Nour M. Moukalled, Rayan Bou-Fakhredin and Ali T. Taher.
Department of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon.
Correspondence to: Ali T. Taher, Department of Internal Medicine,
American University of Beirut Medical Center, P.O. Box 11-0236, Beirut
11072020 Lebanon; T: 00961-1-350000 Extension 5392. E-mail:
ataher@aub.edu.lb
Published: November 1, 2018
Received: October 15, 2018
Accepted: September 15, 2018
Mediterr J Hematol Infect Dis 2018, 10(1): e2018066 DOI
10.4084/MJHID.2018.066
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Thalassemia
incorporates a broad clinical spectrum characterized by decreased or
absent production of normal hemoglobin leading to decreased red blood
cell survival and ineffective erythropoiesis. Chronic iron overload
remains an inevitable complication resulting from regular blood
transfusions (transfusion-dependent) and/or increased iron absorption
(mainly non-transfusion-dependent thalassemia), requiring adequate
treatment to prevent the significant associated morbidity and
mortality. Iron chelation therapy has become a cornerstone in the
management of thalassemia patients, leading to improvements in their
outcome and quality of life. Deferasirox (DFX), an oral iron chelating
agent, is approved for use in transfusion dependent and
non-transfusion-dependent thalassemia and has shown excellent efficacy
in this setting. We herein present an updated review of the role of
deferasirox in thalassemia, exploring over a decade of experience,
which has documented its effectiveness and convenience; in addition to
its manageable safety profile.
|
Introduction
Thalassemia
is characterized by genetic disorders leading to defective synthesis of
the normal globin subunits of human hemoglobin.[1]
Various mutations might affect one or both of the β-globin genes
located on chromosome 11 causing β-thalassemia, and/or any of the four
α-globin genes located on chromosome 16 leading to α- thalassemia.[2]
The clinical spectrum of this condition is determined by the type of
mutation which either causes a decrease in or absence of the affected
globin chain, the number/type of genes involved; as well as the
coinheritance of other genetic abnormalities, with cases ranging from
asymptomatic to severe transfusion-dependent anemia. High incidence of
thalassemia has been reported in the Mediterranean region, the Middle
East, the tropical and subtropical regions of Africa among others, thus
creating a significant heath burden.[3] The initial
exploration of this disease identified the abnormal synthesis of one of
the globin chains and the excess of the other, as the primary
pathophysiological mechanism leading to shortened red blood cell (RBC)
survival, ineffective erythropoiesis, associated with medullary and
extramedullary erythroid proliferation.[2]
Nonetheless, knowledge gained over the past few years has led to
further understanding of the associated physiological and pathological
alterations which result in significant morbidity/mortality in this
population of patients. Among those, iron overload (IOL), related to
blood transfusion; in addition to increased intestinal absorption,
further complicates the management of those patients and significantly
affects their outcome. IOL leads to deposition of iron in different
vital organs including the liver, heart, endocrine glands among others,
leading to various clinical manifestations.[4]
Multiple previous studies have documented cardiomyopathy related to IOL
as a significant cause of mortality in β- thalassemia major patients.[5]
Extensive evidence supports the initiation of iron chelation therapy
(ICT) according to specific criteria, and the availability of oral
chelator agents has further improved patients’ compliance with this
essential therapy. ICT has become an essential component in the
management of thalassemic patients with a significant impact on their
survival and quality of life. Multiple iron chelators have been
utilized in this setting, including the parenteral agent deferoxamine
(DFO) which has been the standard of care, in addition to oral
deferiprone (DFP) and DFX that have led to better patients’ compliance
which might reflect into better efficacy. The choice regarding the
optimal agent depends on the severity of iron burden, the organs
affected, and patients’ comorbidities. We herein present an updated
overview of the role of DFX, an oral iron chelator, in the management
of transfusion- dependent (TDT) and non-transfusion-dependent
thalassemia (NTDT), discussing its pharmacological characteristics,
efficacy; as well as safety.
Pathophysiology of IOL in Thalassemia
The
predominant mechanisms underlying the development of IOL in thalassemia
include increased iron accumulation secondary to transfusion therapy
(main cause in TDT) and enhanced intestinal absorption secondary to
ineffective erythropoiesis and hepcidin suppression (mainly in NTDT).
Iron level is generally tightly controlled by multiple regulatory
proteins which modify iron absorption and release as required to
maintain a balance between iron influx (resulting from recycled
erythrocytes or dietary absorption) and excretion. In plasma, iron is
transported by transferrin which binds to its receptors (TFR1expressed
in most tissues and TFR2 that is uniquely expressed in the liver and
intestine).[6] Transferrin saturation is sensed by
TFR1 and 2 to modulate the production of specific regulatory molecules
such as hepcidin through complicated molecular pathways. In TDT,
transfusional iron usually amounts to 0.3 to 0.6 mg/kg per day (d) with
a monthly transfusion rate of 2 to ≥ 4 units packed RBC (200 to 250 mg
elemental iron per unit). Senescent transfused RBCs are phagocytized by
the reticuloendothelial macrophages, leading to the release of cellular
iron into plasma. The human body lacks a physiological mechanism for
removal of the excess iron load resulting from blood transfusion.[7]
So, iron accumulation occurs with increased transferrin saturation
resulting in non-transferrin- bound iron (NTBI) which is readily
transported through calcium channels leading to iron deposition in
hepatocytes, cardiac myocytes, and/or endocrine glands, with variable
clinical complications related to the production of reactive oxygen
species (including the active labile plasma iron-LPI).[6,7] This accumulation results in cellular dysfunction, apoptosis, and necrosis at the level of affected organs.[7,8]
Even in the absence of regular RBC transfusions, IOL develops in many
patients with NTDT, indicating a role for increased absorption of iron
in the development of hemosiderosis in thalassemic patients.[9]
The conditions of anemia and hypoxia that result from ineffective
erythropoiesis influence the level of the serum protein hepcidin, the
main regulator of intestinal iron absorption.[10-13]
Hepcidin negatively regulates iron absorption by reducing the
expression of ferroportin, a transmembrane protein responsible for
exporting intracellular iron into circulation at the level of the
basolateral membranes of the intestinal epithelia, macrophages and
sinusoidal surfaces of hepatocytes.[14] Hepcidin levels decline when iron sequestration for erythropoiesis increases,[12]
and this, in turn, results in upregulated ferroportin which causes an
increase in the release of iron from the reticuloendothelial system,
leading to depletion of macrophage iron.[15,16] The downregulation of hepcidin in NTDT can also be mediated by elevated levels of growth differentiation factor-15 (GDF-15),[17] a member of the transforming growth factor-β (TGF-β) family,[15,18,19] and twisted gastrulation factor.[20]
Recent studies have highlighted the role of GDF-15 in further
exacerbating IOL in NTDT. GDF-15 is normally upregulated during
ineffective erythropoiesis, causing the downregulation of hepcidin. The
variability in the pathophysiological etiology of IOL across TDT and
NTDT affects the rate of iron accumulation and explains the associated
variance in the deposition of iron in different vital organs.6
Remarkably, it has been noted that iron accumulation preferentially
occurs in the liver in patients with NTDT rather than the myocardium.
This was established after observational studies showed the absence of
cardiac siderosis even in patients with severely elevated liver iron
content (LIC).[9]
Diagnosis and Quantification of IOL
Multiple
noninvasive methods have become available for the evaluation and serial
monitoring of IOL in thalassemic patients. These have largely replaced
the initial standard method that included tissue biopsy for
pathological examination. Each of those methods has its advantages and
drawbacks. Nowadays, clinicians use a combination of serum ferritin
(SF), LIC and cardiac iron evaluation as detected by magnetic resonance
imaging (MRI), for the documentation of IOL; as well as clinical
monitoring for patients started on chelation therapy. While SF
evaluation is simple, widely available, and inexpensive, it might
underestimate the actual iron load in many patients (specifically
NTDT).[21,22] Given its reliability and
reproducibility, measuring LIC using MRI is currently among the
forefront strategies for the estimation of hepatic iron accumulation
and has been validated against LIC detected by liver biopsy.[23]
Myocardial T2* below 10 ms and 20 ms have been associated with an
increased risk of heart failure and arrhythmia respectively.[24] SF has been shown to predict hepatic iron burden but does not correlate with cardiac iron trends.[25]
In addition, the post-hoc analysis from one of the major
chelation trials has documented decreases in LIC by > 1 mg/g dry
weight in 52% of patients without a serum ferritin response, and a
correlation between SF and LIC changes only when SF <4000 ng/ml.[26]
The current recommendations thus include measuring SF every three
months and LIC using MRI annually for both TDT and NTDT, in addition to
yearly cardiac T2* MRI in TDT patients only.[27]
Pharmacokinetics of DFX
DFX binds iron in a 2:1 ratio (tridentate agent).[28] Its
use is characterized by a convenient administration for all age groups,
good oral bioavailability (reaching 70%), in addition to its high
affinity and specificity to iron[29] DFX is characterized by a long half-life reaching around 8-16 hours[30] is metabolized in the liver[31] and leads to iron excretion mainly through the fecal route.[32]
Pharmacokinetic parameters are unique in a specific population of
patients, with dose adjustments recommended in hepatic impairment, or
with concurrent uses of strong UDP- glucuronosyltransferase inducers
and/or bile acid sequestrants, and continuous transfusion burden.[33]
Long-term therapeutic outcomes are affected by the DFX to iron complex
formation ratio, which has been recently suggested as an indicator of
efficacy.[34]
Evidence in TDT
There
has been an extensive evaluation of DFX in TDT patients, either as
monotherapy or in combination with other chelator agents in cases of
severe IOL or when single agents do not lead to adequate efficacy.Monotherapy. DFX at 20 mg/kg/d had shown similar efficacy to DFO at 40 mg/kg/d in reducing LIC.[35]
The prospective ESCALTOR trial reported sustained reduction in LPI
levels in a group of β- thalassemia patients with significant IOL with
a mean decrease in LIC by 3.8 mg iron/g dw, and SF by 517 ng/ml.[30,36]
Two-year treatment with DFX leads to a reduction of iron levels in
those whose baseline LIC ≥7 mg iron/g dw while maintaining iron levels
in those with baseline LIC < 7 mg/g dw.[37] An
initial phase II trial in pediatric patients with TDT had shown that
low doses of DFX were associated with limited efficacy.[4]
These results were also replicated in a large randomized phase III
study including 586 pediatric and adult patients with β-thalassemia who
achieved a significant reduction in SF and LIC with DFX at doses of
20-30 mg/kg/d, while doses of 10 mg/kg/d showed inadequate efficacy in
regularly transfused patients.[38] Sustained
improvements in iron burden were noted with follow up to 5 years, where
83% achieved SF ≤2500 ng/ml, 47.3% of patients reaching SF≤1000 ng/ml
after 4 years, with more than half of patients receiving a final dose
of DFX ≥25 mg/kg/d during the extension period.[39]
While doses of 20 mg/kg/d have maintained an LIC below 7 mg/g dw,
higher doses of around 30 mg/kg/d have been required to achieve a net
reduction in iron levels in those with LIC ≥ 7 mg/g dw.[40,41]
This large prospective trial included 1115 patients with β-thalassemia
and showed a statistically significant decrease in SF with DFX therapy.
In addition, it indicated the need to choose a starting dose that
correlates with the patient’s transfusion requirements, and that needs
to be titrated in a timely manner. At least three years of DFX lead to
reversal or stabilization of liver fibrosis in TDT patients showing
evidence of IOL.[42] A systematic review and
meta-analysis including 1520 patients with TDT also showed increases in
SF at lower DFX doses, but no significant difference in the change in
SF with DFX at 30 mg/kg as compared to DFO.[43]
JaiSwal et al. later reported a significant mean reduction in SF of
1207.11 ng/ml (32.38% decrease) after 12 months therapy with DFX at a
mean dose of 38 mg/kg/d in 45 heavily transfused thalassemia patients.[44]
A prospective observational study including 176 patients with TDT
(total 267), reported long-term results in pediatric patients treated
with DFX and documented a decrease in median SF of 575 ng/ml after five
years of DFX therapy with a mean dose of 25.8 mg/kg/d.[45]
A Cochrane review reported by Bollig et al. indicated similar efficacy
of DFX as compared to DFO (depending on the ratio of DFX to DFO dose,
generally showing similar results at a mean ratio of 1 mg of DFX to 1.8
mg of DFO).[46]DFX
has also been effective in the management of cardiac siderosis. Wood et
al. have reported an improvement in myocardial T2* in patients with
severe cardiac siderosis treated for 18 months with DFX at doses up to
40 mg/kg/d (13 patients).[47] DFX also led to normalization of cardiac
T2* in 68% of patients with a baseline level of 10-20 ms, and sustained
improvements with prolonged duration of therapy.[48]
The phase II CORDELIA trial documented non-inferiority of DFX compared
to DFO in the management of β-thalassemia major asymptomatic patients
with cardiac IOL (T2* 6-20 ms), with a 12% increase in the geometric
mean (Gmean ) myocardial T2*.[49] On the other
hand, the MILE study has shown that DFX at similar doses lead to a 10%
relative improvement in myocardial T2*, with the most significant
results noted in those with moderate cardiac siderosis and those with
lower baseline LIC, while no significant changes were reported in cases
of severe cardiac iron deposition.[50] Greater
improvement in LIC and myocardial T2* were noted with higher doses of
DFX (above 30 mg/kg/d). This study also showed statistically
significant improvement in LIC, specifically those with baseline LIC ≥7
mg/g dw, with a statistically insignificant reduction in SF, and no
major safety concerns. DFX has also resulted in the greatest
improvement in the prevalence of endocrinopathy, in addition to a
significant improvement noted on bone mineral density evaluation as
compared to DFO, DFP and DFO combined with DFP in a retrospective
study.[51]
Safety of DFX
Common
adverse events (AEs) noted in trials evaluating DFX included
gastrointestinal (GI) disturbances, skin rash, elevation in serum
creatinine and/or liver transaminases among others.[52]
Abdominal pain was reported in 4.8% of thalassemia patients in the EPIC
trial, nausea in 3.8% and diarrhea in 7.8% of patients, while
elevations in creatinine >33% above baseline were noted in 3.6% of
the thalassemia cohort.[40] Most of the AEs occurred
with higher doses of DFX (specifically >25 mg/kg/d) including
increases in creatinine by ≥ 30% which has been reported in around 38%
of patients.[38] DFX causes a short-term effect on renal hemodynamics with a reversible reduction in glomerular filtration rate.[53] Elevations in liver transaminases have been more commonly reported in TDT patients.[40,54] Porter et al. have related GI AEs to lower baseline LIC (<7 mg/g dw).[41] Similar AEs have been reported during prolonged follow up periods extending beyond five years.[39]Less common AEs include ocular and visual disturbances, cytopenia, and Fanconi syndrome.[55]
For pediatric patients, Vichinsky et al. reported AEs with suspected
relation to DFX in 39.1% of patients with nine patients having a
serious AE (3.4%), with a gradual decline in the incidence of AEs over
time.[45] Osborne et al. recently reported the
utilization and safety of DFX using an observational post-marketing
study conducted in England. Beta-thalassemia was the second
most frequent reason for prescribing DFX (26 patients; 21.3%), and
increased creatinine was noted in only two patients out of 122 (1.6%).[56] The EPIC trial had reported a 0.6% of proteinuria after 1-year follow up.[40]
Bayhana et al later evaluated the prevalence and need for monitoring of
proteinuria in thalassemia patients on DFX therapy, where a
retrospective single center analysis including 37 total patients (36
with thalassemia major), reported proteinuria in 7 patients (18.9%) at
a mean follow up of 44 months, all of which resolved with follow up.
This analysis identified younger age (below 23) and higher doses of DFX
(above 29 mg/kg/d) as risk factors for the development of
proteinuria.[57] A retrospective chart review
recently reported safety data for prolonged follow-up periods reaching
13 years, including 282 patients, with no significant or persistent
nephrotoxicity noted and only non-progressive and reversible increases
in creatinine.[58]
DFO and DFX
It
has become more common over the past few years to utilize combination
chelation therapy, whether sequentially or concurrently, especially in
cases of severe cardiac IOL. A quasi- experimental study conducted in
Iran included 32 patients with TDT with severe IOL not responding to
monotherapy, who received DFX (30-40 mg/kg/d with DFO 40-50 mg/kg/d for
2 days per week, showing a significant reduction in mean SF from
4031±1955 to 2416±1653 ng/mL after 12 months of treatment, with no
major safety concerns.[59] In an open label trial
that included 18 patients, the combination of DFO (35-50 mg/kg for 3-7
days) and DFX (20-30 mg/kg/d) led to a statistically significant
decrease in median LIC by 5.4
mg/g dw, with a tatistically insignificant improvement of cardiac T2*
by 2.7 ms in 6 patients with baseline T2* below 20 ms.[60]
Cassinerio et al. have also reported improvements in ferritin level,
hepatic and cardiac MRI T2* among seven patients receiving DFO at 32
mg/kg for 3-4 days/week with DFX at an initial dose of 20 mg/kg/d,
after one year of treatment.[61] Aydinok et al.
conducted a third trial combining DFO at 37.4 mg/kg/d for five
days/week with DFX 29.6 mg/kg/d. They included 60 patients with T2*
5-10 ms but a left ventricular ejection fraction ≥56%, moreover,
reported improvements in mean LIC from 33.4 to 18.2 mg/g dw at 24
months, and in cardiac T2* by 9% after one year, and to 9.5 ms at 24 months (baseline 7.2 ms).[62]
These trials all showed the feasibility of combining DFO and DFX with
no unexpected toxicity noted. Recently, a multiple treatment comparison
network meta- analyses and sequential trial analysis included 32
clinical trials and noted that DFX with DFO led to better improvement
in SF level compared to monotherapy or the combination DFO/DFP.[63]
DFP and DFX
Farmaki
et al. initially reported significant improvements in SF level and
hepatic iron in 16 patients with the low iron burden (baseline LIC
1.6±1.1mg/g dw) using DFP combined with DFX.[64] A
significant reduction in SF by 3275 μg/l was also noted among 36
pediatric/young adult thalassemia patients who had shown
suboptimal response to monotherapy with either DFP or DFX, after 1 year
treatment with a combination of DFP and DFX, with AEs including GI
disturbances, arthropathy and increases in creatinine.[65]
They also reported transient elevations in liver enzymes by> 5 times
the upper limit in 11% of patients. In a randomized controlled trial
assessing the combination of DFP 72
mg/kg/d with either DFO or DFX at 23 mg/kg/d, Elalfy et al.[66]
reported a significant reduction in LIC from 12.52 ± 2.28 mg/g dw to
10.17 ± 2.23 mg/g dw with an increase of cardiac T2* from 16.59 ± 1.85
ms to 19.75 ± 2.65 ms. A more rapid rate improvement in cardiac T2* was
attained with DFX and DFP compared to DFO and DFP.[66]
They noted arthropathy in 16.6% of patients, increases in alanine
aminotransferase (ALT) in around 8% of patients, and in creatinine in
6.2% of patients. Karami et al reported results of combining DFP (mean
dose of 53.9±22.2 mg/kg/d) with DFX (mean dose of 29.3±6.8 mg/kg/d) in
6 patients with TDT after failing monotherapy, and showed
non-significant increases in SF with a significant effect on LIC
(change by 7.59±3.16 mg/g dw).[67] Furthermore, 33
patients with TDT who failed monotherapy were evaluated in a
prospective study at a single center in India, with 12 patients
continuing the 2- year treatment with 75 to 100 mg/kg/d DFP (divided
into three doses) and 20 to 40 mg/kg/d DFX.[68] This
regimen showed a reduction in the mean SF by 44.67%±13.78% at two years
and was well tolerated with GI disturbances noted in around 6% of
patients, and elevations in creatinine >33%
above the baseline on two consecutive occasions noted in around 85% of
patients. Pinto et al. have recently reported successful iron chelation
in 8 patients who were intolerant to mono or combination therapies,
using alternating DFP (starting dose 75 mg/kg/day) and DFX (25
mg/kg/day).[69] With a median follow up of 52 months,
this alternating regimen lead to decrease in the mean ferritin by 587
ng/ml, in addition to effective removal of excess cardiac and hepatic
iron, with no moderate to severe.
DFX in the Post-transplantation Setting
Hematopoietic
stem cell transplantation (HSCT) remains the only widely available
curative therapy for thalassemic patients. Inati et al.[70]
conducted a prospective randomized trial comparing DFX (12 patients)
versus phlebotomy (14 patients) in thalassemia patients with IOL post
HSCT (LIC >3 mg/g dw or SF >300 µg/L), showing a median reduction
in SF by 498 µg/L and a mean decrease in LIC by 5.8 mg/g dw after 1
year of DFX therapy. A significantly greater reduction in LIC was
obtained with DFX compared to phlebotomy in those with baseline SF
above 1000 µg/L.70 Yesilipek et al. recently reported results from a
phase II multicenter (in Turkey), single arm study including 27
patients with thalassemia who underwent HSCT within a period of 6
months to 2 years prior to enrollment, and had evidence of IOL (SF>
1000 µg/L, cardiac MRI T2* <20 ms, or LIC ≥5
mg/g dw). DFX was given at an initial dose of 10 mg/kg/d then increased
to 20 mg/kg/d, and resulted in a significant and continued reduction in
median LIC (from 8.6 to 4.1 mg/g dw), increase in median cardiac T2*
from 26 to 28 ms over a 52 week period.[71] This
trial reported serious AEs in only 11.1% of patients, none of which
were related to DFX, with no evidence of hepatotoxicity or
nephrotoxicity in this population of patients.
DFX in NTDT
All
available iron chelators have proven their efficacy in TDT patients.
However, having received Food and Drug Administration (FDA) and
European Medicines Agency (EMA) approval, DFX remains to be the only
drug used in NTDT patients, mostly based on results from the THALASSA
trial.[72,73] In this trial, 1-year treatment
with DFX in NTDT patients > 10 years was shown to decrease LIC at a
daily dose of 5 and 10 mg/kg, respectively, compared to placebo.[73]
Further analysis showed that DFX at starting doses of 5 and 10 mg/kg/d
led to consistent reductions in LIC across all patients, irrespective
of baseline LIC, SF, splenectomy status, underlying NTDT form or
demographics.[73] Greater reductions in LIC were also
achieved in those patients that were dose-escalated at six months from
10 mg/kg/d starting dose to 20 mg/kg/d.[73]
Furthermore, to assess a period of 2 years of DFX treatment, a 1-year
extension phase was then carried out. Patients continued to respond and
showed a decrease in SF and LIC over two years.[73]
Moreover, data from the THETIS study later showed that a starting dose
of 10 mg/kg/d of DFX is effective in reducing IOL in these patients and
that a dose escalation up to 30 mg/kg/d should be considered starting
at week four based on LIC response.[74] DFP has not
been extensively studied in NTDT. However, single-arm, open-label
studies with small sample sizes and a more recent randomized controlled
trial showed significant decreases in SF and LIC with DFP therapy.[75]
Although DFO has not been systematically studied in NTDT, studies with
short durations and small sample sizes have shown an increase in
urinary excretion of iron and a decrease in SF upon DFO administration.Guidelines
with specific indications/thresholds have been established to determine
the appropriate time for initiation, dose escalation, and termination
of ICT in NTDT patients. DFX with an initial starting dose of 10
mg/kg/d should be started in patients ≥10 years of age if their LIC ≥ 5
mg iron/g dw, or if their SF concentration was found to be ≥ 800 μg/L
(if LIC is not available due to MRI unavailability).[76]
To monitor iron levels, LIC should be repeated six months after therapy
initiation, with follow up every 6–12 months, and SF levels should be
measured every three months.[76] If LIC levels at six
months are still greater than 7 mg/g dw (or SF >1500 μg/L only if
LIC is unavailable) with less than 15% reduction in baseline values,
dose escalation should be considered up to 20 mg/kg/d.[76]
DFX therapy can be safely discontinued when patients reach an LIC value
of 3 mg/g dry weight (or SF level of 300 μg/L only if LIC is
unavailable).[76] In the realm of NTDT, it is recommended to intensify ICT if the LIC after six months of treatment >7 mg/g dw, SF >1500–2000
ng/mL or in case of <15% decrease from baseline. Indications to stop
ICT in NTDT include a SF < 300 ng/mL and/or LIC < 3 mg/g dry wt.
liver. Adherence to ICT
Compliance with ICT is often associated with effective IOL control and improved patient survival.[77,78]
Moreover, adherence to long-term ICT is crucial in preventing
IOL-related complications. Poor adherence to ICT is associated with
shorter life expectancy and increased morbidity. There are several
factors that might affect adherence to ICT including the fact that
early iron overload is asymptomatic, the challenges related to the
transition from childhood to adolescence, the possible inconvenience of
administration of chelation therapy, the lack of subjective awareness
of improvement by patients or recurrence of symptoms after
discontinuing a chelator agent, in addition to the AEs associated with
various treatments. Adherence to ICT remains a challenge
for thalassemia patients, and is a multidimensional
issue, involving several factors including patient-related factors
(attitudes and beliefs, perceptions of severity, expectations from
treatment), disease-related factors (acute, chronic, physical state,
emotional state), demographic factors (age, gender, culture, religion,
socioeconomic status), and therapy-related factors (frequency of
dosage, complexity of regimen, administration route, palatability, AEs).DFO
therapy, owing largely to its cumbersome administration, has a
detrimental impact on multiple areas of patients’ lives, including
their emotional well-being, physical functioning, self- esteem, among
others. Treatment with DFO is demanding. The drug has poor oral
bioavailability and a short plasma half-life. Therefore, slow
subcutaneous infusions are necessary 3–7 times weekly. Because of
injection-site reactions and pain, the administration is inconvenient,
and the necessary equipment is not available in many countries. These
factors lead to poor compliance, which in turn leads to increased
mortality.[6] Treatment satisfaction and adherence are generally
greater with oral ICT than with parenteral infusion. One study showed
that adherence to oral DFX monotherapy was significantly higher than
DFO infusion (96% vs. 92%; p<0.001).[79] Adherence
to oral DFX on DFO/DFX combination therapy was lower than that of
monotherapy (90% vs. 96%; p<0.001). Adherence to DFO infusion on
DFO/DFP combination therapy was non- significantly lower than that of
monotherapy or DFO/DFX combination therapy (88% vs. 92%; p=0.25).
Adherence did not significantly change over follow-up period except
that an increase in adherence was seen after a change in chelation from
DFO infusion to oral DFX (p=0.03, paired t- test). In a qualitative
examination of the reported patient adherence over time after this iron
chelator change, no evidence of a ‘honeymoon’ phase was seen, with
temporary high adherence to the new chelator. In an open-ended comment
section, many participants commented on the benefits of oral chelation
and their improved adherence.[79]Another
study by Cappellini et al., compared patient-reported
outcomes (PROs) during receipt of DFO infusions or once-daily DFX oral
therapy.[80] PRO questionnaires were completed by
patients, their parents or legal guardian at different time points:
baseline, week 4, week 24, and end of study (EOS). Patients assessed
their satisfaction level with study treatment (very satisfied,
satisfied, neutral, dissatisfied, or very dissatisfied) and rated its
convenience.[80] At baseline, 289 and 282 patients in
the DFX and DFO groups, respectively, had previous experience
with DFO (7 and 8 patients, respectively were DFO-naïve); of
these patients, 45.3% and 45.0%, respectively, reported that they
were very satisfied or satisfied with DFO
treatment, while 32.5% and 33.0% reported being dissatisfied or very
dissatisfied.[80] There were no significant
differences in the satisfaction ratings between the groups at baseline.
At week 4, week 24, and EOS, significantly more patients
receiving DFX reported being very satisfied or satisfied with
treatment compared with those receiving DFO (92.0% vs 50.4% and 89.6%
vs 44.0%, respectively; P < 0.001).[80] At each
time point, more patients receiving DFO reported being dissatisfied or
very dissatisfied with treatment compared with those receiving DFX
(28.0% vs. 0.7% and 31.2% vs. 2.4%).[80] When
considering only those patients who responded to the question at EOS,
the overall proportion of patients who were satisfied with treatment
was 88.8% (246/277) for DFX and 40.5% (109/269) for DFO.[80]
Results of this study suggested that DFX had a positive impact on
patients' daily lives. A recent Cochrane review exploring the
interventions needed for improving adherence to ICT in patients with
thalassemia or sickle cell disease concluded that real-life data is
required to assess specific adherence strategies and thus make
recommendations in this setting.[81] Recent Advances in ICT
The
efficacy and safety of DFX dispensible tablet (DT) has been well
portrayed through extensive clinical trial programs in patients with a
variety of anemias, including thalassemia, sickle-cell disease,
myelodysplastic syndromes (MDS), and other rare anemias,[4,35,38,82,83]
and has been used widely used in clinical practice all over the world
for over a decade. Nevertheless, barriers to optimal patient acceptance
of treatment still exist with DFX-DT including preparation time,
palatability, the need to take the drug in a fasting state, and
drug-related side effects, notably GI tolerability.[84] DFX film coated tablet (FCT)
An
improved FCT formulation of DFX has been therefore developed for oral
administration.[84] Both DFX FCT and DT are once-daily, oral iron
chelators that are dosed based on body weight. DFX FCT contains the
same active substance, dose-adjusted to achieve comparable exposure to
that achieved with the DT. Because of the increased bioavailability of
the FCT, doses required to achieve the same chelation effect are 30%
lower than the DT. DFX DT has a chalky consistency and is taken on an
empty stomach, at least 30 min before the next meal, and its
administration requires careful dispersion of the tablets in a glass of
water, orange juice, or apple juice.[84] DFX FCT, on
the other hand, can be taken orally on an empty stomach or with a light
meal, offering more convenient and simpler mode of administration, and
potentially improved GI tolerability.The
open-label, phase II ECLIPSE study evaluated the overall safety
profile, as measured by the frequency and severity of AEs and changes
in laboratory values, and the pharmacokinetics (PK), and PROs of DFX
FCT and DT formulations in patients aged ≥10 years with TDT or
very-low-, low-, or intermediate-risk MDS, requiring ICT.[84] The
overall incidence of AEs was similar between treatments, but there were
fewer serious AEs with FCT. FCT recipients consistently reported better
adherence, greater satisfaction, and fewer concerns, with a safety
profile consistent with the known DT formulation.[84]
Taking the dose conversion factor into account, which was
required because the two formulations have differing bioavailability,
patients received similar mean doses of the active ingredient in DFX.
However, at the end of the 24-week trial period, FCT patients had a
higher observed median absolute reduction in SF from baseline,
suggesting a possible association between the treatment arm and
observed efficacy.[84] A potential explanatory factor
of this difference in efficacy could be better treatment adherence
among the patients receiving FCT compared with DT. These findings
suggest a preference in favor of the new formulation, with better
patient satisfaction and adherence translating into reduced IOL-related
complications.Table 1 includes significant trials evaluating DFX in TDT and NTDT, and figure 1A and B includes recommendations regarding chelation with DFX and suggested monitoring and/or adjustments.
 |
Table 1.
Significant trials evaluating DFX in TDT and NTDT. |
 |
Figure 1A. DFX in thalassemia. |
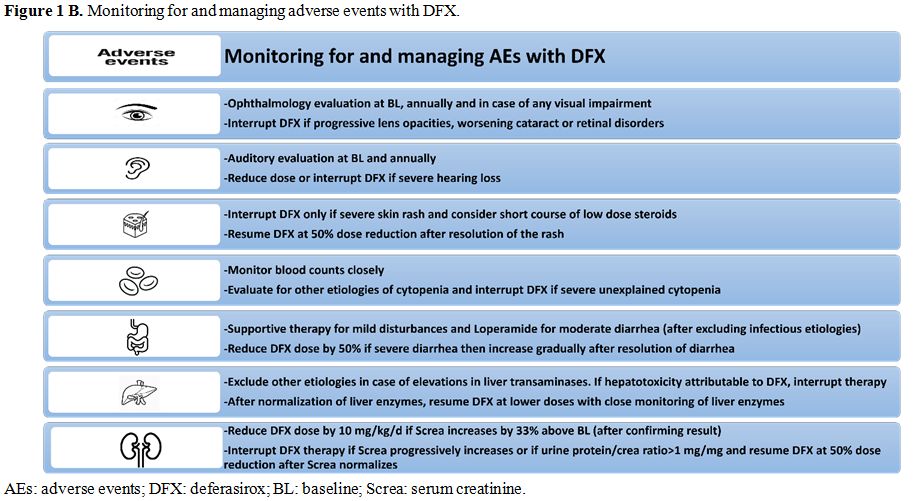 |
Figure 1B. Monitoring for and managing adverse events with DFX. |
Conclusions
ICT
has become a cornerstone in the management of thalassemia patients (TDT
and NTDT). The choice of the optimal chelator agent and schedule
depends on patients’ characteristics and comorbidities; in addition to
the burden of IOL, the organs affected and the presence of symptoms.
Over a decade of experience with DFX in thalassemia patients has
documented its efficacy and manageable safety profile. Extensive
evidence suggests the need to tailor the dose of DFX to the severity of
IOL; as well as the frequency of ongoing transfusions. Recent advances
with the oral formulation might lead to better results related to
optimized compliance, awaiting results with longer follow up duration.
Future trials exploring different combination or sequential chelating
regimens are awaited. In addition, current and future efforts might
lead to improvements in the management of the degree of anemia in
thalassemia, thus reducing the needs for transfusion and further
ameliorating the continuous exacerbation in IOL.
References
- Rachmilewitz E and Giardina P. How I treat thalassemia. Blood 2011; 118 (13): 3479-3487. https://doi.org/10.1182/blood-2010-08-300335 PMid:21813448
- Giardina
P, Forget B. Thalassemia syndromes. In: Hoffman R, Benz E, Shattil S,
et al, eds. Hematology: Basic Principles and Practice (5th ed).
Philadelphia, PA: Churchill Livingstone; 2008:535-563.
- Weatherall DJ. The inherited diseases of hemoglobin are an emerging global health burden. Blood. 2010; 115 (22):4331-4336. https://doi.org/10.1182/blood-2010-01-251348 PMid:20233970 PMCid:PMC2881491
- Galanello
R, Piga A, Forni GL, et al. Phase II clinical evaluation of
deferasirox, a once-daily oral chelating agent, in pediatric patients
with beta-thalassemia major. Haematologica. 2006; 91(10): 1343- 1351.
PMid:17018383
- Borgna-Pignatti C, Rugolotto S,
De Stefano P, Piga A, Di Gregorio F, Gamberini MR, Sabato V, Melevendi
C, Cappellini MD, Verlato G. Survival and disease complications in
thalassemia major. Ann N Y Acad Sci 1998; 850:227–31. https://doi.org/10.1111/j.1749-6632.1998.tb10479.x PMid:9668544
- Taher
AT, Saliba AN. Iron overload in thalassemia: different organs at
different rates. Hematology Am Soc Hematol Educ Program 2017;
2017(1):265-71.
- Saliba A, Taher A. Iron overload in transfusion-dependent thalassemia. Hematology (Amsterdam, Netherlands). 2015;20(5):311- 2. https://doi.org/10.1179/1024533215Z.000000000365
- In:
rd, Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V, editors.
Guidelines for the Management of Transfusion Dependent Thalassaemia
(TDT). Nicosia (CY): Thalassaemia International Federation (c) 2014
Thalassaemia International Federation; 2014.
- Taher
AT, Musallam KM, Wood JC, Cappellini MD. Magnetic resonance evaluation
of hepatic and myocardial iron deposition in transfusion-independent
thalassemia intermedia compared to regularly transfused thalassemia
major patients. American Journal of Hematology. 2010;85(4):288-90. https://doi.org/10.1002/ajh.21626 PMid:20143405
- Pootrakul
P, Kitcharoen K, Yansukon P, Wasi P, Fucharoen S, Charoenlarp P, et al.
The effect of erythroid hyperplasia on iron balance. Blood.
1988;71(4):1124-9. PMid:3355891
- Tanno T, Miller JL. Iron Loading and Overloading due to Ineffective Erythropoiesis. Adv Hematol. 2010;2010:358283. https://doi.org/10.1155/2010/358283 PMid:20467559 PMCid:PMC2868182
- Pigeon
C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, et al. A new
mouse liver-specific gene, encoding a protein homologous to human
antimicrobial peptide hepcidin, is overexpressed during iron overload.
J Biol Chem. 2001;276(11):7811-9. https://doi.org/10.1074/jbc.M008923200 PMid:11113132
- Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425-33. https://doi.org/10.1182/blood-2011-01-258467 PMid:21346250 PMCid:PMC3099567
- Nemeth
E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al.
Hepcidin regulates cellular iron efflux by binding to ferroportin and
inducing its internalization. Science. 2004;306(5704):2090-3. https://doi.org/10.1126/science.1104742 PMid:15514116
- Nicolas
G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, et al. The gene
encoding the iron regulatory peptide hepcidin is regulated by anemia,
hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037-44. https://doi.org/10.1172/JCI0215686 PMid:12370282 PMCid:PMC151151
- Origa
R, Galanello R, Ganz T, Giagu N, Maccioni L, Faa G, et al. Liver iron
concentrations and urinary hepcidin in beta-thalassemia. Haematologica.
2007;92(5):583-8. https://doi.org/10.3324/haematol.10842 PMid:17488680
- Camaschella
C, Nai A. Ineffective erythropoiesis and regulation of iron status in
iron loading anaemias. Br J Haematol. 2016;172(4):512- 23. https://doi.org/10.1111/bjh.13820 PMid:26491866
- Musallam
KM, Taher AT, Duca L, Cesaretti C, Halawi R, Cappellini MD. Levels of
growth differentiation factor-15 are high and correlate with clinical
severity in transfusion-independent patients with beta thalassemia
intermedia. Blood Cells Mol Dis. 2011;47(4):232-4. https://doi.org/10.1016/j.bcmd.2011.07.005 PMid:21865063
- Tanno
T, Bhanu NV, Oneal PA, Goh SH, Staker P, Lee YT, et al. High levels of
GDF15 in thalassemia suppress expression of the iron regulatory protein
hepcidin. Nat Med. 2007;13(9):1096-101. https://doi.org/10.1038/nm1629 PMid:17721544
- Tanno
T, Porayette P, Sripichai O, Noh SJ, Byrnes C, Bhupatiraju A, et al.
Identification of TWSG1 as a second novel erythroid regulator of
hepcidin expression in murine and human cells. Blood.
2009;114(1):181-6. https://doi.org/10.1182/blood-2008-12-195503 PMid:19414861 PMCid:PMC2710947
- Taher
A, El Rassi F, Isma'eel H, et al. Correlation of liver iron
concentration determined by R2 magnetic resonance imaging with serum
ferritin in patients with thalassemia intermedia. Haematologica.
2008;93(10):1584–1586. https://doi.org/10.3324/haematol.13098 PMid:18728025
- Pakbaz
Z, Fischer R, Fung E, et al. Serum ferritin underestimates liver iron
concentration in transfusion independent thalassemia patients as
compared to regularly transfused thalassemia and sickle cell patients.
Pediatr Blood Cancer. 2007;49(3):329–332). https://doi.org/10.1002/pbc.21275 PMid:17554789
- Cheng
HL, Holowka S, Moineddin R, et al. Liver iron overload assessment by t
*2 magnetic resonance imaging in pediatric patients: an accuracy and
reproducibility study. Am J Hematol. 2012;87(4):435–437. https://doi.org/10.1002/ajh.23114 PMid:22286974
- Kirk
P, Roughton M, Porter JB, et al. Cardiac t2* magnetic resonance for
prediction of cardiac complications in thalassemia major. Circulation.
2009;120(20):1961–1968. https://doi.org/10.1161/CIRCULATIONAHA.109.874487 PMid:19801505 PMCid:PMC2784198
- Aubart
M, ou P, Elie C et al. Longitudinal MRI and Ferritin Monitoring of Iron
Overload in Chronically Transfused and Chelated Children With Sickle
Cell Anemia and Thalassemia Major. J Pediatr Hematol Oncol 2016; 38:
497-502. https://doi.org/10.1097/MPH.0000000000000595 PMid:27548334
- Porter
J, Elalfy M, taher A et al. Limitations of serum ferritin to predict
liver iron concentration responses to deferasirox therapy in patients
with transfusion-dependent thalassaemia. Eur J Hematol 2016; 98:
280-288. https://doi.org/10.1111/ejh.12830 PMid:27859648
- Saliba
A, El Rassi F, Taher A. Clinical monitoring and management of
complications related to chelation therapy in patients with β-
thalassemia. Expert Review of Hematology, 9:2, 151-168. https://doi.org/10.1586/17474086.2016.1126176 PMid:26613264
- Cappellini
MD, Taher A. Long-term experience with deferasirox (ICL670), a
once-daily oral iron chelator, in the treatment of transfusional iron
overload. Expert Opin Pharmacother. 2008; 9(13): 2391-2402. https://doi.org/10.1517/14656566.9.13.2391 PMid:18710363
- Nick H. Deferasirox (Exjade®, ICL670) Preclinical overview. Semin Hematol. 2007; 44: S12-15. https://doi.org/10.1053/j.seminhematol.2007.03.005
- Daar
S, Pathare A, Nick H, et al. Reduction in labile plasma iron during
treatment with deferasirox, a once-daily oral iron chelator, in heavily
iron-overloaded patients with beta-thalassaemia. Eur J Haematol. 2009;
82(6): 454-457. https://doi.org/10.1111/j.1600-0609.2008.01204.x PMid:19191863 PMCid:PMC2730549
- Waldmeier
F, Bruin GJ, Glaenzel U, et al. Pharmacokinetics, metabolism, and
disposition of deferasirox in beta-thalassemic patients with
transfusion-dependent iron overload who are at pharmacokinetic steady
state. Drug Metab Dispos. 2010; 38(5): 808- 816. https://doi.org/10.1124/dmd.109.030833 PMid:20097723
- Nick
H, Acklin P, Lattmann R, et al. Development of tridentate iron
chelators: from desferrithiocin to ICL670. Curr Med Chem. 2003;
10(12):1065-1076. https://doi.org/10.2174/0929867033457610
PMid:12678677
- Tanaka C. Clinical Pharmacology of Deferasirox. Clin Pharmacokinet (2014) 53:679–694. https://doi.org/10.1007/s40262-014-0151-4 PMid:24996374
- Lu
M, Lin T, Chiang P, et al. Deferasirox–Iron Complex Formation Ratio as
an Indicator of Long-term Chelation Efficacy in b- Thalassemia Major.
The Drug Monit 2017; 39: 185-191. https://doi.org/10.1097/FTD.0000000000000378 PMid:28141745
- Piga
A, Galanello R, Forni GL, et al. Randomized phase II trial of
deferasirox (Exjade, ICL670), a once-daily, orally-administered iron
chelator, in comparison to deferoxamine in thalassemia patients with
transfusional iron overload. Haematologica. 2006;91(7):873–880.
PMid:16818273
- Taher A, El-Beshlawy A, Elalfy
MS, et al. Efficacy and safety of deferasirox, an oral iron chelator,
in heavily iron-overloaded patients with beta-thalassaemia: the
ESCALATOR study. Eur J Haematol. 2009; 82(6): 458-465. https://doi.org/10.1111/j.1600-0609.2009.01228.x PMid:19187278 PMCid:PMC2730551
- Taher
A, Elalfy MS, Kusai AZ, et al. Achieving treatment goals of reducing or
maintaining body iron burden with deferasirox in patients with
β-thalassemia: results from the ESCALATOR study.Eur J Haematol. 2011;
87(4):349-354. https://doi.org/10.1111/j.1600-0609.2011.01661.x PMid:21668501 PMCid:PMC3229710
- Cappellini
MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox
(icl670), a once-daily oral iron chelator, in patients with
beta-thalassemia. Blood. 2006;107 (9):3455–3462. https://doi.org/10.1182/blood-2005-08-3430 PMid:16352812
- Cappellini
MD, Bejaoui M, Agaoglu L, et al. Iron chelation with deferasirox in
adult and pediatric patients with thalassemia major: efficacy and
safety during 5 years' follow-up. Blood. 2011; 118(4): 884-893. https://doi.org/10.1182/blood-2010-11-316646 PMid:21628399
- Cappellini
MD, Porter J, El-Beshlawy A, et al. Tailoring iron chelation by iron
intake and serum ferritin: the prospective epic study of deferasirox in
1744 patients with transfusion-dependent anemias. Haematologica.
2010;95(4):557–566. https://doi.org/10.3324/haematol.2009.014696 PMid:19951979 PMCid:PMC2857545
- Porter
JB, Elalfy MS, Taher AT, et al. Efficacy and safety of deferasirox at
low and high iron burdens: results from the epic magnetic resonance
imaging substudy. Ann Hematol. 2013;92(2):211–219. https://doi.org/10.1007/s00277-012-1588-x PMid:23086508 PMCid:PMC3542426
- Deugnier
Y, Turlin B, Ropert M, et al. Improvement in liver pathology of
patients with beta-thalassemia treated with deferasirox for at least 3
years. Gastroenterology. 2011;141(4):1202-1211, 1211.e1201-1203.
- Maggio
A, Filosa A, Vitrano A et al. Iron chelation therapy in thalassemia
major: A systematic review with meta-analyses of 1520 patients included
on randomized clinical trials. Blood Cells, Molecules, and Diseases
2011; 47: 166–175. https://doi.org/10.1016/j.bcmd.2011.07.002 PMid:21843958
- JaiSwal
S, Hishikar R, Khandwal O et al. Efficacy of Deferasirox as an Oral
Iron Chelator in Paediatric Thalassaemia Patients. Journal of Clinical
and Diagnostic Research. 2017 Feb, Vol-11(2): FC01-FC03.
- Vichinsky
E, El-Beshlawy A, AlZoebie A et al. Long term safety and efficacy of
deferasirox in young pediatric patients with transfusional
hemosiderosis: Results from a 5-year observational study (ENTRUST).
Pediatr Blood Cancer. 2017;64:e26507. https://doi.org/10.1002/pbc.26507 PMid:28296163
- Bollig
C, Schell LK, Rücker G et al. Deferasirox for managing iron overload in
people with Thalassaemia. Cochrane Database of Systematic Reviews
2017(8): 1-216. https://doi.org/10.1002/14651858.CD007476.pub3
- Wood
JC, Glynos T, Thompson A, Giardina P, Harmatz P, Kang BP, Paley C,
Coates TD. Relationship between labile plasma iron, liver iron
concentration and cardiac response in a deferasirox monotherapy trial.
Haematologica 2011; 96:1055–8. https://doi.org/10.3324/haematol.2010.032862 PMid:21393329 PMCid:PMC3128226
- Pennell
DJ, Porter JB, Cappellini MD, et al. Deferasirox for up to 3 years
leads to continued improvement of myocardial t2* in patients with
beta-thalassemia major. Haematologica. 2012;97(6):842–848. https://doi.org/10.3324/haematol.2011.049957 PMid:22271905 PMCid:PMC3366648
- Pennell
DJ, Porter JB, Piga A, et al. A Multicenter, Randomized, Open-Label
Trial Evaluating Deferasirox Compared with Deferoxamine for the Removal
of Cardiac Iron in Patients with β- Thalassemia Major and Iron Overload
(CORDELIA). Blood 2012;121(21).
- Ho P J, Tay L,
Teo J et al. Cardiac iron load and function in transfused
patients treated with deferasirox (the MILE study). Eur J Haem 2016; 9:
9-105.
- Poggi M, Sorrentino F, Pugliese P et
al. Longitudinal changes of endocrine and bone disease in adults with
β-thalassemia major receiving different iron chelators over 5 years.
Ann Hematol (2016) 95:757–763. https://doi.org/10.1007/s00277-016-2633-y PMid:26957357
- EXJADE® (deferasirox) US Prescribing Information. 2013. Available from http://www.pharma.us.novartis.com/product/pi/pdf/exjade.pdf
- Piga
A, Fracchia S, Lai ME, et al. Effect of deferasirox on renal
haemodynamics in patients with beta-thalassaemia: first interim
analysis [abstract]. Haematologica. 2010; 95 (Suppl 2): 1798.
- Taher
AT, Porter J, Viprakasit V, et al. Deferasirox reduces iron overload
significantly in non-transfusion dependent thalassemia: 1- year results
from a prospective, randomized, double-blind, placebo- controlled
study. Blood. 2012;120(5):970–977. https://doi.org/10.1182/blood-2012-02-412692 PMid:22589472
- Rafat
C, Fakhouri F, Ribeil JA, Delarue R, Le Quintrec M. Fanconi syndrome
due to deferasirox. American Journal of Kidney Diseases 2009;54
(5):931–4. https://doi.org/10.1053/j.ajkd.2009.03.013 PMid:19493602
- Osborne
V, Davies M, Layton D, Shakir AS. Utilisation and Safety of
Deferasirox: Results from an Observational Cohort Study in England.
Drug Saf (2018) 41:267–275. https://doi.org/10.1007/s40264-017-0606-2 PMid:29019038
- Bayhana
T, Ünal S, Ünlü O, Küçüker H, Tutal Ad, Karabulut E et al. The
questioning for routine monthly monitoring of proteinuria in patients
with β-thalassemia on deferasirox chelation. Hematology, 2017 VOL. 22,
NO. 4, 248–251.
- Origa R, Piga A, Tartaglione
I et al. Renal safety under long-course deferasirox therapy in iron
overloaded transfusion-dependent b- thalassemia and other anemias. AJH
2018; 1-3.
- Arandi N, Haghpanah S, Safaei S et
al. Combination therapy – deferasirox and deferoxamine – in thalassemia
major patients in emerging countries with limited resources.
Transfusion Medicine 2015, 25, 8–12. https://doi.org/10.1111/tme.12188 PMid:25801075
- Lal,
A., J. Porter, N. Sweeters, et al. 2013.Combined chelation therapy with
deferasirox and deferoxamine in thalassemia. Blood Cells Mol Dis. 50:
99–104. https://doi.org/10.1016/j.bcmd.2012.10.006 PMid:23151373 PMCid:PMC3592978
- Cassinerio,
E., N. Orofino, A. Roghi, et al. 2014. Combination of deferasirox and
deferoxamine in clinical practice: an alternative scheme of chelation n
in thalassemia major. Blood Cells Mol Dis. 53: 164–167. https://doi.org/10.1016/j.bcmd.2014.04.006 PMid:24846580
- Aydinok,
Y., A. Kattamis, M.D. Cappellini, et al. 2015. Effects of
deferasirox–deferoxamine onmyocardial and liver iron in patients
with severe transfusional iron overload. Blood 125: 3868–3877. https://doi.org/10.1182/blood-2014-07-586677 PMid:25934475 PMCid:PMC4490296
- Sridharan
K, Sivaramakrishnan G. Efficacy and safety of iron chelators in
thalassemia and sickle cell disease: a multiple treatment comparison
network meta-analysis and trial sequential analysis. Exp Rev Clinic
Pharmacol 2018; 11 (6): 641–650. https://doi.org/10.1080/17512433.2018.1473760 PMid:29727586
- Farmaki,
K., I. Tzoumari, C. Pappa. 2011. Oral chelators in
transfusion-dependent thalassemia major patients may prevent or reverse
iron overload complications. Blood Cells Mol. Dis. 47: 33–40. https://doi.org/10.1016/j.bcmd.2011.03.007 PMid:21531154
- Totadri,
S., D. Bansal, P. Bhatia, et al. 2015. The deferiprone and deferasirox
combination is efficacious in iron overloaded patients with
β-thalassemia major: a prospective, single center, open-label study.
Pediatr. Blood Cancer 62: 1592–1596. https://doi.org/10.1002/pbc.25533 PMid:25820920
- Elalfy,
M.S., A.M. Adly, Y. Wali, et al. 2015. Efficacy and safety of a novel
combination of two oral chelators deferasirox/deferiprone over
deferoxamine/deferiprone in severely iron overloaded young beta
thalassemia major patients. Eur J. Haematol. 95: 411–420. https://doi.org/10.1111/ejh.12507 PMid:25600572
- Karami
H, Kosaryan M, Amree Ah et al. Combination iron chelation therapy with
deferiprone and deferasirox in iron-overloaded patients with
transfusion dependent β-thalassemia major. Clinics and Practice 2017;7
(912): 11-14.
- Parakh N, Chandra J, Sharma S et
al. Efficacy and Safety of Combined Oral Chelation With Deferiprone and
Deferasirox in Children With b-Thalassemia Major: An Experience From
North India. J Pediatr Hematol Oncol 2017;39: 209–213. https://doi.org/10.1097/MPH.0000000000000780 PMid:28221264
- Pinto
VM, Balocco M, Quintino S, Bacigalupo L, Gianesin B, Rizzi M, Malagò R,
De Franceschi L, Forni GL. Daily alternating deferasirox and
deferiprone therapy successfully controls iron accumulation in
untreatable transfusion-dependent thalassemia patients. Am J Hematol.
2018 Jul 22. doi: 10.1002/ajh.25222. [Epub ahead of print]. https://doi.org/10.1002/ajh.25222
- Inati
A, kahale M, Sbeiti N, Cappellini MD, Taher AT, kousa S, Nasr T,
Musalem KM, Abbas HA, Porter J. One‐year results from a prospective
randomized trial comparing phlebotomy with deferasirox for the
treatment of iron overload in pediatric patients with thalassemia major
following curative stem cell transplantation. Pediatr Blood Cancer
2017; 64: 188-196. https://doi.org/10.1002/pbc.26213 PMid:27576370
- Yesilipek
MA, Karasu G, Kaya Z, Kuskonmaz BB, Uygun V, Dag I, Ozudogru, ertem M.
A Phase II, Multicenter, Single-Arm Study to Evaluate the Safety and
Efficacy of Deferasirox after Hematopoietic Stem Cell Transplantation
in Children with β-Thalassemia Major. Biol Blood Marrow
Transplant 24 (2018) 608–632. https://doi.org/10.1016/j.bbmt.2017.11.006 PMid:29155313
- Musallam
KM, Rivella S, Vichinsky E, Rachmilewitz EA. Non- transfusion-dependent
thalassemias. Haematologica. 2013;98(6):833- 44. https://doi.org/10.3324/haematol.2012.066845 PMid:23729725 PMCid:PMC3669437
- Taher
AT, Porter JB, Viprakasit V, Kattamis A, Chuncharunee S, Sutcharitchan
P, et al. Deferasirox effectively reduces iron overload in
non-transfusion-dependent thalassemia (NTDT) patients: 1-year extension
results from the THALASSA study. Ann Hematol. 2013;92(11):1485-93. https://doi.org/10.1007/s00277-013-1808-z PMid:23775581 PMCid:PMC3790249
- Taher
AT, Cappellini MD, Aydinok Y, Porter JB, Karakas Z, Viprakasit V, et
al. Optimising iron chelation therapy with deferasirox for
non-transfusion-dependent thalassaemia patients: 1-year results from
the THETIS study. Blood Cells Mol Dis. 2016; 57:23-9. https://doi.org/10.1016/j.bcmd.2015.11.002 PMid:26852651
- Calvaruso
G, Vitrano A, Di Maggio R, Lai E, Colletta G, Quota A, et al.
Deferiprone versus deferoxamine in thalassemia intermedia: Results from
a 5-year long-term Italian multicenter randomized clinical trial. Am J
Hematol. 2015;90(7):634-8. https://doi.org/10.1002/ajh.24024 PMid:25809173
- Taher
A, Vichinsky E, Musallam K, Cappellini M-D, Viprakasit V. Guidelines
for the management of non-transfusion dependent thalassaemia (NTDT):
Thalassaemia International Federation, Nicosia, Cyprus; 2013.
- Delea
TE, Edelsberg J, Sofrygin O, Thomas SK, Baladi JF, Phatak PD, et al.
Consequences and costs of noncompliance with iron chelation therapy in
patients with transfusion-dependent thalassemia: a literature review.
Transfusion. 2007;47(10):1919-29. https://doi.org/10.1111/j.1537-2995.2007.01416.x PMid:17880620
- Gabutti V, Piga A. Results of long-term iron-chelating therapy. Acta Haematol. 1996;95(1):26-36. https://doi.org/10.1159/000203853 PMid:8604584
- Trachtenberg
FL, Gerstenberger E, Xu Y, Mednick L, Sobota A, Ware H, et al.
Relationship among chelator adherence, change in chelators, and quality
of life in thalassemia. Qual Life Res. 2014; 23(8):2277-88. https://doi.org/10.1007/s11136-014-0671-2 PMid:24682717 PMCid:PMC4315322
- Cappellini
MD, Bejaoui M, Agaoglu L, Porter J, Coates T, Jeng M, et al.
Prospective evaluation of patient-reported outcomes during treatment
with deferasirox or deferoxamine for iron overload in patients with
beta-thalassemia. Clin Ther. 2007;29(5):909-17. https://doi.org/10.1016/j.clinthera.2007.05.007 PMid:17697909
- Fortin
PM, Fisher SA, Madgwick KV, Trivella M, Hopewell S, Doree C, Estcourt
LJ. Interventions for improving adherence to iron chelation therapy in
people with sickle cell disease or thalassaemia (Review). Cochrane
Database of Systematic Reviews 2018, Issue 5. Art. No.: CD012349. DOI:
10.1002/14651858.CD012349.pub2 https://doi.org/10.1002/14651858.CD012349.pub2
- Porter
J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Cappellini MD, et
al. Relative response of patients with myelodysplastic syndromes and
other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr
prospective study. Eur J Haematol. 2008;80(2):168- 76. PMid:18028431
PMCid:PMC2268958
- Vichinsky E, Onyekwere O,
Porter J, Swerdlow P, Eckman J, Lane P, et al. A randomised comparison
of deferasirox versus deferoxamine for the treatment of transfusional
iron overload in sickle cell disease. Br J Haematol. 2007;136(3):501-8.
https://doi.org/10.1111/j.1365-2141.2006.06455.x PMid:17233848 PMCid:PMC1974786
- Taher
AT, Origa R, Perrotta S, Kourakli A, Ruffo GB, Kattamis A, et al. New
film-coated tablet formulation of deferasirox is well tolerated in
patients with thalassemia or lower-risk MDS: Results of the randomized,
phase II ECLIPSE study. Am J Hematol. 2017;92(5):420-8. https://doi.org/10.1002/ajh.24668 PMid:28142202
- Pennell
DJ, Porter JB, Cappellini MD, et al. Efficacy of deferasirox in
reducing and preventing cardiac iron overload in beta thalassemia.
Blood. 2010; 115(12): 2364-2371. https://doi.org/10.1182/blood-2009-04-217455 PMid:19996412
- Pennell
DJ, Porter JB, Cappellini MD, et al. Continued improvement in
myocardial T2* over two years of deferasirox therapy in beta-
thalassemia major patients with cardiac iron overload. Haematologica.
2011; 96(1): 48-54. https://doi.org/10.3324/haematol.2010.031468 PMid:21071497 PMCid:PMC3012764
- Pennell
DJ, Porter JB, Piga A et al. Sustained improvements in myocardial T2*
over 2 years in severely iron-overloaded patients with beta thalassemia
major treated with deferasirox or deferoxamine. Am J Hematol. 2015;
90(2):91-6. https://doi.org/10.1002/ajh.23876 PMid:25345697
[TOP]


