisiting old friends: Developments in understanding Histoplasma
capsulatum pathogenesis. J Microbiol. 2016 Mar;54(3):265-76. https://doi.org/10.1007/s12275-016-6044-5 PMid:26920886

- Woods
JP1, Heinecke EL, Luecke JW, Maldonado E, Ng JZ, Retallack DM,
Timmerman MM. Pathogenesis of Histoplasma capsulatum. Semin Respir
Infect. 2001 Jun;16(2):91-101. PMid:11521241

- Taylor
ML, Díaz S, González PA, Sosa AC, Toriello C. Relationship between
pathogenesis and immune regulation mechanisms in histoplasmosis: a
hypothetical approach. Rev Infect Dis. 1984 Nov-Dec;6(6):775-82. https://doi.org/10.1093/clinids/6.6.775 PMid:6240756

- Sepúlveda
VE, Williams CL1, Goldman WE2. Comparison of phylogenetically distinct
Histoplasma strains reveals evolutionarily divergent virulence
strategies. MBio. 2014 Jul 1;5(4):e01376-14. https://doi.org/10.1128/mBio.01376-14 PMCid:PMC4161242

- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007 Jan;20(1):115-32. https://doi.org/10.1128/CMR.00027-06 PMid:17223625 PMCid:PMC1797635

- Sizemore TC. Rheumatologic manifestations of histoplasmosis: a review. Rheumatol Int. 2013 Dec;33(12):2963-5. https://doi.org/10.1007/s00296-013-2816-y PMid:23835880

- Unis G, Pêgas KL, Severo LC. [Pulmonary histoplasmoma in Rio Grande do Sul]. Rev Soc Bras Med Trop. 2005 Jan-Feb;38(1):11-4. https://doi.org/10.1590/S0037-86822005000100003 PMid:15717088

- Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Surv Ophthalmol. 2015 Jul-Aug;60(4):279-95. https://doi.org/10.1016/j.survophthal.2015.02.005 PMid:25841248

- Oliveira
CC. Peripheral T-Cell Lymphoma in Mediastinum Lymph Nodes and Lung
Associated to Histoplasmosis in a Patient with Chronic Lymphoid
Leukemia/Small Lymphocytic Lymphoma. Mediterr J Hematol Infect Dis.
2017 Jun 20;9(1):e2017044. https://doi.org/10.4084/mjhid.2017.044 PMid:28698787 PMCid:PMC5499492

- Garbee
DD, Pierce SS, Manning J. Opportunistic Fungal Infections in Critical
Care Units. Crit Care Nurs Clin North Am. 2017 Mar;29(1):67-79. https://doi.org/10.1016/j.cnc.2016.09.011 PMid:28160958

- De
D, Nath UK. Disseminated Histoplasmosis in Immunocompetent Individuals-
not a so Rare Entity, in India. Mediterr J Hematol Infect Dis. 2015 Apr
20;7(1):e2015028. https://doi.org/10.4084/mjhid.2015.028 PMid:25960856 PMCid:PMC4418405

- Focà
E, Odolini S, Brianese N, Carosi G. Malaria and hiv in adults: when the
parasite runs into the virus. Mediterr J Hematol Infect Dis.
2012;4(1):e2012032. https://doi.org/10.4084/mjhid.2012.032 PMid:22708047 PMCid:PMC3375742

- Adenis
A, Nacher M, Hanf M, Vantilcke V, Boukhari R, Blachet D, Demar M, Aznar
C, Carme B, Couppie P. HIV-associated histoplasmosis early mortality
and incidence trends: from neglect to priority. PLoS Negl Trop Dis.
2014 Aug 21;8(8):e3100. https://doi.org/10.1371/journal.pntd.0003100 PMid:25144374 PMCid:PMC4140672

- Untanu
RV, Akbar S, Graziano S, Vajpayee N. Histoplasmosis-Induced
Hemophagocytic Lymphohistiocytosis in an Adult Patient: A Case Report
and Review of the Literature. Case Rep Infect Dis. 2016;2016:1358742. https://doi.org/10.1155/2016/1358742

- Henter
JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S,
McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and
therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr
Blood Cancer. 2007 Feb;48(2):124-31. https://doi.org/10.1002/pbc.21039 PMid:16937360

- Townsend
JL, Shanbhag S, Hancock J, Bowman K, Nijhawan AE.
Histoplasmosis-Induced Hemophagocytic Syndrome: A Case Series and
Review of the Literature. Open Forum Infect Dis. 2015 Apr
15;2(2):ofv055. https://doi.org/10.1093/ofid/ofv055

- Nyalakonda
H, Albuerne M, Suazo Hernandez LP, Sarria JC. Central Nervous System
Histoplasmosis in Acquired Immunodeficiency Syndrome. Am J Med Sci.
2016 Feb;351(2):177-86. https://doi.org/10.1016/j.amjms.2015.11.016 PMid:26897273

- Wheat
LJ, Musial CE, Jenny-Avital E. Diagnosis and management of central
nervous system histoplasmosis. Clin Infect Dis. 2005 Mar
15;40(6):844-52. https://doi.org/10.1086/427880 PMid:15736018

- Soza
GM, Patel M, Readinger A, Ryan C. Disseminated cutaneous histoplasmosis
in newly diagnosed HIV. Proc (Bayl Univ Med Cent). 2016
Jan;29(1):50-1.36 Klein IP, Martins MA, Martins MD, Carrard VC.
Diagnosis of HIV infection on the basis of histoplasmosis-related oral
ulceration. Spec Care Dentist. 2016 Mar-Apr;36(2):99-103.

- Koene
RJ, Catanese J, Sarosi GA. Adrenal hypofunction from histoplasmosis: a
literature review from 1971 to 2012. Infection. 2013 Aug;41(4):757-9. https://doi.org/10.1007/s15010-013-0486-z PMid:23771479

- Nimitvilai
S, Thammaprasert W, Vinyuvat S. Histoplasmosis myositis: a case report
and literature review. Southeast Asian J Trop Med Public Health. 2015
Jul;46(4):738-42. PMid:26867394

- Mambie
A, Pasquet A, Melliez H, Bonne S, Blanc AL, Patoz P, Ajana F. A case of
immune reconstitution inflammatory syndrome related to a disseminated
histoplasmosis in an HIV-1 infected patient. AIDS. 2013 Aug
24;27(13):2170-2. https://doi.org/10.1097/01.aids.0000432448.53110.e3 PMid:24384595

- Silva
TC, Treméa CM, Zara AL, Mendonça AF, Godoy CS, Costa CR, Souza LK,
Silva MR. Prevalence and lethality among patients with histoplasmosis
and AIDS in the Midwest Region of Brazil. Mycoses. 2017
Jan;60(1):59-65. https://doi.org/10.1111/myc.12551 PMid:27625302

- Gupta
N, Arora SK, Rajwanshi A, Nijhawan R, Srinivasan R. Histoplasmosis:
cytodiagnosis and review of literature with special emphasis on
differential diagnosis on cytomorphology. Cytopathology. 2010
Aug;21(4):240-4. https://doi.org/10.1111/j.1365-2303.2009.00693.x PMid:19843140

- Qin
L, Zhao L, Tan C, Chen XU, Yang Z, Mo W. A novel method of combining
Periodic Acid Schiff staining with Wright-Giemsa staining to identify
the pathogens Penicillium marneffei, Histoplasma capsulatum, Mucor and
Leishmania donovani in bone marrow smears. Exp Ther Med. 2015
May;9(5):1950-1954. https://doi.org/10.3892/etm.2015.2357 PMid:26136921 PMCid:PMC4471769

- Mukunyadzi
P, Johnson M, Wyble JG, Scott M. Diagnosis of histoplasmosis in urine
cytology: reactive urothelial changes, a diagnostic pitfall. Case
report and literature review of urinary tract infections. Diagn
Cytopathol. 2002 Apr;26(4):243-6. https://doi.org/10.1002/dc.10049 PMid:11933270

- Goldani
LZ, Klock C, Diehl A, Monteiro AC, Maia AL. Histoplasmosis of the
thyroid. J Clin Microbiol. 2000 Oct;38(10):3890-1. PMid:11015430
PMCid:PMC87503

- Koley
S, Mandal RK, Khan K, Choudhary S, Banerjee S. Disseminated Cutaneous
Histoplasmosis, an Initial Manifestation of HIV, Diagnosed with Fine
Needle Aspiration Cytology. Indian J Dermatol. 2014 Mar;59(2):182-5. https://doi.org/10.4103/0019-5154.127681 PMid:24700939 PMCid:PMC3969680

- Rajeshwari
M, Xess I, Sharma MC, Jain D. Acid-Fastness of Histoplasma in Surgical
Pathology Practice. J Pathol Transl Med. 2017 Sep;51(5):482-487. https://doi.org/10.4132/jptm.2017.07.11 PMid:28934824 PMCid:PMC5611531

- Antinori S. Histoplasma capsulatum: more widespread than previously thought. Am J Trop Med Hyg. 2014 Jun;90(6):982-3. https://doi.org/10.4269/ajtmh.14-0175 PMid:24778192 PMCid:PMC4047757

- Wheat LJ, Azar MM, Bahr NC, Spec A, Relich RF, Hage C. Histoplasmosis. Infect Dis Clin North Am. 2016 Mar;30(1):207-27. https://doi.org/10.1016/j.idc.2015.10.009 PMid:26897068

- Guarner
J, Brandt ME. Histopathologic diagnosis of fungal infections in the
21st century. Clin Microbiol Rev. 2011 Apr;24(2):247-80. https://doi.org/10.1128/CMR.00053-10 PMid:21482725 PMCid:PMC3122495

- Mukhopadhyay
S, Katzenstein AL. Biopsy findings in acute pulmonary histoplasmosis:
unusual histologic features in 4 cases mimicking lymphomatoid
granulomatosis. Am J Surg Pathol. 2010 Apr;34(4):541-6. https://doi.org/10.1097/PAS.0b013e3181d4388b PMid:20351490

- Ollague
Sierra JE, Ollague Torres JM. New clinical and histological patterns of
acute disseminated histoplasmosis in human immunodeficiency
virus-positive patients with acquired immunodeficiency syndrome. Am J
Dermatopathol. 2013 Apr;35(2):205-12. https://doi.org/10.1097/DAD.0b013e31822fd00a PMid:22157244

- McLeod
DS, Mortimer RH, Perry-Keene DA, Allworth A, Woods ML, Perry-Keene J,
McBride WJ, Coulter C, Robson JM. Histoplasmosis in Australia: report
of 16 cases and literature review. Medicine (Baltimore). 2011
Jan;90(1):61-8. https://doi.org/10.1097/MD.0b013e318206e499 PMid:21200187

- Baddley
JW, Sankara IR, Rodriquez JM, Pappas PG, Many WJ Jr. Histoplasmosis in
HIV-infected patients in a southern regional medical center: poor
prognosis in the era of highly active antiretroviral therapy. Diagn
Microbiol Infect Dis. 2008 Oct;62(2):151-6. https://doi.org/10.1016/j.diagmicrobio.2008.05.006 PMid:18597967

- Couppié
P, Aznar C, Carme B, Nacher M. American histoplasmosis in developing
countries with a special focus on patients with HIV: diagnosis,
treatment, and prognosis. Curr Opin Infect Dis. 2006 Oct;19(5):443-9. https://doi.org/10.1097/01.qco.0000244049.15888.b9 PMid:16940867

- Akpek
G, Lee SM, Gagnon DR, Cooley TP, Wright DG. Bone marrow
aspiration,biopsy, and culture in the evaluation of HIV-infected
patients for invasive mycobacteria and histoplasma infections. Am J
Hematol. 2001 Jun;67(2):100-6. https://doi.org/10.1002/ajh.1086 PMid:11343381

- Nightingale
SD, Parks JM, Pounders SM, Burns DK, Reynolds J, Hernandez JA.
Disseminated histoplasmosis in patients with AIDS. South Med J.
1990Jun;83(6):624-30. https://doi.org/10.1097/00007611-199006000-00007 PMid:2356493

- Boyce
KJ, Andrianopoulos A.Fungal dimorphism: the switch from hyphae to yeast
is a specialized morphogenetic adaptation allowing colonization of a
host. FEMS Microbiol Rev. 2015 Nov; 39(6):797-811. https://doi.org/10.1093/femsre/fuv035 PMid:26253139

- Arango-Bustamante
K, Restrepo A, Cano LE, de Bedout C, Tobón AM, González A. Diagnostic
value of culture and serological tests in the diagnosis of
histoplasmosis in HIV and non-HIV Colombian patients. Am J Trop Med
Hyg. 2013 Nov;89(5):937-42. https://doi.org/10.4269/ajtmh.13-0117 PMid:24043688 PMCid:PMC3820340

- Prechter GC, Prakash UB. Bronchoscopy in the diagnosis of pulmonary histoplasmosis. Chest 1989; 95:1033. https://doi.org/10.1378/chest.95.5.1033

- Chalupová
J, Raus M, Sedlářová M, Sebela M. Identification of fungal
microorganisms by MALDI-TOF mass spectrometry. Biotechnol Adv. 2014
Jan-Feb;32(1):230-41. https://doi.org/10.1016/j.biotechadv.2013.11.002 PMid:24211254

- Powers-Fletcher MV, Hanson KE. Non-culture diagnostics in fungal disease. Infect Dis Clin North Am. 2016; 30(1): 37-49. https://doi.org/10.1016/j.idc.2015.10.005 PMid:26897062

- Swartzentruber
S, Rhodes L, Kurkjian K, Zahn M, Brandt ME, Connolly P, WheatLJ.
Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin
InfectDis. 2009 Dec 15;49(12):1878-82. https://doi.org/10.1086/648421 PMid:19911965

- Scheel
CM, Samayoa B, Herrera A, Lindsley MD, Benjamin L, Reed Y, Hart J, Lima
S, Rivera BE, Raxcaco G, Chiller T, Arathoon E, Gómez BL. Development
and evaluation of an enzyme-linked immunosorbent assay to detect
Histoplasma capsulatum antigenuria in immunocompromised patients. Clin
Vaccine Immunol. 2009 Jun;16(6):852-8. https://doi.org/10.1128/CVI.00066-09 PMid:19357311 PMCid:PMC2691040

- Cloud
JL, Bauman SK, Neary BP, Ludwig KG, Ashwood ER. Performance
characteristics of a polyclonal enzyme immunoassay for the quantitation
of Histoplasma antigen in human urine samples. Am J Clin Pathol.
2007Jul;128(1):18-22. https://doi.org/10.1309/Q7879TYDW5D93QK7 PMid:17580268

- Theel
ES, Harring JA, Dababneh AS, Rollins LO, Bestrom JE, Jespersen DJ.
Reevaluation of commercial reagents for detection of Histoplasma
capsulatum antigen in urine. J Clin Microbiol. 2015 Apr;53(4):1198-203.
https://doi.org/10.1128/JCM.03175-14 PMid:25631806 PMCid:PMC4365218

- Theel
ES, Jespersen DJ, Harring J, et al. Evaluation of an enzyme immunoassay
for detection of Histoplasma capsulatum antigen from urine specimens. J
Clin Microbiol 2013;51:3555. https://doi.org/10.1128/JCM.01868-13 PMid:23966508 PMCid:PMC3889733

- Prattes
J, Heldt S, Eigl S, Hoenigl M. Point of care testing for the diagnosis
of fungal infections: are we there yet? Curr Fungal Infect Rep. 2016;
10: 43-50. https://doi.org/10.1007/s12281-016-0254-5 PMid:27358661 PMCid:PMC4896970

- Raja
HA, Miller AN, Pearce CJ, Oberlies NH Fungal Identification Using
Molecular Tools: A Primer for the Natural Products Research Community.
1 J Nat Prod. 2017 Mar 24;80(3):756-770. https://doi.org/10.1021/acs.jnatprod.6b01085 PMid:28199101 PMCid:PMC5368684

- Babady
NE, Buckwalter SP, Hall L, Le Febre KM, Binnicker MJ, Wengenack
NL.Detection of Blastomyces dermatitidis and Histoplasma capsulatum
from culture isolates and clinical specimens by use of real-time PCR. J
Clin Microbiol. 2011Sep;49(9):3204-8. https://doi.org/10.1128/JCM.00673-11 PMid:21752970 PMCid:PMC3165572

- Bialek
R, Cirera AC, Herrmann T, Aepinus C, Shearn-Bochsler VI, Legendre AM.
Nested PCR assays for detection of Blastomyces dermatitidis DNA
inparaffin-embedded canine tissue. J Clin Microbiol. 2003
Jan;41(1):205-8. https://doi.org/10.1128/JCM.41.1.205-208.2003 PMid:12517849 PMCid:PMC149559

- Molecular Identification of Fungi anuary 2010. Publisher: Springer ISBN: 978-3-642-05041-1
- Panel
on Opportunistic Infections in HIV-Infected Adults and Adolescents.
Guidelines for the prevention and treatment of opportunistic infections
in HIV-infected adults and adolescents: recommendations from the
Centers for Disease Control and Prevention, the National Institutes of
Health, and the HIV Medicine Association of the Infectious Diseases
Society of America. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed (25/07/2017)
- Johnson
PC, Wheat LJ, Cloud GA, Goldman M, Lancaster D, Bamberger DM, Powderly
WG, Hafner R, Kauffman CA, Dismukes WE; U.S. National Institute of
Allergy and Infectious Diseases Mycoses Study Group. Safety and
efficacy of liposomal amphotericin B compared with conventional
amphotericin B for induction therapy of histoplasmosis in patients with
AIDS. Ann Intern Med. 2002 Jul 16;137(2):105-9. https://doi.org/10.7326/0003-4819-137-2-200207160-00008 PMid:12118965

- Goldman
M, Zackin R, Fichtenbaum CJ, Skiest DJ, Koletar SL, Hafner R, Wheat LJ,
Nyangweso PM, Yiannoutsos CT, Schnizlein-Bick CT, Owens S, Aberg JA;
AIDS Clinical Trials Group A5038 Study Group. Safety of discontinuation
of maintenance therapy for disseminated histoplasmosis after
immunologic response to antiretroviral therapy. Clin Infect Dis. 2004
May 15;38(10):1485-9. https://doi.org/10.1086/420749 PMid:15156489

- Consolidated
Guidelines on the Use of Antiretroviral Drugs for Treating and
Preventing HIV Infection: Recommendations for a Public Health Approach.
Geneva: World Health Organization; 2013 Jun. http://www.who.int/hiv/pub/guidelines/arv2013/download/en/
- Focà
E, Odolini S, Sulis G, Calza S, Pietra V, Rodari P, Giorgetti PF, Noris
A, Ouedraogo P, Simpore J, Pignatelli S, Castelli F. Clinical and
immunological outcomes according to adherence to first-line HAART in a
urban and rural cohortof HIV-infected patients in Burkina Faso, West
Africa. BMC Infect Dis. 2014 Mar21;14:153. https://doi.org/10.1186/1471-2334-14-153 PMid:24656065 PMCid:PMC3994430

[TOP]





















 .
. 
























































