Jochen J Frietsch1*, Detlef Michel2*, Thomas Stamminger2, Friederike Hunstig1, Sebastian Birndt1, Ulf Schnetzke1, Sebastian Scholl1, Andreas Hochhaus1 and Inken Hilgendorf1.
* Both authors contributed equally.
1 Klinik für Innere Medizin II, Hämatologie und internistische Onkologie, Universitätsklinikum Jena, Jena, Germany.
2 Institut für Virologie, Universitätsklinikum Ulm, Ulm, Germany.
Published: January 1, 2019
Received: September 21, 2018
Accepted: November 14, 2018
Mediterr J Hematol Infect Dis 2019, 11(1): e2019001 DOI
10.4084/MJHID.2019.001
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
CMV
associated tissue-invasive disease is associated with a considerable
risk of morbidity and mortality after allogeneic hematopoietic stem
cell transplantation (HSCT). Recently, the terminase inhibitor
letermovir (LMV) has been approved for prophylaxis of CMV infection in
HSCT. We hereby report a 60-year-old female experiencing CMV
reactivation after HSCT in a CMV seronegative donor-constellation. Due
to ongoing elevated CMV viral load and drug-associated
myelosuppression, which prevented ganciclovir therapy, treatment was
replaced by foscarnet. Due to nephrotoxicity, foscarnet was switched to
LMV. The patient developed skin GvHD and prednisolone was started.
Subsequently, CMV viremia worsened despite LMV therapy. Genotyping
revealed the mutation C325Y of the CMV UL56 terminase being associated
with high-level resistance against LMV. Prolonged uncontrolled
low-level viremia due to prednisolone treatment may have favored the
selection of drug-resistant CMV. Despite the excellent toxicity profile
of LMV, physicians should be aware of risk factors for the emergence of
resistance.
|
Introduction
Allogeneic
hematopoietic stem cell transplantation (HSCT) remains the only
curative treatment for a huge variety of malignant and even
non-malignant diseases.[1] Viral infections and
reactivations, especially of cytomegalovirus (CMV), and its associated
tissue-invasive disease, remain a serious complication following HSCT.[2]
For a long time, the antiviral drugs ganciclovir (GCV)/valganciclovir,
foscarnet (FOS), cidofovir and acyclovir/valacyclovir have been used
for prophylaxis or pre-emptive therapy but were limited by side effects
and/or the selection of viral mutations that confer antiviral drug
resistance. Recently the armamentarium has been widened by the
administration of maribavir, brincidofovir, and letermovir (LMV).
Targeting the subunit UL56 of the terminase enzyme complex, LMV
specifically inhibits the cleavage and packaging of newly synthesized
viral DNA.[3] A recent phase III trial demonstrated
that LMV prophylaxis after allogeneic HSCT resulted in a significantly
lower risk of active CMV infection compared to placebo.[4]
In addition, LMV seems to be well tolerated without the risk of myelo-
or nephrotoxicity. However, experimental in vitro data suggested that
LMV may possess a low genetic barrier to resistance.[5]
Here we report the case of a patient after allogeneic HSCT with
prolonged CMV viremia with a C325Y mutation-based resistance to LMV,
being selected in vivo.
Case Presentation
We
report a 60-year-old, CMV seropositive female patient with acute
myeloid leukemia (AML). Cytogenetics revealed trisomy 8 in 3 out of 10
metaphases. Verification of mutations in ASXL1, EZH2 and NPM1 led to
high risk classification according to European LeukemiaNet ELN
guidelines.[6] Administering induction and
consolidation chemotherapy (cytarabine 1.000 mg/m², bid, on day 1, 3, 5
and 7, idarubicin 12 mg/m², qd, on days 1-3) resulted in achieving
complete remission. After conditioning therapy with treosulfan (day -6
to -4 at doses of 14 g/m², qd), fludarabine (day -6 to -2 at doses of
30 mg/m², qd) and anti-thymocyte globulin (Grafalon®; neovii Biotech,
days -4 to -2 at a dose of 20 mg/kg body weight (bwt)) the patient
received 6.06 x 106 CD34+ peripheral
blood stem cells/kg bwt from a 24-year-old, unrelated, male, human
leukocyte antigen allele mismatched 9/10, EBV seropositive, CMV
seronegative donor. Prophylaxis of Graft-versus-host disease (GvHD)
consisted of cyclosporine (CsA), starting on day -1, combined with
methotrexate (MTX). CsA was maintained at therapeutic plasma levels.
Acyclovir was administered as antiviral prophylaxis.
Loads of
CMV were routinely monitored once a week by PCR technique using
whole-blood (for detailed information about viral copy numbers see figure 1). CMV reactivation with 7 x 104
copies/ml was detected on day +43. Despite a change in antiviral
medication to GCV at a dosage of 5 mg/kg bwt, bid, viral load kept on
increasing up to 4.8 x 105 copies/ml
after ten days of initiation of treatment. The viral load finally
decreased underuse of FOS at a dosage of 90 mg/kg bwt bid and early but
slow reduction of immunosuppressive therapy. Additionally, we
administered 1 ml/kg bwt CMV Immunoglobulins (Cytotect® CP Biotest) on
day +48. The CMV-treatment schedule is given in figure 1.
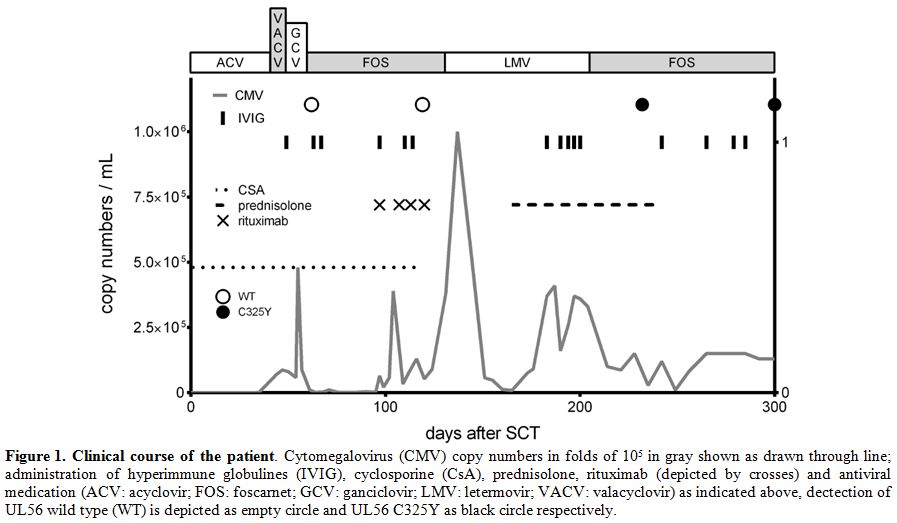 |
Figure 1. Clinical course of the patient.
Cytomegalovirus (CMV) copy numbers in folds of 105 in gray shown as
drawn through line; administration of hyperimmune globulines (IVIG),
cyclosporine (CsA), prednisolone, rituximab (depicted by crosses) and
antiviral medication (ACV: acyclovir; FOS: foscarnet; GCV: ganciclovir;
LMV: letermovir; VACV: valacyclovir) as indicated above, dectection of
UL56 wild type (WT) is depicted as empty circle and UL56 C325Y as black
circle respectively. |
Due
to delayed engraftment, several bone marrow aspirates were obtained,
revealing increasing chimerism from 89% on day +30, 94% on day +54 up
to 100% since day +82. As a result of minimal residual disease (MRD) of
AML, we quickly reduced CsA. This resulted in decreasing leukemia
associated molecular markers. However, CMV copy numbers raised from
1.5-3.5 x 103 copies/ml up to 3.9 x 105 copies/ml (please refer to figure 1, day 88 et seq.) and EBV reactivation (up to 0.83-1.65 x 105)
despite sustained administration of FOS. Except for nephrotoxicity, no
clinical side effects of FOS occurred. EBV reactivation was effectively
treated with the monoclonal CD20 antibody rituximab throughout four
weeks, at a dosage of 375 mg/m² per week (Figure 1).[7]
At
the same time, CMV copy number increased despite the continuation of
treatment with FOS. Therefore, we attempted to exclude the existence of
viral mutations by DNA sequencing following nested PCR amplification.
With modifications, amplification and sequencing of UL56 were performed
as described previously.[8] The method allows
identification of the UL56 coding region from amino acids 1 to 620.
Albeit clinically expected, verifying the viral kinase UL97 and the
viral polymerase UL54 as wild-type, no mutation conveying resistance
was demonstrable. A retrospective analysis revealed no mutation in the
viral terminase region UL56, too. As a consequence, administration of
CsA was terminated at day +118.
Based on delayed engraftment,
drug-associated myelotoxicity and nephrotoxicity, and prolonged
hospitalization, we initiated LMV at a dose of 480 mg qd. The patient
was discharged from stationary treatment, and LMV resulted in an
increase first, and within a treatment period of five weeks in an
impressive decrease of CMV copy numbers (from 90.000 at LMV initiation
up to 1.000.000 to 8.200 per milliliter) as already reported for other
cases.[9] Due to the occurrence of herpes stomatitis,
acyclovir was administered, adapted to renal function. Simultaneously,
the patient developed a maculopapular rash on day +155 affecting the
lower arms and the abdominal skin. Subsequently, a skin biopsy was
performed, and suspected acute GvHD confirmed by histology.
Consequently,
prednisolone 25 mg qd was started at day +165 in the absence of any
signs of gastrointestinal or liver involvement of GvHD and tapered
without recurrence of acute GvHD afterward. However, under the
intensified immunosuppression the viral load increased up to 410.000
copies per milliliter despite the continuation of LMV treatment. Upon
genotyping, mutation C325Y (cytosine at amino acid position 325 was
substituted by tyrosine) was detected within UL56 which is supposed to
confer high-level resistance to LMV.[10]
Consequently,
administration of LMV was stopped, and FOS application, adapted to
renal function, 3.000 mg bid commenced once again in combination with
administration of CMV-hyperimmune globulin. This change in antiviral
treatment resulted in a decrease of viral loads. On day +292, the
patient is alive in complete remission of AML without signs of GvHD or
clinical signs of active CMV infection, still receiving FOS without any
side effects. Of note, during the whole course of treatment, CMV was
below 1 x 103 copies/ml only until day +42, between day +63 and +67 as well as day +75 and +84 after HSCT.
Discussion and Conclusion
CMV
associated tissue-invasive disease (e.g., pneumonitis, retinitis) is
known to cause significant morbidity and mortality in patients after
allogeneic HSCT.[2] However, CMV replication may also
exert anti-leukemic effects after HSCT in AML patients. Based on that
conflict, physicians have to choose appropriate antiviral strategies.[11]
Side
effects like nephrotoxicity, electrolyte disturbances, and
myelotoxicity sometimes restrict the treatment with distinct antiviral
drugs. Concerning its safety profile, the newly approved drug LMV
appears to be superior to other anti-cytomegaloviral substances.
However, since LMV specifically interferes with cleavage and packaging
of viral DNA, without affecting viral DNA replication, this may result
in prolonged detection of CMV DNA after the initiation of LMV therapy.[5]
Furthermore, the case presented in this study underlines that LMV is
highly specific for CMV without an inhibitory effect on related
herpesviruses such as HSV or VZV. Consequently, concomitant prophylaxis
with acyclovir is compulsory in order to prevent disease due to HSV/VZV
reactivation as observed by the HSV-associated stomatitis in our
patient.
Experimental in vitro data suggested an early selection
of cytomegaloviruses with resistance-associated mutations in the
presence of LMV. Thus, it was proposed that CMV may exhibit a low
genetic barrier towards LMV resistance development necessitating
continuous surveillance during treatment.[12] So far, the UL56 V236M mutation has been selected in vivo during two clinical trials.[5,8]
The authors of a subsequent phase III trial stated that the development
of breakthrough CMV viremia with confirmed UL56 mutations had been
observed.[4,13] DNA sequence
analysis of the UL56 and UL89 coding regions was performed on samples
obtained from 28 letermovir-treated patients who had received at least
one dose of study drug and experienced prophylaxis failure. Two
patients were identified as having a letermovir-resistance
substitution, pUL56 V236M or C325W. These substitutions were identified
from on-treatment samples (www.accessdata.fda.gov, Reference ID 4179078, ClinicalTrials.gov Identifier: NCT02137772).[14]
Here, we report for the first time in vivo cytomegalovirus carrying the
UL56 mutation C325Y, which was detected by CMV genotyping upon rapidly
increasing viral loads in a patient under LMV treatment. In vitro data
indicate that this mutation is associated with high-grade LMV
resistance increasing the 50% effective concentration of LMV
>5.000-fold.[12,13] In line with the published risk factors by El Chaer et al.,[15]
it is tempting to suggest, that prolonged uncontrolled low-level CMV
viremia might have favored the emergence of letermovir resistance.
In
the future, the combination of antiviral drugs with different
mechanisms of action may be used synergistically to reduce the
incidence of mutations and side effects. In addition, transfer of ex
vivo-generated CMV-specific T-cells can suppress CMV-reactivation by
re-establishing functional antiviral immune responses in
immunocompromised hosts.[16]
References
- Mohty B, Mohty M. Long-term complications and side
effects after allogeneic hematopoietic stem cell transplantation: an
update. Blood Cancer J. 2011 Apr;1(4):e16. https://doi.org/10.1038/bcj.2011.14
- Gandhi
MK, Khanna R. Human cytomegalovirus: clinical aspects, immune
regulation, and emerging treatments. Lancet Infect Dis.
2004;4(12):725-38. https://doi.org/10.1016/S1473-3099(04)01202-2
- Frange
P, Leruez-Ville M. Maribavir, brincidofovir and letermovir: Efficacy
and safety of new antiviral drugs for treating cytomegalovirus
infections. Med Mal Infect. 2018. https://doi.org/10.1016/j.medmal.2018.03.006 PMid:29650261
- Marty
FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al.
Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell
Transplantation. N Engl J Med. 2017;377(25):2433-44. https://doi.org/10.1056/NEJMoa1706640 PMid:29211658
- Razonable
RR. Role of letermovir for prevention of cytomegalovirus infection
after allogeneic haematopoietic stem cell transplantation. Curr Opin
Infect Dis. 2018. https://doi.org/10.1097/QCO.0000000000000459 PMid:29746444
- Dohner
H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al.
Diagnosis and management of AML in adults: 2017 ELN recommendations
from an international expert panel. Blood. 2017;129(4):424-47. https://doi.org/10.1182/blood-2016-08-733196 PMid:27895058 PMCid:PMC5291965
- Styczynski
J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C,
et al. Management of Epstein-Barr Virus infections and post-transplant
lymphoproliferative disorders in patients after allogeneic
hematopoietic stem cell transplantation: Sixth European Conference on
Infections in Leukemia (ECIL-6) guidelines. Haematologica.
2016;101(7):803-11. https://doi.org/10.3324/haematol.2016.144428 PMid:27365460 PMCid:PMC5004459
- Lischka
P, Michel D, Zimmermann H. Characterization of Cytomegalovirus
Breakthrough Events in a Phase 2 Prophylaxis Trial of Letermovir
(AIC246, MK 8228). J Infect Dis. 2016;213(1):23-30. https://doi.org/10.1093/infdis/jiv352 PMid:26113373
- Stoelben
S, Arns W, Renders L, Hummel J, Muhlfeld A, Stangl M, et al. Preemptive
treatment of Cytomegalovirus infection in kidney transplant recipients
with letermovir: results of a Phase 2a study. Transpl Int.
2014;27(1):77-86. https://doi.org/10.1111/tri.12225 PMid:24164420
- Ligat
G, Cazal R, Hantz S, Alain S. The human cytomegalovirus terminase
complex as an antiviral target: a close-up view. FEMS Microbiol Rev.
2018;42(2):137-45. https://doi.org/10.1093/femsre/fuy004 PMid:29361041 PMCid:PMC5972660
- Elmaagacli
AH, Koldehoff M. Cytomegalovirus replication reduces the relapse
incidence in patients with acute myeloid leukemia. Blood.
2016;128(3):456-9. https://doi.org/10.1182/blood-2016-04-713644 PMid:27216219
- Chou
S. Rapid In Vitro Evolution of Human Cytomegalovirus UL56 Mutations
That Confer Letermovir Resistance. Antimicrob Agents Chemother.
2015;59(10):6588-93. https://doi.org/10.1128/AAC.01623-15 PMid:26259791 PMCid:PMC4576131
- Goldner
T, Hempel C, Ruebsamen-Schaeff H, Zimmermann H, Lischka P. Geno- and
phenotypic characterization of human cytomegalovirus mutants selected
in vitro after letermovir (AIC246) exposure. Antimicrob Agents
Chemother. 2014;58(1):610-3. https://doi.org/10.1128/AAC.01794-13 PMid:24189264 PMCid:PMC3910730
- Letermovir
(MK-8228) Versus Placebo in the Prevention of Clinically-Significant
Cytomegalovirus (CMV) Infection in Adult, CMV-Seropositive Allogeneic
Hematopoietic Stem Cell Transplant Recipients
(MK-8228-001) Available from: https://ClinicalTrials.gov/show/NCT02137772
- El
Chaer F, Shah DP, Chemaly RF. How I treat resistant cytomegalovirus
infection in hematopoietic cell transplantation recipients. Blood.
2016. http://www.bloodjournal.org/content/bloodjournal/early/2016/10/19/blood-2016-06-688432.full.pdf
- Gary
R, Aigner M, Moi S, Schaffer S, Gottmann A, Maas S, et al.
Clinical-grade generation of peptide-stimulated CMV/EBV-specific T
cells from G-CSF mobilized stem cell grafts. J Transl Med.
2018;16(1):124. https://doi.org/10.1186/s12967-018-1498-3 PMid:29743075 PMCid:PMC5941463
[TOP]