Arzouma Paul Yooda1,2,3, Serge Theophile Soubeiga1,2, K. Yacouba Nebie3, Birama Diarra1, Salam Sawadogo3, Abdoul Karim Ouattara1,2, Dorcas Obiri-Yeboah4, Albert Theophane Yonli1,2, Issoufou Tao1,2, Pegdwende Abel Sorgho1,2, Honorine Dahourou3 and Jacques Simpore1,2.
1 Laboratory
of Molecular Biology and Molecular Genetics (LABIOGENE) UFR/SVT,
University Ouaga I Prof. Joseph KI-ZERBO, 03 BP 7021 Ouagadougou
03, Burkina Faso.
2 Biomolecular Research Center Pietro Annigoni (CERBA), 01 BP 364 Ouagadougou 01, Burkina Faso.
3 National Blood Transfusion Center (CNTS), 01 BP 5372 Ouagadougou 01, Burkina Faso.
4 Department of Microbiology and Immunology, School of Medical Sciences, University of Cape Coast, Ghana
Corresponding
author: Professor Jacques Simpore, Laboratory of Molecular Biology and
Molecular Genetics (LABIOGENE), University Ouaga I Prof. Joseph
KI-ZERBO, Burkina Faso. Tel: +226-70230792, E-mail:
jacques.simpore@labiogene.org
Published: July 1, 2018
Received: April 13, 2018
Accepted: June 12, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018041 DOI
10.4084/MJHID.2018.041
This article is available on PDF format at:

This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background and Objective:
The improved performance of serological tests has significantly reduced
the risk of human immunodeficiency and hepatitis B and C viruses
transmission by blood transfusion, but there is a persistence of
residual risk. The objective of this study was to evaluate the impact
of multiplex PCR in reducing the risk of residual transmission of these
viruses in seronegative blood donors in Burkina Faso.
Methods:
This cross-sectional study was conducted from March to September 2017.
The serological tests were performed on sera using ARCHITECTSR i1000
(Abbot diagnosis, USA). Detection of viral nucleic acids was performed
by multiplex PCR on mini-pools of seronegative plasma for HBV, HCV and
HIV using SaCycler-96 Real Time PCR v.7.3 (Sacace Biotechnologies).
Multiplex PCR-positive samples from these mini-pools were then
individually tested by the same method.
Results:
A total of 989 donors aged 17 to 65 were included in the present study.
"Repeat donors" accounted for 44.79% (443/989). Seroprevalences for
HIV, HBV, and HCV were 2.53% (25/989), 7.28% (72/989) and 2.73%
(27/989), respectively. Of the 14 co-infections detected, HBV/HCV was
the most common with 0.71% (7/989) of cases. Of 808 donations tested by
multiplex PCR, 4.70% (38/808) were positive for HBV while no donation
was positive for HIV or HCV. Conclusion:
Our study showed a high residual risk of HBV transmission through blood
transfusion. Due to the high prevalence of blood-borne infections in
Burkina Faso, we recommend the addition of multiplex PCR to serologic
tests for optimal blood donation screening.
|
Introduction
Optimal
transfusion-transmitted infections safety remains a permanent concern
in the world and especially in sub-Saharan Africa. In transfusion
practice, priority is given to the screening of three major pathogenic
viruses that are Human Immunodeficiency Virus (HIV) and Hepatitis B
(HBV) and C (HCV) Viruses. The highest prevalence of these viruses is
found in sub- Saharan Africa,[1,2] where 12.5% of transfused patients are at risk for post-transfusion hepatitis and 5-10% at risk of HIV infection.[1,3]
In Burkina Faso, previous studies have reported seroprevalences between
0.5%-3%, 8%-15% and 1%-9% respectively for HIV, HBV and HCV in the
general population and in blood donors.[4-9]
Today,
the risk of transmission of these viruses through transfusion has
markedly decreased due to the application of preventive measures and
improved performance of serological tests. Nevertheless, there is still
a persistence of a residual risk mainly related to the serological
window periods characterized by low-level serological viral markers,
which are usually undetectable by conventional serological assays.[10]
In order to ensure blood safety, the World Health Organization (WHO)
recommends the recruitment of voluntary, regular and unpaid donors
selected from low-risk populations.[11]
In
Burkina Faso, since its operationalization in 2005, Regional Blood
Transfusion Center of Ouagadougou (CRTS/O) has continuously improved
its blood transfusion safety policy by applying measures aimed at
reducing the residual risk of transfusion, namely: medical selected
donors, recruitment of voluntary, regular and unpaid donors and the use
of fourth-generation serological tests for the biological qualification
of blood donations.
With a view to optimal transfusion safety
research, Burkina Faso is now considering the introduction of PCR in
the screening of blood donations. The application of this technology in
blood transfusion in developed countries has made it possible to reduce
the residual transfusion risk associated with the serological window.[12-14]
Burkina Faso, a developing country (DC) has a high endemicity of these
transfusion-transmitted viral infections but has very little data on
the impact of multiplex PCR in our context. The present study was
conducted at the CRTS/O to evaluate the impact of multiplex PCR in
reducing the risk of residual transfusion-transmitted HIV,
HBV and HCV infections in blood donors seronegative for these three viruses in Burkina Faso.
Material and Methods
Type and population of study.
This was a cross-sectional study that took place from March to
September 2017 at the CRTS/O. The study population consisted of blood
donors of both genders accepted for fixed-site blood donation and
mobile collection at the CRTS/O at the end of the medical selection.
Medical screening was performed by qualified health professionals based
on a standardized pre-donation interview questionnaire designed to gain
insight on risk behaviors for HIV, HBV and HCV infections. Each donor
freely agreed to participate in the study. A "repeat donor" was any
donor who had already given at least one blood donation before this
study. Otherwise, he was considered "first- time donor".
Ethical considerations.
Ethical approval for the study was obtained from Ethics Committee for
Health Research of Burkina Faso (deliberation n° 2015-6-080). Written
informed consent was provided by all study participants.
Sampling.
Venous blood specimens were collected from blood donors on a red-top
and EDTA tubes. After centrifugation at 1500 g for 20 minutes, aliquots
of serum and plasma were performed within 6 hours after samples
collection for laboratory analyzes.
Screening for HIV, HBV and HCV serological markers.
The serological markers of HIV, HBV and HCV were investigated on donor
sera by chemiluminescent microparticle immunoassay (CMIA) using
ARCHITECT i1000 SR (Abbott Diagnosis, USA). The p24 antigen and
antibodies against HIV1/2 were simultaneously investigated using the
ARCHITECT HIV Ag/Ab Combo Kit (Abbott, Wiesbaden Germany). HBV surface
antigen (HBsAg) and anti-HCV antibodies were respectively determined
using the ARCHITECT HBsAg Qualitative II (Abbott, Ireland, Sligo
Ireland) and ARCHITECT Anti-HCV (Abbott GmbH & Co.KG, Wiesbaden
Germany).
Detection for viral genomes of HIV, HBV and HCV.
The detection of viral nucleic acid was performed sequentially on the
samples obtained exclusively from blood donors who tested seronegative
for the three viruses (HIV, HBV, HCV). A first multiplex PCR was
carried out on mini-pools of 8 plasma samples each. The mini- pools
detected positive were reconstituted into 2 mini-pools of 4 plasma
samples for a second multiplex PCR. Finally, an individual PCR assay
was performed on the samples from positive mini- pools to the second
PCR.
Constitution of mini-pools.
Seronegative tested samples were divided into mini-pools of 8 plasma
samples each. Each mini-pool consisted of 160 μL of plasma from each
donation (i.e. 1.28 mL of plasma per mini-pool). Mini-pools of 8 plasma
tested positive at the first assay were reconstituted into mini-pools
of 4 plasma samples of 100 μL of each donation (i.e. 400 μL per
mini-pool).
Extraction and amplification by multiplex PCR in real time.
Molecular analysis was performed at the laboratory of Molecular Biology
and Genetics (CERBA/ LABIOGENE). Extractions of viral nucleic acid were
performed with 100 μL of mini- pool plasma using the Ribo-sorb sacaceTM
Kit (Sacace Biotechnologies®, Como Italy). PCR amplification was
performed on the SaCycler-96 Real Time PCR v.7.3 (Sacace
Biotechnologies) with the HCV/HBV/HIV Real-TM sacaceTM multiplex kit
(Sacace Biotechnologies®, Como, Italy). The PCR was carried out using a
reaction volume of 25 μL (10 μL of DNA/RNA and 15 μL of the mix). The
SaCycler-96 real-time multiplex PCR amplification was performed
according to the following program: 1 cycle of 95°C for 15 s follows by
46 cycles of 95 °C for 15 s and 60°C for 40 s. The sensitivity of the
HCV/HBV/HIV Real-TM kit was respectively 10 IU/mL, 5 IU/mL and 20
copies/mL for HCV, HBV and HIV. After the first two amplifications,
individual real-time PCR for HBV-positive samples of the mini-pools was
performed to find the positive sample (s) from each pool. For this step
the sacaceTM Ribo-Sorb Silica kit was used for extraction and the
sacaceTM HBV Real-TM Qual kit was used for amplification according to
the manufacturer's recommendations.
Statistical analyzes.
The data were analyzed using the Epi Info version 7 software. The
chi-square test was used for comparisons and any value was considered
statistically significant for p ≤ 0.05.
Results
Seroprevalences
of HIV, HBV and HCV according to the socio-demographic characteristics
of blood donors at the CRTS/O. A total of 989 blood donors were
included in this study. The age of donors ranged from 17 to 65 years
with an average of 27.29 +/- 8.81 years. The 21 to 30 age group was the
most represented with 55.31% (547/989) of donors with a sex ratio (M/F)
of 1.96 in the study population. Of all blood donors, 55.21% (546/989)
were first-time donors and 66.90% (632/989) were recruited from mobile
collection sites (Table 1).
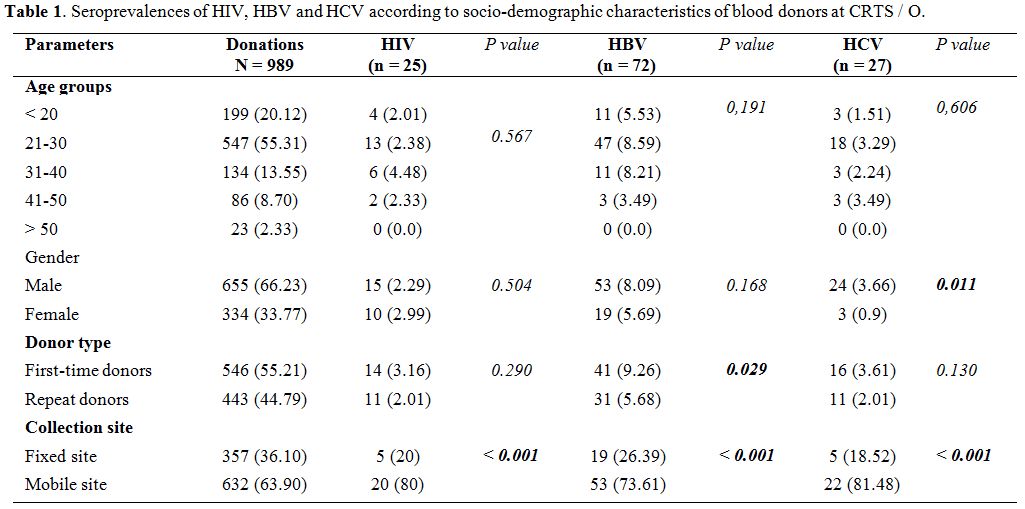 |
Table 1. Seroprevalences of HIV, HBV and HCV according to socio-demographic characteristics of blood donors at CRTS / O. |
Of
989 donations tested for antibodies and/or antigens associated with
HIV, HBV and HCV infection, 865 (87.46%) donations were detected
seronegative versus 124 (12.54%) seropositive cases. HIV infection was
positive in 2.53% (25/989) of blood donors, HBV in 7.28% (72/989) and
HCV in 2.73% (27/989) of cases. HIV/HBV,
HBV/HCV, HIV/HCV and HIV/HBV/HCV
coinfections were
0.4% (4/989), 0.71% (7/989), 0.2% (2/989) and 0.1% (1/989)
respectively. The seroprevalence of transfusion-transmissible
infections was higher among first-time donors and mobile collection
sites (Table 2).
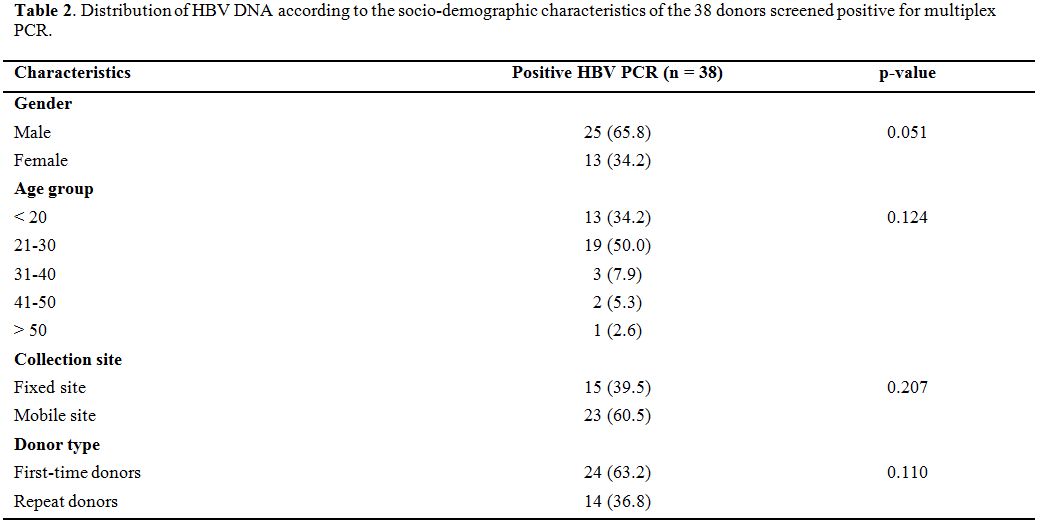 |
Table
2. Distribution of HBV DNA according to the socio-demographic
characteristics of the 38 donors screened positive for multiplex PCR. |
Prevalence
of HIV, HBV, and HCV genomes (DNA, RNA) in seronegative screened blood
donors by the CMIA method. Forty-three (43) mini-pools were detected
positive for HBV out of the 101 mini-pools of 8 plasma samples tested
at the first real-time multiplex PCR. No mini-pool was positive for HIV
and HCV. Of 86 mini-pools of 4 plasma samples reconstituted for the
second multiplex PCR, 42 were positive for HBV. No mini-pool was
positive for HIV and HCV. Of the 168 (42 x 4) samples tested by single
PCR, 38 were positive for HBV. Altogether, 4.70% (38/808) of
seronegative donations were positive by multiplex PCR. These positive
samples were confirmed using the same detection kit on the same device.
Discussion
The
present study which aimed to determine the impact of multiplex PCR in
reducing the residual risk of transfusion- transmitted viral
infections, reported a prevalence of 4.70% (38/808) of HBV viral DNA in
seronegative blood donors. Although the sample size is limited, this
study is the first using multiplex PCR for the seronegative-blood
donors screening in Burkina Faso.
The donations came exclusively
from voluntary and unpaid blood donors. In recent years, family or
replacement donations have been phased out by the CRTS/O in order to
comply with WHO recommendations[11] for better blood
safety. Nevertheless, our study reported seroprevalences of 2.53%,
7.28%, and 2.73% respectively for HIV, HBV, and HCV. The seroprevalence
of 2.53% of HIV reported in this study is similar to that of 2.00%
reported in the general population of the central region of Burkina
Faso in 2016[15] where our study was conducted and it
is markedly higher than the 0.8% reported in the general population of
Burkina Faso by the same study.[15] It is also higher than the 1.8% reported in blood donors from three regional centers in Burkina Faso in 2009[4] but comparable to the 2.21% reported in Koudougou blood donors in 2012.[5]
As for HBV, the prevalence of 7.28% obtained in our study was
lower than those found in previous studies in the general population9
and blood donors in Nouna,[7] Ouagadougou[9] and Koudougou[5] or in specific groups (pregnant women[7] and health workers[6]). The prevalence of 2.73% of HCV infection was also lower than the 4.4% prevalence reported among blood donors in 2014.[8]
Altogether, the results of this study show a high prevalence of HIV and
HCV against a low prevalence of HBV compared to previous studies in
Burkina Faso. This could be explained by the low rate (only 11.3%) of
regular blood donor in Burkina Faso.[4]
This
decrease in HBV prevalence can be attributed to extensive awareness and
screening campaigns during the last decade, significant improvement in
the accessibility and availability of hepatitis B vaccine and expanded
immunization program against HBV for children at 8 weeks after birth
since 2006. In addition, awareness campaigns, which are much more
focused on hepatitis B infection with free screening in recent years,
are increasingly providing a selective population of voluntary donors
at fixed sites who already know their negative serological status for
hepatitis B infection but generally unaware of their serology for HIV
and/or HCV. This could also explain the high seroprevalence of the
latter viruses and low prevalence of HBV infection compared to previous
data in Burkina Faso. Furthermore, unpublished data have shown a
variation in the prevalence of HBV infection between different
districts of Ouagadougou. This observation could be extended to HIV
and/or HCV infections. In this study, the seroprevalences of these
three viruses were higher in first-time donors than in repeat donors. A
similar observation has been made in several studies carried out in
Burkina Faso[4] and other West African countries.[16,17]
The high prevalence of these viral infections in first-time donors can
be attributed to the lower level of knowledge about blood-borne
infections and routes of transmission compared to regular or repeat
donors. However, to cope with the high demand for labile blood products
(LBP), mobile blood collections by CRTS/O is required in high schools,
universities, barracks, places of worship etc. These mobile collections
would encourage the enrollment of first-time donors although the
latter, unlike regular donors, are not always aware of the issue of
transfusion safety.[18] This increases the risk of
collection of infected donors during seroconversion period. In the
present study, 63.90% of blood donors come from mobile sites explaining
the predominance of first- time donors (55.21%).
Likewise,
the seroprevalences of these three infections were higher in the blood
donors at the mobile sites compared to those taken at the fixed site at
the CRTS/O with a statistically significant difference (p < 0.001)
for all these three viral infections. This observation suggests that
our method of medical donor selection is not adapted to our blood
donors’ recruitment policy in mobile sites. Indeed, in mobile sites,
blood collections are often organized without prior awareness-raising
of potential donors on transfusion safety issues. This is especially
true as mobile collections tend to encourage the recruitment of
first-time donors. All of these results show that, despite the medical
selection of blood donors and the computerization of their data,
seroprevalences of HIV, HBV and HCV remain very high among blood donors
in Burkina Faso. This reflects shortcomings in the promotion of blood
donation and donor education prior to blood collection hence the low
proportion of regular donors. These high seroprevalences of HIV, HBV
and HCV in a population of volunteer donors also pose the problem of
the quality of medical selection. Indeed, a well-conducted medical
selection with relevant selection criteria is highly effective in
controlling the risk associated with the silent period in infections.[19-21]
In Burkina Faso, the pre-donation questionnaire and the quality of the
selection have not yet been formally evaluated. Nevertheless, a study
by Kafando et al. showed that there was no significant difference
between the positivity rate among donors accepted to donate and those
who were refused.[22]
Of 808 seronegative HIV,
HBV, and HCV donations tested by multiplex PCR, 38 (4.70%) residual
cases were detected positive. These results are higher than those
obtained in similar studies in other developing countries also
experiencing high prevalence of these three infections. For example: in
Ghana, out of 9372 seronegative screened donors by rapid tests, 3% were
detected HBV-positive DNA; no cases of HIV and HCV were detected.[23]
In South Africa,[24] the residual risk rate of HIV, HBV and HCV was estimated to be 1 per 45,765, 1 per 11, 810 and 1 per 732, 200; in Thailand,[25]
out of 4798 HIV-negative donations, 6 cases of HBV- positive cases were
detected, no HCV and HIV cases were detected. Our results are
considerably higher compared to those obtained in developed countries
where the prevalence of these three infections is low. For example:
United States[14] (HIV: 1 / 2million, HCV: 1 / 270,000), Italy[26] (HCV: 2.5 / million, HIV: 1.8 / million, HBV: 57.8 / million), Germany[27] (HCV: 1 / 10.8 million, HBV: 1/360 000). All 38 donations tested positive by multiplex PCR were only positive for HBV DNA.
These
results suggest that the residual risk of transmission of HBV by blood
transfusion is greater compared to that of HIV and HCV in Burkina Faso.
Otherwise, the contribution of PCR would be more important for HBV
compared to HIV and HCV in our country. The proportion of positive HBV
donations that are only detectable in the PCR (4.72%) obtained in our
study can be explained by the high prevalence and incidence of this
infection in the blood donor population in Burkina Faso.[4]
These high prevalences and incidences are necessarily accompanied by
significant proportions of infections in the early phase from the point
of view of serological markers. Added to this is the high prevalence of
occult hepatitis B reported in several studies in Burkina Faso.[28,29]
Occult hepatitis B is characterized by the presence of HBV
DNA in the serum of a patient who is screened for HBsAg by the usual
serological tests.[30] Although our study did not
allow us to detect residual cases associated with HIV and HCV, the
number of donations detected positive HBV by PCR confirms that PCR is
more sensitive than ELISA. This sensitivity had already
been demonstrated by some studies conducted in blood
donors in Burkina Faso,[31] and in two neighboring countries, Togo[32] and Ghana.[23]
The absence of positive cases of HIV and HCV in our study could be
explained by the relatively low prevalence of these viruses in the
general population but also the limited size of our sample. The
residual risk of HIV estimated by a mathematical model based on
serological tests was 1 for 55,000 donations among blood donors in
Burkina Faso[33] in 2011. Similarly, no cases of
residual HIV and HCV risk detectable by PCR were reported in two other
similar blood donor studies in Ghana,[23] a neighboring country in Burkina Faso and Kenya,[34]
a country of Sub-Saharan Africa like ours. However, cases of residual
risk of HIV and HCV, although relatively low compared to HBV, have been
observed in similar large-sample studies in other developing
countries and developed countries.[13,26,27]
Of
the 38 cases of HBV DNA detected in our study, 24 (63.2%) were
first-time donors versus 14 (36.8%) who were repeat donors. These
results show that the residual risk of transfusion is higher in
first-time donors compared to repeat donors, but without a significant
statistical difference. These results confirm are consistent with the
literature that donations from regular donors would be the most safety.
This is a cornerstone of WHO's strategy of promoting regular donations.
Nevertheless, we note in our study that residual cases of HBV are also
important in repeat donors. From a mathematical model, Nagalo et al.
estimated equally high incidence rates of 3270.2, 5874.1, and 6784.6
per 100,000 donations for HIV-1, HBV, and HCV, respectively, among
repeat donors.[4] Considering a 100% HBV transmission
rate by transfusion of a contaminated LBP, the PCR prevented 38 cases
of HBV transmission or even more if these 38 donations were used for
the preparation of other LBP such as Frozen Fresh Plasmas (FFPs) and
Standard Platelet Concentrates (SPC). All of these results show that in
addition to promoting unpaid and regular voluntary donation, the
implementation of the PCR is useful in the context of Burkina Faso.
Conclusion
The
study reported very high seroprevalences of HIV, HBV, and HCV in the
blood donor population in Burkina Faso. Multiplex PCR has shown
the existence of a high rate of residual cases of HBV associated with
ELISA serological tests, which is a serious concern to transfusion
safety in Burkina Faso. It is imperative for the CRTS/O to adopt a new
screening strategy for blood donations including the Screening of viral
genomes of HIV, HBV and HCV for optimal transfusion safety.
Acknowledgments
A
deep gratitude to the National Blood Transfusion Center (Burkina Faso),
the Regional Blood Center of Ouagadougou and the Biomolecular Research
Center Pietro Annigoni of Ouagadougou (CERBA).
References
- UNAIDS. Global Report on AIDS Epidemic: Treatment and care. 2008. Available at www.unaids.org/sites/default/files/media_asset/jc1510_2008globalrep ort_en_0.pdf. Accessed on 09/04/2018.
- Jayaraman
S, Chalabi Z, Perel P, Guerriero C, Roberts I. The risk of
transfusion‐transmitted infections in sub‐Saharan Africa. Transfusion.
2010;50:433-42. https://doi.org/10.1111/j.1537-2995.2009.002402.x PMid:19843290

- Fasola
F, Otegbayo I. Post-transfusion viral hepatitis in sickle cell anaemia:
retrospective-prospective analysis. Nigerian Journal of Clinical
Practice. 2002;5:16-9.

- Nagalo
BM, Bisseye C, Sanou M, Kienou K, Nebié YK, Kiba A, Dahourou H,
Ouattara S, Nikiema JB, Moret R. Seroprevalence and incidence of
transfusion‐transmitted infectious diseases among blood donors from
regional blood transfusion centres in Burkina Faso, West Africa.
Tropical medicine & international health. 2012;17:247- 53. https://doi.org/10.1111/j.1365-3156.2011.02902.x PMid:21988100

- Nagalo
MB, Sanou M, Bisseye C, Kaboré MI, Nebie YK, Kienou K, Kiba A, Dahourou
H, Ouattara S, Zongo JD. Seroprevalence of human immunodeficiency
virus, hepatitis B and C viruses and syphilis among blood donors in
Koudougou (Burkina Faso) in 2009. Blood transfusion. 2011;9:419.
PMid:21839011 PMCid:PMC3200412

- Pietra
V, Kiema D, Sorgho D, Kabore S, Mande S, Castelli F, Puoti M, Simpore
J. Prévalence des marqueurs du virus de l'hépatite B et des anticorps
contre le virus de l'hépatite C parmi le personnel du District
Sanitaire de Nanoro, Burkina Faso. Science et technique, sciences de la
santé. 2008;31:53-9.

- Collenberg
E, Ouedraogo T, Ganamé J, Fickenscher H, Kynast‐Wolf G, Becher H,
Kouyaté B, Kräusslich HG, Sangaré L, Tebit DM. Seroprevalence of six
different viruses among pregnant women and blood donors in rural and
urban Burkina Faso: a comparative analysis. Journal of medical
virology. 2006;78:683-92. https://doi.org/10.1002/jmv.20593 PMid:16555290

- Zeba
MTA, Sanou M, Bisseye C, Kiba A, Nagalo BM, Djigma FW, Compaoré TR,
Nebié YK, Kienou K, Sagna T. Characterisation of hepatitis C virus
genotype among blood donors at the regional blood transfusion centre of
Ouagadougou, Burkina Faso. Blood Transfusion. 2014;12:s54.
PMid:24599906 PMCid:PMC3934227

- Tao
I, Compaoré TR, Diarra B, Djigma F, Zohoncon TM, Assih M, Ouermi D,
Pietra V, Karou SD, Simpore J. Seroepidemiology of hepatitis B and C
viruses in the general population of burkina faso. Hepatitis research
and treatment. 2014;2014.

- Kleinman
S, Busch MP, Korelitz JJ, Schreiber GB. The incidence/window period
model and its use to assess the risk of transfusion-transmitted human
immunodeficiency virus and hepatitis C virus infection. Transfusion
medicine reviews. 1997;11:155-72. https://doi.org/10.1053/tmrv.1997.0110155 PMid:9243769

- WHO. Blood safety: A strategy for the AFRICAN REGION. 2001. Available at http://apps.who.int/iris/bitstream/handle/10665/95734/AFR_RC51_R2.pdf?sequence=1&isAllowed=y. Accessed on 09/04/2018.
- Davidson
T, Ekermo B, Gaines H, Lesko B, Åkerlind B. The cost‐ effectiveness of
introducing nucleic acid testing to test for hepatitis B, hepatitis C,
and human immunodeficiency virus among blood donors in Sweden.
Transfusion. 2011;51:421-9. https://doi.org/10.1111/j.1537-2995.2010.02877.x PMid:20849409

- Stramer
S, Glynn S, Kleinman S. Detection of Hiv-1 and Hcv infections among
antibody-negative blood donors by nucleic acid amplification testing.
Vox Sanguinis. 2005;88:68-9.

- Zou
S, Dorsey KA, Notari EP, Foster GA, Krysztof DE, Musavi F, Dodd RY,
Stramer SL. Prevalence, incidence, and residual risk of human
immunodeficiency virus and hepatitis C virus infections among United
States blood donors since the introduction of nucleic acid testing.
Transfusion. 2010;50:1495-504. https://doi.org/10.1111/j.1537-2995.2010.02622.x PMid:20345570

- CNLS/IST. Rapport d'activité sur la riposte au SIDA au Burkina Faso. http://www.unaidsorg/sites/default/files/country//BFA_narrative_repo rt_2016pdf. 2016. Accessed on 09/04/2018.
- Mavenyengwa
RT, Mukesi M, Chipare I, Shoombe E. Prevalence of human
immunodeficiency virus, syphilis, hepatitis B and C in blood donations
in Namibia. BMC Public Health. 2014;14:424. https://doi.org/10.1186/1471-2458-14-424 PMid:24884633 PMCid:PMC4012713

- Noubiap
JJN, Joko WYA, Nansseu JRN, Tene UG, Siaka C. Sero- epidemiology of
human immunodeficiency virus, hepatitis B and C viruses, and syphilis
infections among first-time blood donors in Edéa, Cameroon.
International Journal of Infectious Diseases. 2013;17:e832-e7. https://doi.org/10.1016/j.ijid.2012.12.007 PMid:23317526

- Nébié
K, Olinger C, Kafando E, Dahourou H, Diallo S, Kientega Y, Domo Y,
Kienou K, Ouattara S, Sawadogo I. Faible niveau de connaissances des
donneurs de sang au Burkina Faso; une entrave potentielle à la sécurité
transfusionnelle. Transfusion clinique et biologique. 2007;14:446-52. https://doi.org/10.1016/j.tracli.2007.12.005 PMid:18295528

- Stokx
J, Gillet P, De Weggheleire A, Casas EC, Maendaenda R, Beulane AJ, Jani
IV, Kidane S, Mosse CD, Jacobs J. Seroprevalence of
transfusion-transmissible infections and evaluation of the pre-
donation screening performance at the Provincial Hospital of Tete,
Mozambique. BMC infectious diseases. 2011;11:141. https://doi.org/10.1186/1471-2334-11-141 PMid:21605363 PMCid:PMC3120673

- Seck
M, Dieye B, Guèye Y, Faye B, Senghor A, Toure S, Dieng N, Sall A, Touré
A, Dièye T. Évaluation de l'efficacité de la sélection médicale des
donneurs de sang dans la prévention des agents infectieux. Transfusion
Clinique et Biologique. 2016;23:98-102. https://doi.org/10.1016/j.tracli.2015.11.001 PMid:26681660

- Tagny
CT, Kouao MD, Touré H, Gargouri J, Fazul AS, Ouattara S, Anani L,
Othmani H, Feteke L, Dahourou H. Transfusion safety in francophone
African countries: an analysis of strategies for the medical selection
of blood donors. Transfusion. 2012;52:134-43. https://doi.org/10.1111/j.1537-2995.2011.03391.x PMid:22014098 PMCid:PMC3668689

- Kafando
E, Nébié Y, S S, Kienou K, Dahourou H, Simpore J. Risk Behavior among
Ineligible Blood Donors in a Blood Transfusion Center (Burkina Faso). J
Hematol Blood Transfus Disord. 2017;4:015.

- Owusu‐Ofori
S, Temple J, Sarkodie F, Anokwa
M, Candotti D, Allain JP. Predonation screening of blood
donors with rapid tests: implementation and efficacy of a novel
approach to blood safety in resource‐poor settings. Transfusion.
2005;45:133-40. https://doi.org/10.1111/j.1537-2995.2004.04279.x PMid:15660820

- Vermeulen
M, Lelie N, Sykes W, Crookes R, Swanevelder J, Gaggia L, Le Roux M,
Kuun E, Gulube S, Reddy R. Impact of individual‐ donation nucleic acid
testing on risk of human immunodeficiency virus, hepatitis B virus, and
hepatitis C virus transmission by blood transfusion in South Africa.
Transfusion. 2009;49:1115-25. https://doi.org/10.1111/j.1537-2995.2009.02110.x PMid:19309474

- Nantachit
N, Thaikruea L, Thongsawat S, Leetrakool N, Fongsatikul L, Sompan P,
Fong YL, Nichols D, Ziermann R, Ness P. Evaluation of a multiplex
human immunodeficiency virus‐1, hepatitis C virus, and hepatitis B
virus nucleic acid testing assay to detect viremic blood donors in
northern Thailand. Transfusion. 2007;47:1803-8. https://doi.org/10.1111/j.1537-2995.2007.01395.x PMid:17880604

- Velati
C, Romanò L, Fomiatti L, Baruffi L, Zanetti AR. Impact of nucleic acid
testing for hepatitis B virus, hepatitis C virus, and human
immunodeficiency virus on the safety of blood supply in Italy: a 6‐
year survey. Transfusion. 2008;48:2205-13. https://doi.org/10.1111/j.1537-2995.2008.01813.x PMid:18631163

- Hourfar
MK, Jork C, Schottstedt V,
Weber‐Schehl M, Brixner V, Busch MP, Geusendam G,
Gubbe K, Mahnhardt C, Mayr‐Wohlfart U. Experience of German Red Cross
blood donor services with nucleic acid testing: results of screening
more than 30 million blood donations for human
immunodeficiency virus‐1, hepatitis C virus,
and hepatitis B virus. Transfusion. 2008;48:1558-66. https://doi.org/10.1111/j.1537-2995.2008.01718.x PMid:18466173

- Somda
K, Sermé A, Coulibaly A, Cissé K, Sawadogo A, Sombié A, Bougouma A.
Hepatitis B Surface Antigen Should Not Be the Only Sought Marker to
Distinguish Blood Donors towards Hepatitis B Virus Infection in High
Prevalence Area. Open Journal of Gastroenterology. 2016;6:362. https://doi.org/10.4236/ojgas.2016.611039

- Diarra
B, Yonli AT, Sorgho PA, Compaore TR, Ouattara AK, Zongo WA, Tao I,
Traore L, Soubeiga ST, Djigma FW. Occult hepatitis B virus infection
and associated genotypes among HBsAg-negative subjects in Burkina Faso.
Mediterranean journal of hematology and infectious diseases. 2018;10. https://doi.org/10.4084/mjhid.2018.007

- Raimondo
G, Allain J-P, Brunetto MR, Buendia M-A, Chen D-S, Colombo M, Craxì A,
Donato F, Ferrari C, Gaeta GB. Statements from the Taormina expert
meeting on occult hepatitis B virus infection. Journal of hepatology.
2008;49:652-7. https://doi.org/10.1016/j.jhep.2008.07.014 PMid:18715666

- Nagalo
B, Bisseye C, Sanou M, Nebié Y, Kiba A, Kienou K, Zongo J, Simporé J.
Diagnostic moléculaire du virus de l'immunodéficience humaine acquise
(VIH) sur les pools de plasmas de donneurs de sang au centre régional
de transfusion sanguine de Ouagadougou (CRST- 0), Burkina Faso.
Médecine tropicale. 2011;71:137-41.

- Assih
M, Feteke L, Bisseye C, Ouermi D, Djigma F, Karou SD, Simpore J.
Molecular diagnosis of the human immunodeficiency, hepatitis B and C
viruses among blood donors in lomé (Togo) by multiplex real time PCR.
The Pan African Medical Journal. 2016;25. https://doi.org/10.11604/pamj.2016.25.242.7096

- Lefrère
JJ, Dahourouh H, Dokekias AE, Kouao MD, Diarra A, Diop S, Tapko JB,
Murphy EL, Laperche S, Pillonel J. Estimate of the residual
risk of transfusion‐transmitted human
immunodeficiency virus infection in sub‐Saharan Africa: a multinational
collaborative study. Transfusion. 2011;51:486-92. https://doi.org/10.1111/j.1537- 2995.2010.02886.x PMid:20880002

- Basavaraju
S, Mwangi J, Nyamongo J, Zeh C, Kimani D, Shiraishi R, Madoda R, Okonji
J, Sugut W, Ongwae S. Reduced risk of
transfusion‐transmitted HIV in
Kenya through centrally co‐
ordinated blood centres, stringent donor selection and effective p24
antigen‐HIV antibody screening. Vox sanguinis. 2010;99:212-9. https://doi.org/10.1111/j.1423-0410.2010.01340.x PMid:20497410

[TOP]


































