Alessandro Re, Chiara Cattaneo and Giuseppe Rossi.
Ematologia, Spedali Civili di Brescia.
Correspondence to: Alessandro Re, Ematologia, Spedali Civili di
Brescia, Piazzale Spedali Civili n 1, 25123 Brescia, Italy. Tel:
+390303995438, Fax: +300303996135. E-mail:
alessandro.re@asst-spedalicivili.it
Published: January 1, 2019
Received: September 12, 2018
Accepted: November 23, 2018
Mediterr J Hematol Infect Dis 2019, 11(1): e2019004 DOI
10.4084/MJHID.2019.004
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Patients
infected with human immunodeficiency virus (HIV) are at increased risk
for developing both non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma
(HL). Even if this risk has decreased for NHL after the introduction of
combination antiretroviral therapy (cART), they remain the most common
acquired immune deficiency syndrome (AIDS)-related cancer in the
developed world. They are almost always of B-cell origin, and some
specific lymphoma types are more common than others. Some of these
lymphoma types can occur in both HIV-uninfected and infected patients,
while others preferentially develop in the context of AIDS.
HIV-associated lymphoma differs from lymphoma in the HIV negative
population in that they more often present with advanced disease,
systemic symptoms, and extranodal involvement and are frequently
associated with oncogenic viruses (Epstein-Barr virus and/or human
herpesvirus-8). Before the introduction of cART, most of these patients
could not tolerate the treatment strategies routinely employed in the
HIV-negative population. The widespread use of cART has allowed for the
delivery of full-dose and dose-intensive chemotherapy regimens with
improved outcomes that nowadays can be compared to those seen in
non-HIV infected patients. However, a great deal of attention should be
paid to opportunistic infections and other infectious complications,
cART-chemotherapy interactions, and potential cumulative toxicity. In
the context of relatively sparse prospective and randomized trials, the
optimal treatment of AIDS-related lymphomas remains a challenge,
particularly in patients with severe immunosuppression. This paper will
address epidemiology, pathogenesis, and therapeutic strategies in
HIV-associated NHL and HL.
|
Introduction
Since
the beginning of the acquired immune deficiency syndrome (AIDS)
epidemic, in the early eighties, the association between lymphomas and
the acquired immunodeficiency became evident and was reported before
the discovery of human immunodeficiency virus (HIV) as the responsible
agent for the syndrome. Diffuse large B-cell lymphoma (DLBCL), Burkitt
lymphoma (BL) and primary central nervous system lymphoma (PCNSL) were
soon recognized as AIDS-defining event in patients who lived with HIV
infection (PLWH).[1] Most of these patients could not tolerate the
dosage of chemotherapy (CT) routinely employed in the HIV-negative
population, and the majority died of these diseases. After the advent
of combination antiretroviral therapy (cART) in 1996, the death rate
from AIDS dramatically decreased as the risk of new opportunistic
infections and the incidence of Kaposi’s Sarcoma (KS). The incidence of
lymphomas, however, did not decrease as sharply, and they became the
most common AIDS-related cancer in the developed world.[2] The
widespread use of cART has given PLWH the opportunity to receive and
tolerate a standard dose of CT and has increased the probability of
cure. However, in the context of relatively sparse prospective and
randomized trials, the optimal treatment of AIDS-related lymphomas
(ARL) remains a challenge, particularly in patients with severe
immunosuppression. In this review, we report the main information
concerning epidemiology and pathogenesis of ARL and summarize the
therapeutic strategies in Hodgkin (HL) and non-Hodgkin lymphoma (NHL),
analyzing the lymphoma subtypes individually. We also briefly discuss
some specific aspects of ARL clinical management, such as the use of
concomitant cART, infectious prophylaxis, and prophylaxis of central
nervous system (CNS) involvement by NHL. We also describe the main
results with autologous (ASCT) and allogeneic stem cell transplantation
(AlloSCT).
Epidemiology
ARL
usually present with advanced-stage disease and follow an aggressive
clinical course. They are almost always of B-cell origin, and some
specific lymphoma types are more common than others.[3] Some of these
lymphoma types can occur in both HIV-uninfected and infected patients,
while others preferentially develop in the context of AIDS or in
patients with other immunodeficiencies (Table 1).[4]
In an early phase of the HIV epidemic, the relative risk to develop NHL
for AIDS patients was >100-fold higher compared to the general
population.[5,6] After entering the cART era, the incidence of ARL has
substantially decreased; however, they remain clearly higher than in
the general population.[7] In Italy, 500 fold higher risk to develop
NHL than in the general population was reported in persons with AIDS
between 1986-1996 and 90 fold higher between 1997-2004.[8] Actually, a
wide range of increased risks for lymphoma has been reported in
population-based studies, mainly depending on the population under
observation and the calendar years examined. In the latest studies
conducted in Switzerland and in the USA the relative increase in
patients with HIV/AIDS appears lower, ranging between 10-20 fold higher
than in the general population.[9,10] A consortium of North American
cohorts estimated that the probability to develop NHL (i.e., cumulative
incidence) among PLWH in the cART era is 4%, even if it appears
declining across 96-2009.[11] However, the advent of cART had a
different impact on the epidemiology of the various subtypes of NHL.
While PCNSL dramatically decreased, the decline in DLBCL incidence was
less impressive, and BL was not substantially affected.[7-10] The
incidence of classical HL in PLWH is approximately 5 fold to 20 fold
higher than in the general HIV negative population, and the risk of HL
has remained stable or even increased since the introduction of
cART.[12] Even in the cART era, it appears that people with AIDS and
NHL or HL have a significantly reduced survival in comparison with an
HIV-negative population with the same diseases.[13-15] Report from the
Italian Cancer Registry showed, for the period 1996-2005, 5-year
survival of 64% among HIV-uninfected patients with NHL, compared to 25%
among AIDS patients with NHL, and respectively 86% vs. 42% among
patients with HL.[13]
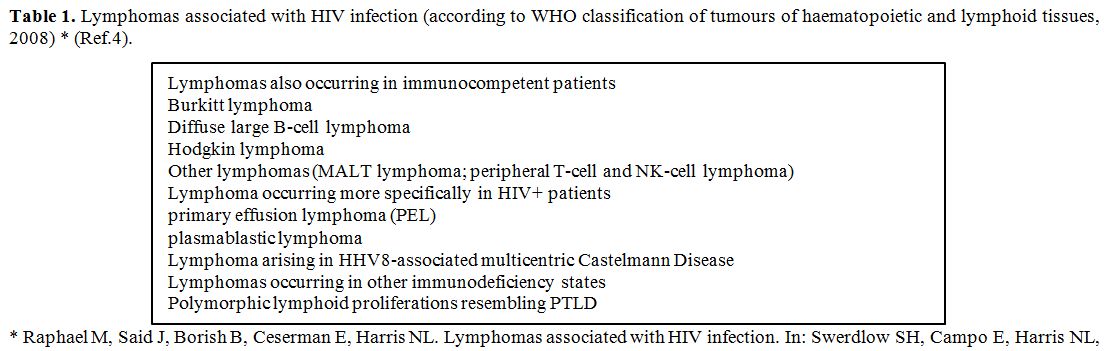 |
Table 1. Lymphomas
associated with HIV infection (according to WHO classification of
tumours of haematopoietic and lymphoid tissues, 2008) * (Ref.4). |
Pathogenesis
While
it is clear that HIV increases the lymphoma risk, there is no evidence
that HIV infection by itself leads to cell transformation.[16] Only
recently a possible direct effect of HIV through secreted or
transmitted viral proteins has been hypothesised: some experimental
evidence support oncogenic functions of HIV Tat, and specific variants
of HIV p17 has been found to be associated with the development of
lymphoma.[17,18] However, HIV does not infect the lymphoma cells and is
thought to have mainly an indirect role in lymphomagenesis, primarily
causing immunosuppression, with the consequent attenuation of tumor
surveillance. Indeed, an inverse association between CD4+ cell count
and NHL onset has been demonstrated by several studies.[19,20] However,
as the risk of lymphoma in PLWH remains high even after the widespread
use of cART, the relationship between immune status and lymphoma
development appears more complex. HIV-associated DLBCL and PCNSL
are often associated with Epstein-Barr virus (EBV) infection and tend
to occur when immunosuppression is more pronounced.
In contrast,
HIV-associated BL tends to occur earlier in the course of the illness
when CD4 counts are somewhat better preserved.[21] HL occurs with
relatively high frequency during the first few months after initiation
of cART as the CD4 cell counts are increasing, suggesting that HL may
be driven by immune recovery rather than by cell count depletion, at
least in some cases.[22] Anyway, while elimination of HIV from
peripheral blood can be achieved with cART, viral replication can still
occur in lymphoid tissues.[23] Moreover, the specific and direct role
of other oncogenic viruses, such as EBV and human herpesvirus-8
(HHV-8), in ARL pathogenesis is well documented, and most lymphomas
with excess risk among PLWH are associated with these virus infections (Table 2).
The incidence of some EBV-associated lymphomas, including BL and HL,
remains high in the cART era and rates of HHV8-associated primary
effusion lymphoma (PEL) and multicenter Castleman’s disease (MCD) are
unaffected by the use of cART.[24] It is also known that chronic
inflammation could contribute to lymphomagenesis; in clinical
observation, even with long-term virological suppression, inflammatory
biomarkers remain at high levels in HIV-infected people.[25] In
conclusion, HIV creates an environment in which chronic antigen
stimulation, cytokine dysregulation, and coinfection with oncogenic
viruses, in the background of genetic abnormalities and disrupted
immune surveillance to tumor antigens, can lead to the emergence of
monoclonal B cells.[26,27]
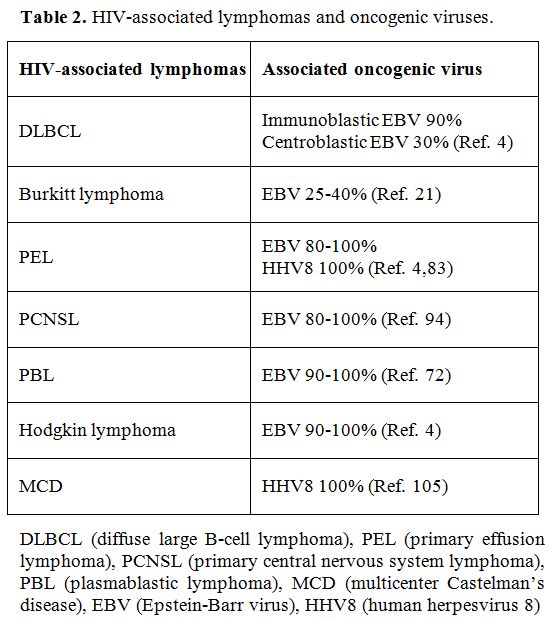 |
Table 2. HIV-associated lymphomas and oncogenic viruses. |
Clinical Management
After
a diagnosis of an ARL, in addition to the usual evaluation of lymphoid
malignancy, assessment of HIV disease status including CD4 cell counts,
HIV viral load, sensitivity of the virus to available antiretroviral
drugs and prior history of AIDS-related complications is necessary.
Understanding the prospects for successful long-term HIV management is
essential. A patient with a high viral load and poor immune function,
who is cART naïve, is likely to respond well to cART and have fewer
complications of CT compared with a patient who has resistant HIV as a
result of having had multiple cART regimens.
Combination of HIV treatment and chemotherapy.
Several HIV medications and CT agents have overlapping side effects,
such as renal and hepatic toxicity, myelosuppression and peripheral
neuropathy.[28] In addition, many CT drugs and HIV medications are
metabolized through the cytochrome p450 (CYP) enzyme system of the
liver. The cART can augment or inhibit the clearance of CT agents and
which can lead to either increased CT-associated toxicity or a decrease
in treatment efficacy.[29,30] Notably, the HIV protease inhibitor
ritonavir is a particularly potent inhibitor of the CYP system that can
diminish the clearance of vinca alkaloids and should be avoided during
ABVD therapy for HL.[29] Several authors propose antiretroviral
discontinuation during lymphoma treatment.[31] However, a retrospective
analysis of the trial AMC034 showed that in patients treated with
concurrent cART dose adjusted-EPOCH + rituximab (R-DA-EPOCH) is
well-tolerated and allows for faster recovery of immune function
compared to consecutive CT and cART.[32] A meta-analysis of 1546
patients with HIV-associated NHL demonstrated that concurrent cART and
CT was associated with statistically improved complete remission (CR)
rates with a trend toward improved overall survival (OS).[33]
Currently, it is suggested that all HIV-infected patients with
malignancies should continue cART during CT.[29,30] There is some
evidence of the detrimental effect of protease inhibitor (PI)-based
cART, due to excess of toxicity and the use of integrase inhibitors
might bring advantages concerning drug-drug interactions and allows for
a faster decline of the viremia.[34]
Infection prophylaxis.
No comparative studies exist, and only one guideline has been published
for opportunistic infections (OI) prophylaxis in HIV-associated
malignancies.[35] Cotrimoxazole prophylaxis against P. jiroveci
pneumonia and toxoplasmosis should be administered during
immuno-suppressive treatment regardless of the CD4 cell count.[36]
Other infections’ prophylaxis is generally recommended at least in
particular circumstances (low CD4 count, prolong and profound
neutropenia, prolonged use of steroids).[37]
Diffuse large B cell lymphoma.
DLBCL, the most frequent ARL, often presents at an advanced stage and
with B symptoms and extranodal tissue is frequently involved, mainly in
severely immunosuppressed patients. Prognosis is determined by
patient-, lymphoma- and HIV-specific factors. The International
Prognostic Index (IPI) has been extensively validated and remain a
reliable predictor of outcomes. Low CD4 counts have been reported as
predictors of poor survival in several studies, while others have not
found such an association, especially in the cART era.[38,39] An
AIDS-related lymphoma IPI has been recently developed, that employs the
Age Adjusted-IPI and an HIV severity score incorporating CD4 count,
viral load, and prior history of AIDS to risk-stratify ARL.[40] No
consensus has emerged in the HIV setting for distribution and relation
to outcome of biologically distinct subtypes of DLBCL, germinal center
B-cell and activated B-cell[41-44] and the proportion and outcome of
“double hit” (characterized by rearrangement of c-myc and either bcl-2
or bcl-6) and “double-expresser” DLBC lymphoma (overexpression of c-myc
and bcl-2) has not been extensively studied. Treatment recommendations
for DLBCL in HIV infected patients are mostly based on evidence from
phase 2 trials, retrospective series or expert opinion. Interpretation
of the literature is complicated by the fact that in many early studies
patients with different subtypes of aggressive NHL were all treated
with the same regimens and frequently composite outcomes were reported.
A significant positive impact on outcomes for HIV-related DLBCL has
been reported after the introduction of cART. North American and
European cooperative group trials reported CR rates of 48-63% and
1-year overall survival (OS) of 60-80% with CHOP in the cART
era.[45,46] Moreover, infusional regimens were explored; CDE results
were in line with what seen with CHOP,[47] while 39 patients (79% with
DLBCL and 18% BL) treated at National Cancer Institute (NCI) with
DA-EPOCH, obtained a CR rate of 74%, and a median OS of 60%, comparable
with HIV negative population treated with the same regimen at NCI at
the same time.[31] After successful addition of the CD20-directed
monoclonal antibody, rituximab to CHOP therapy in HIV negative
patients, a direct comparison of CHOP vs. rituximab-CHOP (R-CHOP) in
HIV-associated NHL have been conducted in the AMC010 trial. One hundred
and fifty HIV-positive patients with intermediate and high-grade
CD20-pos NHL (80% of patients had DLBCL) were randomized in a 1:2
fashion to receive either CHOP or R-CHOP followed by three-monthly
rituximab maintenance. Despite higher CR/CR unconfirmed rate (58% vs.
47%), and less lymphoma-related deaths in the R-CHOP arm (14% vs. 29%),
there were no statistical differences in progression-free survival
(PFS) (median t10 months vs. 9) and OS (32 months vs. 25). A possible
explanation for the lack of benefit from rituximab might be the high
treatment-related mortality (14% in the rituximab arm vs. 2%; P=0.03),
which was particularly high (36%) for pts with CD4 count < 50/mcL in
R-CHOP arm. Moreover, 40% of the infectious deaths was during rituximab
maintenance, and in this study routine neutropenic antibiotic
prophylaxis was not employed.[48] Several phase 2 trials along with a
pooled analysis from 19 trials demonstrated that the addition of
rituximab to the CHOP regimen was beneficial (CR rate ranged between
58-77%) and did not lead to a higher rate of death from infectious
complications.[33,49,50] Similarly, the addition of rituximab to CDE
resulted in higher remission rate (RR) and improved survival.[51] Then,
all trials in CD20 positive ARL nowadays include rituximab. Some trials
exclude patients with CD4 < 50/mcL; however, rituximab has been used
safely in patients with < 50/mcL CD4 count in many studies.[52,53]
Several prospective trials combined rituximab with EPOCH. In AMC034, a
randomized phase 2 trial, rituximab was given either consecutively with
EPOCH or sequentially.[54] One hundred and 6 patients were enrolled
(75% with DLBCL and 25% with BL or BL-like); CR was 73% in the
concurrent arm (71% for DLBCL) vs. 55% in the sequential arm (46% for
DLBCL). 2 years OS and PFS were 70% vs. 67% and 66% vs. 63% in the
concurrent vs. sequential arm. The NCI explored short-course-EPOCH with
dose-dense rituximab (SC-EPOCH-RR) in 33 patients, with rituximab given
on day 1 and 5 of each cycle. Patients received one cycle after
18fluorodeoxyglucose positron emission tomography (PET) negativity,
with PET evaluated each cycle after the second. CR was 91% after a
median of 3 cycles, with PFS and OS at five years 84% and 68%
respectively.[43] Indirect evidence from retrospective analyses
suggests that in ARL EPOCH might be more efficacious than CHOP. In
DLBCL an improved OS was found in a retrospective analysis of pooled
data with DA-EPOCH vs. CHOP; however, the difference between CHOP plus
rituximab vs. EPOCH plus rituximab was not significant.[33] Moreover, no
randomized trial comparing R-CHOP vs R-EPOCH has been performed in HIV
positive patients and in HIV negative population a randomized
prospective trial showed DA-R-EPOCH and R-CHOP to be equally
effective.[53] Table 3 shows
the results of V the main studies investigating the first-line
treatment of HIV-related DLBCL in the cART era. At present both
regimens are considered a valid choice of CT for patients with
HIV-associated DLBCL and outcomes approach those for HIV negative
patients in the current era.[56,57]
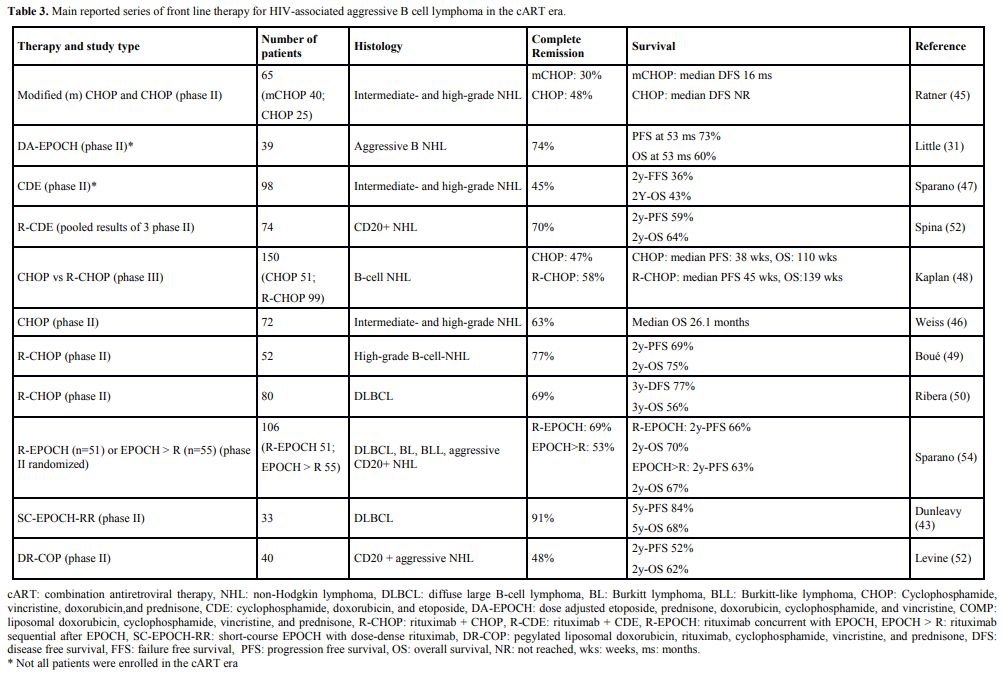 |
Table 3. Main reported series of front line therapy for HIV-associated aggressive B cell lymphoma in the cART era. |
Therapeutic options for relapsed/refractory (R/R) disease have been poorly investigated.
The
standard of care in HIV-negative population is salvage therapy followed
by high-dose CT and ASCT. The one year OS of patients with R/R ARL who
did not undergo ASCT was only 37%, in a retrospective series of
patients treated by several American institutions; patients who
underwent ASCT as part of their salvage therapy lived longer (1 year OS
63.2%).[58] Since in the cART era several clinical trials have
demonstrated that HIV-infected patients can safely and successfully
undergo ASCT,[59] there is consensus to approach HIV positive patients
with R/R DLBCL like immunocompetent patients. High dose salvage
regimens such as ICE, DHAP, ESHAP, GDP, with rituximab appear to have
similar efficacy, and patients with chemosensitive disease who are
transplant eligible should proceed to ASCT (see dedicated paragraph on
ASCT in the HIV setting). At present, we treat HIV positive patients
with DLBCL in the first line with R-CHOP and consider consolidation
with HDT in patients with high risk disease according to IPI, in cART
responding patients with permissive immune status. In R/R disease we
use conventional salvage CT (we prefer ESHAP) followed by ASCT in
responding patients.
Burkitt lymphoma.
BL, the second commonest subtype of ARL, occurs in individuals with
relatively preserved CD4 counts. Patients typically present with poor
Performance Status (PS) and high lactate dehydrogenase level.
Extra-nodal involvement is common, and the incidence of CNS involvement
ranges from 8 to 28%.[60] In the pre-cART era, HIV patients with BL
were usually treated with the same non-intensive chemotherapy regimens
as for DLBCL, with similar unsatisfactory results. After the advent of
cART, survival in BL remained poor, with CHOP or M-BACOD-based
therapies.[61] Spina et al. demonstrated that BL had a worse prognosis
with R-CDE compared to DLBCL.[51] This led to the investigation of the
intensive regimens commonly used in immunocompetent patients with BL
(HyperCVAD, CODOX-M/IVAC, LMB-86), and several retrospective, and phase
II studies showed their feasibility and efficacy in the HIV setting (CR
rates 63-92% and OS 47-73%), even if they appeared more toxic than in
the general population.[62-64] Moreover, several studies demonstrated
the feasibility of adding rituximab to intensive regimens.[65-67]
Ribera et al. reported the results of B-ALL/NHL2002 study, that showed
comparable outcome in patients with and without HIV (CR 82% vs 87% and
4 years OS 63% vs 78%) in spite of a higher incidence of severe
mucositis and infections in HIV positive patients, with 13% of patients
who died in induction.[67] To reduce the toxicity of dose-intensive
regimens, the AIDS Malignancy Consortium (AMC) conducted a study
(AMC048) with a modified CODOX-M/IVAC-R regimen in 34 HIV-positive
patients. A 2 years OS of 69% was reported with no severe mucositis and
only one treatment-related death.[68] Dunleavy et al. treated 11
patients with SC-EPOCH-RR with an excellent OS 90% at 73 months of
follow-up.[69] Recently Ferreri AJM et al. reported the safety and
activity of the Carmen Trial, a phase II trial including a dose-dense
and short-term chemoimmunotherapy program, with ASCT as first-line
consolidation for patients who did not achieve CR after induction.[70] Table 4
shows the results of the main studies investigating the treatment of
HIV-related BL in the cART era. We suggest treating HIV positive
patients with the regimens specifically designed for HIV-positive
subjects with BL.[68-70] As an alternative, the same intensive regimens
commonly used for immunocompetent patients in a specific center,
including rituximab, can be used; however, dose-adjustment might be
required, at least in patients with advanced HIV disease.
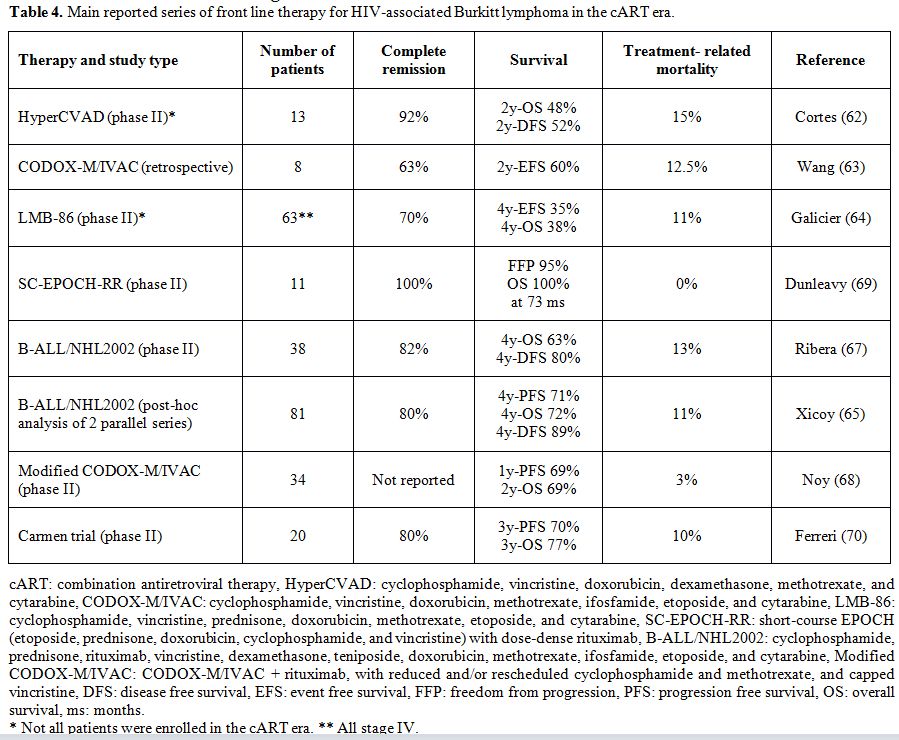 |
Table 4. Main reported series of front line therapy for HIV-associated Burkitt lymphoma in the cART era. |
Plasmablastic lymphoma.
PBL was initially described in the late nineties as a rare variant of
DLBCL, with plasmacytoid appearance, affecting primarily mucosal sites,
particularly the oropharynx, and occurring predominantly in HIV
positive patients.[71] Median CD4 count at presentation ranges from
87-206 cells/ml. It is characterized by loss of the mature B cell
markers including CD20, and an elevated proliferation index. It is
almost always associated with EBV, and up to 50% of the cases carry a
translocation involving c-MYC, which might have a negative prognostic
impact.[72,73] No prospective trials have been conducted in patients
with PBL, and the majority of the studies report poor OS (5-17 months)
with a variety of regimens, including CHOP, CHOP-like regimens, EPOCH,
CDE, CODOX-M/IVAC.[74-76] Castillo et al. reported no OS benefit of
intensive regimens like CODOX-M/IVAC vs. CHOP or CHOP-like regimens.[75]
Ibrahim et al. reported a single institution experience on 25 patients
showing improved OS with DA-EPOCH vs. CHOP (17 vs. 7 months).[77] More
encouraging results have been recently reported in a small series by
Ariela Noy et al. (CR 70% achieved with either CHOP- or EPOCH-based
regimens and one year OS 67%).[78] Reasons for different outcomes
reported by different authors are not clear. Cattaneo et al. reported
their single institution experience, showing 67% three years OS in 13
patients with PBL treated during the cART era.[79] In this series
treatment strategy included CHOP-14 regimen and extensive use of
radiotherapy (RT); moreover, five patients received ASCT as
consolidation, an approach that seems promising in this setting.[80] We
suggest treating PBL patients with intensive chemotherapy (CHOP-14 as
an option) followed by RT, at least in a localized stage. Early
consolidation with ASCT might be an option for advanced stage patients
and the cART should be concurrently used.[33]
However, new
therapies seem advisable to improve outcomes and should be investigated
in first and/or salvage setting. Bortezomib is of particular interests
as it is also particularly effective against multiple myeloma, which
shares many molecular and immunohistochemical features with PBL;
several reports have documented activity of bortezomib in PBL.[81]
Moreover, lenalidomide as a single agent has been used with some
success in relapsed/refractory PBL and in combination with CT in the
first-line setting.[82]
Primary effusion lymphoma.
PEL is a rare B-cell lymphoma characterized by effusions involving the
pleura, pericardium, and/or peritoneum; however, a rare solid
extra-cavitary variant has been described. Severe immunosuppression
with low CD4+ cell counts is common. Most PELs have lymphocyte
activation markers (CD30 and CD38) without normal B-cell markers (CD19
and CD20). HHV8 has a pathogenetic role and is present in almost 100%
of cases. Other HHV8-related complications such as KS or MCD may
precede or occur concurrently with PEL.[83,84] The most used CT regimen
is CHOP that allows achieving CR rate of 40-50%, with median survival
around six months.[84-86] However, some patients do achieve
long-term remissions, but predictive factors have not been identified.
Reports of outcomes using more intensive CT are controversial and seem
to indicate that intensifying CT is of limited benefit.[87] Anecdotal
reports of cases responding to cART alone have been reported. The
effect of anti-HHV-8 therapy remains unproven.[88] Bortezomib has been
used in combination with conventional CT with promising results.[89] A
case report describes sustained remission in an HIV negative patients
treated with single-agent lenalidomide.[90] Recently, Shah NN et al.
has reported the successful use of daratumumab, a CD38-directed human
IgG1κ monoclonal antibody, to treat a case of HIV-related PEL.[91]
Anti-CD30 directed treatment also showed promise.[92]
In
conclusion, the optimal first-line treatment for HIV-related PEL is
undefined. Standard CHOP therapy or more intensive CT regimens in young
patients with advanced disease are acceptable approaches. Even if newer
therapies are advocated, no specific strategy can be recommended at
present.
Primary central nervous system lymphoma.
PCNSL is a subtype of DLBCL with a post-germinal center phenotype. The
immunophenotype of PCNSL in HIV positive subjects differs from
immunocompetent patients; EBV is almost always detectable in lymphoma
cells and cerebrospinal fluid, while it is rarely present in PCNSL in
the HIV negative population.[93,94] Clinical findings and standard
radiological investigations cannot provide a definitive diagnosis, that
usually requires brain biopsy; however, the combination of detectable
EBV in cerebrospinal fluid (CSF) and consistent radiological findings
in a severely immunosuppressed HIV positive patients may be sufficient
in selected cases. HIV positive patients with PCNSL usually have
advanced immunosuppression and CD4 count < 50/mmc, making impossible
the administration of high dose (HD) methotrexate (MTX) and cytosine
arabinoside, as employed in immunocompetent patients,[95] in a high
proportion of patients. Whole brain RT was used extensively as the only
therapy in the pre-cART era, but with dismal outcomes and survival of a
few months. The advent of cART led to a modest improvement in
survival.[96-99] Anecdotal literature suggests that the prompt
implementation of cART in patients with HIV-PCNSL could result in
long-term remission; however, this procedure should be reserved for
carefully selected patients, not eligible for intensive CT.[100] At
least two retrospective study showed the feasibility and efficacy of
combined cART plus HD-MTX, at least in selected patients.[101,102]
Moulignier et al. analyzed 51 patients consecutively treated in France
with HD-MTX (3 gr/ms) and cART and reported a median OS of 5.7 years.
No one died of acute treatment-related toxicity.[102] Gupta et al.
reported on 20 patients treated with cART plus MTX-based regimens from
several centers in the US; median OS was not yet reached after a median
follow-up of 27 months. In this experience CD4 reconstitution with cART
administered during HD-MTX correlates with long-term survival;
rituximab did not add untoward toxicity while the addition of other
agents to HD-MTX did not improve outcome and was associated with an
increased rate of neutropenic complications and a more attenuated rate
of CD4 recovery.[101] Thus, in the absence of prospective studies, we
suggest treating cART responding patients with HD MTX and rituximab. If
induction treatment is well tolerated and a response is documented,
consolidation with HDT and ASCT could be considered in selected
patients. Indeed, ASCT seems to have a beneficial role in HIV positive
PCNSL patients, and which deserves further evaluation.[103]
Multicentric Castleman's Disease.
Even if a polyclonal disease, MCD is an aggressive B-cell
lymphoproliferative disorder with an increased incidence in PLWH, that
can be life-threatening, either through multiple organ failure or the
development of NHL.[104] It presents with various clinical features and
lymph nodes and spleen enlargements, with usually B symptoms, weakness,
and malaise. A hemophagocytic syndrome may complicate the clinical
course in a non-negligible number of cases.[105] Almost all MCD cases in
HIV positive subjects are associated with lytically active HHV-8
infection. HHV-8 encodes a viral IL-6 that plays a major role in the
pathophysiology of the disease and the level of plasma HHV8 DNA is a
helpful biomarker to monitor disease activity and response to
therapy.[106] A variety of treatment strategies have been reported, and
there is no widely accepted standard of care. Usually, the treatment
approach is designed according to the severity of the disease. The
prognosis has improved in recent years, mainly after the introduction
of cART (even if MCD can occur or worsen soon after initiation of cART)
and treatment with rituximab.[107] Rituximab showed its efficacy in 2
prospective trials,[109] and there is evidence that rituximab decreases
the risk of subsequent development of NH.[110] Notably, an association
with KS has been reported up to 70% of cases, and KS may reactivate
during treatment with rituximab. Cytotoxic CT as a single agent
(etoposide seems to give the best results) or in combination are
effective and are considered the therapy of choice in patients with
severe disease.[101] The utility of antiherpes agents in MCD has not been
demonstrated. We usually treat patients with a combination of cART,
rituximab, and antiviral therapy such as valganciclovir, reserving
combination CT (such as CHOP) + rituximab in severe or not responding
disease. Targeting IL-6 and IL6 receptor with monoclonal antibodies
appears as an attractive approach and could be considered at least in
selected patients.[112]
Hodgkin lymphoma.
HL in PLWH frequently presents with unfavorable features such as
advanced-stage, extranodal disease, and bone marrow involvement, and is
associated with EBV in 80-100% of cases. The mixed cellularity subtype
is the most commonly observed. Median CD4 counts at HL diagnosis ranges
between 150 and 260 cells/mcL, and its incidence has remained stable or
may have even increased after the advent of cART.[12] Before the
introduction of cART, the prognosis was poorer compared to the general
population, mainly for poor tolerance of CT, with high rates of OI and
toxic deaths.[113,114] CR and OS rates improved significantly in patients
responding to cART; indeed, the low CD4 count remains an independent
adverse prognostic factor.[115,116] While a prospective study with
Stanford V and concomitant cART resulted in 3-year OS 51%,[117] higher
cure rates have been reported with ABVD and cART. Three large
retrospective studies reported CR rate of 74-87% and five years OS of
76-81%. Notably, in two of these studies, a comparison was made with
HIV negative patients, and the HIV status, which did not result to
affect the outcome.[118-120] A relatively large prospective study on a
stage- and risk-adapted treatment strategy, including ABVD, baseline
BEACOPP, and involved field RT has been reported by Hentrich et al. CR
rates were respectively 96%, 100%, and 86% for early favorable-, early
unfavorable-, and advanced-stage disease and 2 years OS 95.7%, 100%,
and 86.8%. However, BEACOPP was toxic, dose delays and dose reductions
were common, and treatment-related mortality was 7% in patients with
advanced disease.[121] Then, nowadays prognosis for patients with HL and
HIV infection approaches that of patients without HIV infection, and a
stage adapted treatment appears feasible. ABVD with or without RT (with
the same indication for RT as in HIV negative population) is now seen
as the standard of care for front-line therapy. However, a higher
incidence of toxicity might be expected compared to the general
population. The role of BEACOPP is not clear as the experience are very
limited and contrasting results have been reported.[122] Table 5 shows
the results of the main studies investigating the first-line treatments
of HIV-related HL in the cART era.
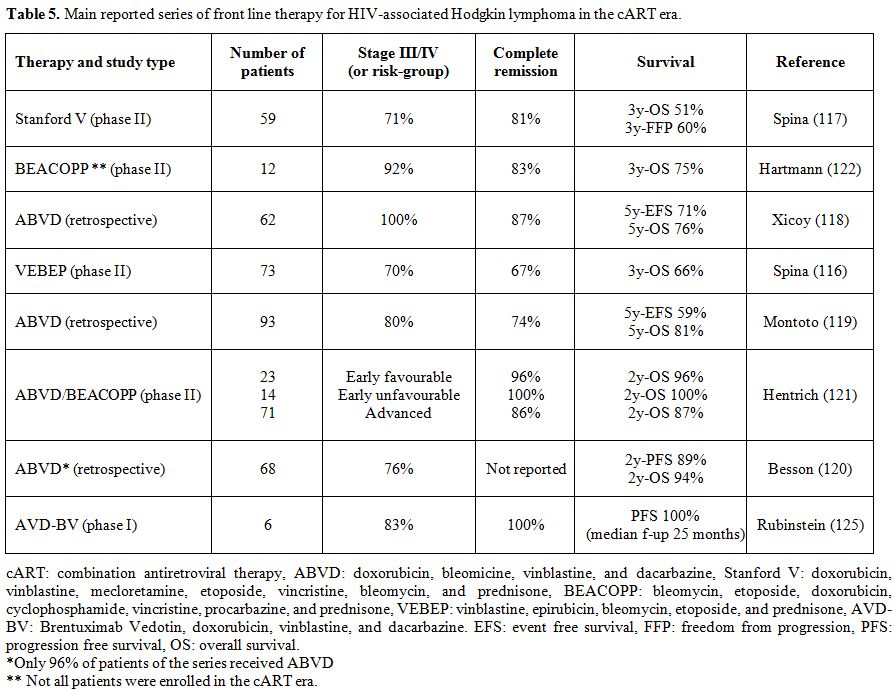 |
Table 5. Main reported series of front line therapy for HIV-associated Hodgkin lymphoma in the cART era. |
Patients who relapse or have
primary refractory disease should be considered for conventional
salvage CT followed by ASCT as several experiences have reported the
feasibility and efficacy of this approach in the HIV setting (see
dedicated paragraph). Only limited evidence on the role of PET scans in
the diagnosis of HL, and interim evaluation is available in HIV
positive patients. As a general rule, PET scan results should be
interpreted with caution as PET can be falsely positive in particular
in cART- naïve or viremic patients. A recent Intergroup Cooperative
trial that used FDG-PET after cycle 2 of ABVD to guide further therapy
included 12 pts with HIV infection; even if based on a very small
experience, the investigators concluded that it might be appropriate to
include HIV patients in further studies of response-adapted therapy.[123]
Novel agents, such as the CD30-directed immunoconjugate brentuximab
vedotin (BV), are under evaluation in the HIV
setting.[124]
The
combination of BV with doxorubicin, vinblastine, and
dacarbazine showed safety in newly
diagnosed HIV-associated HL in a phase I study (no dose-limiting
toxicity was found and six patients who completed therapy achieved
CR);[125] the phase II portion of
this trial is ongoing (NCT01771107). Immunomodulatory approaches, such
as checkpoint inhibition with anti-PD-1 agents, may also be
investigated in future studies, with some cautions due to the
peculiarity of the HIV setting.[126]
We treat HIV positive patients
in the first line with ABVD +/- RT in a stage- and risk-adapted
strategy, according to standard guidelines we use for HIV negative
population. We perform the PET-2 evaluation and evaluate case by case
if PET-2 is positive. In R/R disease we use conventional salvage CT (we
prefer BeGEV) followed by ASCT in responding patients.
Prophylaxis of CNS involvement by NHL.
CNS involvement by systemic NHL has been reported up to 25% in HIV
positive patients, and the use of intrathecal (i.t.) prophylaxis with
MTX and/or ARA-C has been long considered a mandatory part of the
systemic treatment of all HIV infected patients with aggressive
NHL,[6,127] even if any formal studies to evaluate the role of i.t.
prophylaxis have been conducted.[128] However, the CNS involvement has
decreased since the introduction of cART and the widespread use of
rituximab.[129,130] A recent retrospective review of pooled data from 886
patients was recently published by Barta et al.[131] At presentation CNS
involvement was found in 13% of patients, and CNS relapses were rare,
but occurred early and had poor outcomes (median OS 1.6 months). More
than 90% of patients had received i.t. MTX prophylaxis. Then, the use
of i.t. prophylaxis in all HIV positive patients with NHL in an era of
better systemic lymphoma control remains to be defined. Most experts
recommend that CNS prophylaxis, in the context of an effective cART,
should be given following the same criteria as in HIV negative
patients, according to different sites of involvement, stage, and
histological subtype.
Autologous stem cell transplant.
High dose therapy (HDT) and ASCT has been considered prohibitive in HIV
positive patients for several years, at least until the introduction of
cART, when groups from Europe and USA began to offer ASCT to HIV
positive patients with R/R lymphoma.[132,133] Then, ASCT has been
demonstrated to be feasible and efficacious in several series of HIV
positive patients with NHL and HL.[59,132-134] Patients were sent to the
ASCT mainly because of R/R and in few cases of high risk first CR. The
results of the main series of ARL receiving ASCT are shown in Table
6.[135-141] These studies showed low transplant-related mortality and
durable remissions. After variable follow-up periods, PFS varied from
29-85% and OS from 36-87%, with results that mainly depended on the
status of disease at the time of transplantation and an overall outcome
comparable to their HIV negative counterparts. The HIV positive
patients seem to experience more infectious complications in the first
few months after transplant than patients without HIV that didn’t
translate into a significant difference in survival, while the risk of
relapse showed a trend in favor of HIV positive patients.[140,142,143]
However, these studies were mainly retrospective or recruited patients
at the time of stem cell collection. In the Italian study,[138] patients
with relapsed or refractory lymphoma were recruited at the time of
treatment failure, before salvage CT. 54% of the entire series of 50
patients could proceed to ASCT, with satisfactory outcome in patients
who actually received transplantation (overall survival 75%) as well as
good results in the entire series, with 50% of patients alive after a
median follow-up of 45 months (Figure 1). A recent prospective trial
from Italy analyzed the use of ASCT as upfront consolidation after
R-CHOP, in patients with aggressive B cell lymphoma at high risk
according to the IPI, and reported promising results. Of 27 enrolled
patients, 15 patients received ASCT according to the protocol, and 14
are alive and relapse-free after several years from the transplant.[144]
Nowadays, HIV infection should not preclude lymphoma patients from
undergoing ASCT. The same eligibility criteria as established for HIV
negative lymphoma patients should be adopted for patients with HIV and
the second-line therapy as induction before ASCT should consist of the
same salvage regimens used for the HIV-negative population.
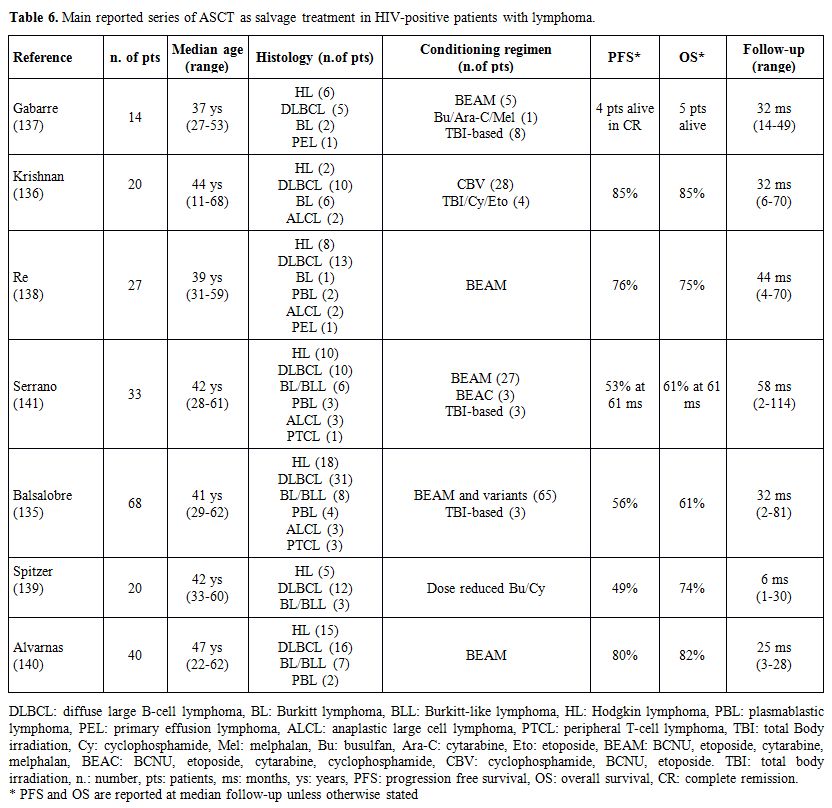 |
Table 6.
Main reported series of ASCT as salvage treatment in HIV-positive patients with lymphoma. |
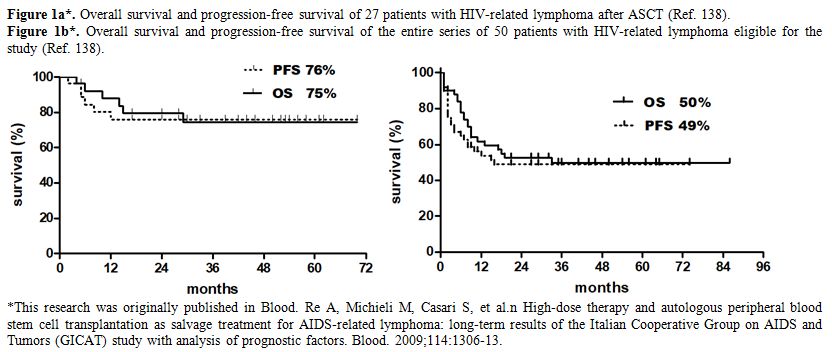 |
Figure 1.
Figure 1a*. Overall survival and progression-free survival of 27 patients with HIV-related lymphoma after ASCT (Ref. 138).
Figure
1b*. Overall survival and progression-free survival of the entire
series of 50 patients with HIV-related lymphoma eligible for the study
(Ref. 138).
|
Allogeneic stem cell transplant.
Reports of alloSCT for HIV infected patients date back to the early
eighties. However, prior to effective antiretroviral therapy, alloSCT
outcomes were extremely poor, with patients dying because of
treatment-related toxicity or relapse. After the advent of cART,
single-institution, retrospective studies with a small number of
patients suggest that alloSCT may be feasible and beneficial in HIV
positive patients with hematologic malignancies.[145] A Center for
International Blood & Marrow Transplant Research (CIBMTR), a
registry study, reported outcomes of 23 patients receiving alloSCT
(including matched related or unrelated donor transplants) for several
different hematologic disorders and found that 4 of 9 patients survived
in the cART era.[146] Cumulative incidences of acute and chronic graft
versus host disease (GVHD) did not appear much different than would be
expected from HIV negative patients. Major causes of death were
regimen-related toxicities and infections. Ten patients of this series
had an NHL; however, the outcomes were not analysed separately.
The
first prospective cooperative group trial of matched related or
unrelated alloSCT[147] has been recently reported. Myeloablative or
nonmyeloablative regimen were used at the investigator’s discretion.
Seventeen patients underwent alloSCT for treatment of acute myeloid
leukemia (9), acute lymphoblastic leukemia (2), myelodysplasia (2) or
lymphoma (4). There was no non-relapse mortality at 100 days. Grade
II-IV GVHD developed in 41% of patients. At 24 months of median
follow-up, one year OS was 57%; cause of death included disease relapse
(5), acute GVHD (1), liver failure (1), and adult respiratory distress
syndrome (1).
Even if data supporting the use of alloSCT are
limited, most authors conclude that alloSCT should be considered for
HIV patients with evidence of treatable HIV infection and standard
indications for alloSCT.
Acknowledgments
We
would like to thank Dr Carlo Brugnara (Boston Children’s Hospital,
Harvard Medical School, Boston, MA; USA) for fruitful discussion and
manuscript revision.
Competing Interests and Funding
The Authors
declare that they have no conflict of interest. This work was supported
by FUR-UNIVR (LDF).
References
- AIDS: 1987 revision of CDC/WHO case definition.
Bull World Health Organ. 1988; 66(2): 259-63, 269-73. PMid:2840220
PMCid:PMC2491057
- Simard EP, Engels EA. Cancer as a cause of death
among people with AIDS in the United States. Clin Infectious Disease.
2010; 51: 957-962 (PubMed: 20825305) https://doi.org/10.1086/656416 PMid:20825305 PMCid:PMC2943990
- Cote TR, Biggar RJ, Rosenberg PS, Devesa SS, Percy
C, Yellin FL, et al. Non-Hodgkin's lymphoma among people with AIDS:
incidence, presentation and public health burden. AIDS/Cancer Study
Group. Int J Cancer. 1997; 73(5):645-650. https://doi.org/10.1002/(SICI)1097-0215(19971127)73:5<645::AID-IJC6>3.0.CO;2-X
- Raphael M, Said J, Borish B, Ceserman E, Harris NL.
Lymphomas associated with HIV infection. In: Swerdlow SH, Campo E,
Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, editors. WHO
classification of tumours of haematopoietic and lymphoid tissues. 4th
ed. Lyon: IARC Press; 2008.
- Goedert JJ. The epidemiology of acquired
immunodeficiency syndrome malignancies. Semin Oncol. 2000
Aug;27(4):390-401. PMid:10950365
- Beral V, Peterman T, Berkelman R, Jaffe H. AIDS-associated non-Hodgkin lymphoma. Lancet. 1991 Apr 6;337(8745):805-9. https://doi.org/10.1016/0140-6736(91)92513-2
- Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel
TS, Scoppa SM, Biggar RJ. Trends in cancer risk among people with AIDS
in the United States 1980-2002. AIDS 2006, 20:1645-1654. https://doi.org/10.1097/01.aids.0000238411.75324.59 PMid:16868446
- 8. Dal Maso L, Polesel J,
Serraino D, Lise M, Piselli P, Falcini F, et al. Pattern of cancer risk
in persons with AIDS in Italy in the HAART era. Br J Cancer.
2009;100:840-847.
https://doi.org/10.1038/sj.bjc.6604923 PMid:19223894 PMCid:PMC2653754
- Franceschi S, Lise M, Clifford GM, Rickenbach M,
Levi F, Maspoli M, et al. Changing patterns of cancer incidence in the
early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer.
2010;103:416-422. https://doi.org/10.1038/sj.bjc.6605756 PMid:20588274 PMCid:PMC2920013
- Gibson TM, Morton LM, Shiels MS, Clarke CA, Engels
EA. Risk of non-Hodgkin lymphoma subtypes in HV-infected people during
the HAART era: a population-based study. AIDS. 2014;28:2313-2318. https://doi.org/10.1097/QAD.0000000000000428 PMid:25111081 PMCid:PMC4260326
- Silverberg MJ, Lau B, Achenbach CJ, Jing Y,
Althoff KN, D'Souza G, et al. Cumulative Incidence of Cancer Among
Persons With HIV in Nort America: A Cohort Study. Ann Intern Med. 2015;
163:507-518.
https://doi.org/10.7326/M14-2768 PMid:26436616 PMCid:PMC4711936
- Shiels MS, Koritzinsky EH, Clarke CA, Suneja G,
Morton LM, Engels EA. Prevalence of HIV Infection among U.S. Hodgkin
Lymphoma cases. Cancer Epidemiol Biomarkers Prev. 2014;23:274-281. https://doi.org/10.1158/1055-9965.EPI-13-0865 PMid:24326629 PMCid:PMC3946161
- Dal Maso L, Suligoi B, Franceschi S, Braga C,
Buzzoni C, Polesel J, et al. Survival after cancer in Italian persons
with AIDS, 1986-2005: a population-based estimation. J Acquir Immune
Defic Syndr. 2014;66:428-435 https://doi.org/10.1097/QAI.0000000000000184 PMid:24798769
- Chao C, Xu L, Abrams D, et al. Survival of
non-Hodgkin lymphoma patients with and without HIV infection in the era
of combined antiretroviral therapy. AIDS 2010;24:1765-1770.
https://doi.org/10.1097/QAD.0b013e32833a0961 PMid:20453630 PMCid:PMC2895006
- Cingolani A, Cozzi Lepri A, Teofili L, Galli L,
Mazzotta V, Baldin GM, Hohaus S, Bandera A, Alba L, Galizzi N, Castagna
A, D'arminio Monforte A, Antinori A; ICONA Foundation Study Group.
Survival and predictors of death in people with HIV-associated lymphoma
compared to those with a diagnosis of lymphoma in general population.
PLoS One. 2017 Oct 31; 12(10), 1-15. https://doi.org/10.1371/journal.pone.0186549 PMid:29088223 PMCid:PMC5663375
- IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans. Biological agents. Volume 100B. A Review
of human carcinogenesis. IARC Monogr Eval Carcinog Risks Hum. 2012;
100(pt B):1-441.
- Kundu RK, Sangiorgi F, Wu LY, Pattengale PK,
Hinton DR, Gill PS, Maxson R. Expression of the human immunodeficiency
virus-Tat gene in lymphoid tissues of transgenic mice is associated
with B-cell lymphoma. Blood. 1999;94:275-282. PMid:10381523
- Dolcetti R, Giagulli C, He W, Selleri M, Caccuri
F, Eyzaguirre LM, Mazzuca P, Corbellini S, Campilongo F, Marsico S,
Giombini E, Muraro E, Rozera G, De Paoli P, Carbone A, Capobianchi MR,
Ippolito G, Fiorentini S, Blattner WA, Lu W, Gallo RC, Caruso A (2015)
Role of HV-1 matrix protein p17 variants in lymphoma pathogenesis. Proc
Natl Acad Sci USA 112:14331-14336. https://doi.org/10.1073/pnas.1514748112 PMid:26578780 PMCid:PMC4655530
- Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA
(2007) AIDS-related cancer and severity of immunosuppression in persons
with AIDS. J Natl Cancer Inst 99:962-972. https://doi.org/10.1093/jnci/djm010 PMid:17565153
- Polesel J, Clifford GM, Rickenbach M, Dal Maso L,
Battegay M, Bouchardy C, Furrer H, Hasse B, Levi F, Probst-Hensch NM
(2008) Non Hodgkin lymphoma incidence in the Swiss HIV cohort study
before and after highly active antiretroviral therapy. AIDS 22:301-306.
https://doi.org/10.1097/QAD.0b013e3282f2705d PMid:18097233
- Clayton A, Mughal T. The changing face of
HIV-associated lymphoma: what can we learn about optimal therapy in the
post highly active antiretroviral therapy era? Hematol Oncol
2004;22:111-120.
https://doi.org/10.1002/hon.735 PMid:15991221
- Lanoy E, Rosenberg PS, Fily F, et al.
HIV-associated Hodgkin Lymphoma during the first months on combination
antiretroviral therapy. Blood 2011;118:44-49. https://doi.org/10.1182/blood-2011-02-339275 PMid:21551234 PMCid:PMC3139385
- Totonchy J and Cesarman E. Does persistent HIV
replication explain continued lymphoma incidence in the era of
effective antiretroviral therapy? Curr Opin Virol. 2016 October;
20:71-77.
https://doi.org/10.1016/j.coviro.2016.09.001 PMid:27665065 PMCid:PMC5102761
- Parka LS, Hernandez-Ramirez RU, Silverberg MJ,
Crothers KA, Dubrow R (2016) Prevalence of non-HIV cancer risk factors
in persons living with HIV/AIDS. AIDS 30:273-291. https://doi.org/10.1097/QAD.0000000000000922 PMid:26691548 PMCid:PMC4689318
- Hunt PW (2012) HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 9:139-147. https://doi.org/10.1007/s11904-012-0118-8 PMid:22528766
- Carbone A. Emerging pathways in the development of AIDS-related lymphomas. Lancet Oncol. 2003;4:22-29. https://doi.org/10.1016/S1470-2045(03)00957-4
- Gaidano G, Carbone A, Dalla-Favera R. Genetic
basis of acquired immunodeficiency syndrome-related lymphomagenesis. J
Natl Cancer Inst Monogr. 1998;23:95-100.
https://doi.org/10.1093/oxfordjournals.jncimonographs.a024181
- Rudek MA, Flexner C, Ambinder RF. Use of
antineoplastic agents in patients with cancer who have HIV/AIDS. Lancet
Oncol 2011; 12:905-912. https://doi.org/10.1016/S1470-2045(11)70056-0
- Rubinstein PG, Aboulafia DM, Zloza A. Malignancies
in HIV/AIDS: From epidemiology to therapeutic challenges. AIDS. 2014
February 20; 28(4): 453-465 https://doi.org/10.1097/QAD.0000000000000071 PMid:24401642 PMCid:PMC4501859
- Mounier N and Rudek MA. Chemotherapy and
interactions with combination antiretroviral therapy. In:
HIV-associated Hematological Malignancies. M.Hentrich, S.K. Barta
(eds.) Cap. 17; pag. 207-214. Springer international Publishing
Switzerland 2016. https://doi.org/10.1007/978-3-319-26857-6_17
- Little RF, Pittaluga S, Grant N, Steinberg SM,
Kavlick MF, Mitsuya H, et al. Highly effective treatment of acquired
immunodeficiency syndrome-related lymphoma with dose-adjusted EPOCH:
impact of antiretroviral therapy suspension and tumor biology. Blood.
2003; 101:4653-4659 https://doi.org/10.1182/blood-2002-11-3589 PMid:12609827
- Tan CRC, Barta SK, Lee Jeannette, Rudek MA,
Sparano JA and Noy A. Combination antiretroviral therapy accelerates
immune recovery in patients with HIV-related lymphoma treated with
EPOCH: a comparison within one prospective trial AMC034. Leukemia and
Lymphoma 2017, Nov 21:1-10.
- Barta SK, Xue X, Wang D, et al. Treatment factors
affecting outcomes in HIV-associated non –Hodgkin lymphomas: a pooled
analysis of 1546 patients. Blood 2013; 122:3251-3262. https://doi.org/10.1182/blood-2013-04-498964 PMid:24014242 PMCid:PMC3821722
- Focà E, Cavaglià G, Rusconi S, Cascavilla A,
Cenderello G, Re A, Casari S, van den Bogaart L, Zinzani PL, Caracciolo
D, Di Perri G, Bonito A, Lucchini A, Cassola G, Viale P, Calcagno A.
Survival in HIV-infected patients with lymphoma according to the choice
of antiretroviral treatment: an observational multicentre study. HIV
Med. 2018 Jun 4 [Epub ahead of print] https://doi.org/10.1111/hiv.12624 PMid:29862615
- Bower M, Palfreeman A, Alfa-Wali M, Bunker C,
Burns F, Churchill D, Collins S, Cwynarski K, Edwards S, Fields P, Fife
K, Gallop-Evans E, Kassam S, Kulasegaram R, Lacey C, Marcus R, Montoto
S, Nelson M, Newsom-Davis T, Orkin C, Shaw K, Tenant-Flowers M, Webb A,
Westwell S, Williams M; British HIV Association. British HIV
association guidelines for HIV-associated malignancies 2014. HIV Med.
2014;15:85-90.
- EACS: European AIDS Clinical Society Guidelines version 8.1, Part V: Opportunistic Infections.
http://www.eascociety.org/files/guidelines_8.1-english.pdf. October 2016.
- Hentrich M. Infection prophylaxis. In: M.Hentrich,
S.K. Barta, eds. HIV-associated Hematological Malignancies. Springer
international Publishing Switzerland. 2016; 223-226. https://doi.org/10.1007/978-3-319-26857-6_19
- Rossi G, Donisi A, Casari S, Re A, Cadeo G, Carosi
G. The International Prognostic Index can be used as a guide to
treatment decisions regarding patients with human immunodeficiency
virus-related systemic non-Hodgkin lymphoma. Cancer. 1999;86:2391-7. https://doi.org/10.1002/(SICI)1097-0142(19991201)86:11<2391::AID-CNCR29>3.0.CO;2-0
- Lim ST, Karim R, Tulpule A, Nathwani BN, Levine
AM. Prognostic factors in HIV-related duffuse large B-cell lymphoma:
before versus after highly active antiretroviral therapy. J Clin Oncol.
2005;23:8477-82. https://doi.org/10.1200/JCO.2005.02.9355 PMid:16230675
- Barta SK, Xue X, Wang D, Lee JY, Kaplan LD, Ribera
JM, Oriol A, Spina M, Tirelli U, Boue F, Wilson WH, Wyen C, Dunleavy K,
Noy A, Sparano JA. A new prognostic score for AIDS-related lymphomas in
the rituximab-era. Haematologica. 2014;99:1731-37. https://doi.org/10.3324/haematol.2014.111112 PMid:25150257 PMCid:PMC4222464
- Hoffmann C, Tiemann M, Schrader C, Janssen D, Wolf
E, Vierbuchen M, Parwaresch R, Ernestus K, Plettenberg A, Stoehr A,
Fatkenheuer G, Wyen C, Oette M and Horst HA. AIDS-related B-cell
lymphoma (ARL): correlation of prognosis with differentiation profiles
assessed by immunophenotyping. Blood. 2005;106:1762-69. https://doi.org/10.1182/blood-2004-12-4631 PMid:15905193
- Chadburn A, Chiu A, Lee JY, Chen X, Hyjek E,
Banham AH, Noy A, Kaplan LD, Sparano JA, Bhatia K, Cesarman E.
Immunophenotypic analysis of AIDS-related diffuse large B-cell lymphoma
and clinical implications in patients from AIDS malignancies consortium
clinical trials 010 and 034. J Clin Oncol. 2009;27:5039-48. https://doi.org/10.1200/JCO.2008.20.5450 PMid:19752343 PMCid:PMC2799056
- Dunleavy K, Little RF, Pittaluga S, Grant N, Wayne
AS, Carrasquillo JA, Steinberg SM, Yarchoan R, Jaffe ES, Wilson WH. The
role of tumor histogenesis, FDG-PET, and short-course EPOCH with
dose-dense rituximab (SC-EPOCH-RR) in HIV-associated diffuse large
B-cell lymphoma. Blood. 2010;115:3017-24. https://doi.org/10.1182/blood-2009-11-253039 PMid:20130244 PMCid:PMC2858473
- Baptista M, Tapia G, Munoz-Marmol A, Muncunill J,
Montoto S, Gribben J, Calaminici M, Martinez A, Gonzalez-Farre B,
Lopez-Guillermo A, Gonzalez-Barca E, Terol M, Miralles P, Alcoceba M,
Vall-Llovera F, Briones J, Abrisqueta P, Abella E, Provencio M,
Garcia-Ballesteros C, Moraleda J, Sancho J, Ribera J, Mate J, Navarro
J. Application of Cell-of-origin subtypes determined by digital gene
expression in HIV-related diffuse large B-cell lymphomas. Hematological
Oncology. 2017;35(S2): abstract n.151.
- Ratner L, Lee J, Tang S, Redden D, Hamzeh F,
Herndier B, Scadden D, Kaplan L, Ambinder R, Levine A, Harrington W,
Grochow L, Flexner C, Tan B, Straus D; AIDS Malignancy Consortium.
Chemotherapy for human immunodeficiency virus-associated non-Hodgkin's
lymphoma in combination with highly active antiretroviral therapy. J
Clin Oncol. 2001;19:2171-78.
https://doi.org/10.1200/JCO.2001.19.8.2171 PMid:11304769
- Weiss R, Mitrou P, Arasteh K, Schuermann D,
Hentrich M, Duehrsen U, Sudeck H, Schmidt-Wolf IG, Anagnostopoulos I,
Huhn D. Acquired immunodeficiency syndrome-related lymphoma:
simultaneous treatment with combined cyclophosphamide, doxorubicin,
vincristine, and prednisone chemotherapy and highly active
antiretroviral therapy is safe and improves survival-results of the
German Multicenter Trial. Cancer. 2006;106:1560-80. https://doi.org/10.1002/cncr.21759 PMid:16502436
- Sparano JA,Lee S, Chen MG, Nazeer T, Einzig A,
Ambinder RF, Henry DH, Manalo J, Li T, Von Roenn JH. Phase II trial of
infusional cyclophosphamide, doxorubicine, and etoposide in patients
with HIV-associated non-Hodgkin's lymphoma: an Eastern Cooperative
Oncology Group Trial (E1494). J Clin Oncol. 2004;22:1491-500. https://doi.org/10.1200/JCO.2004.08.195 PMid:15084622
- Kaplan LD, Lee JY, Ambinder RF, Sparano JA,
Cesarman E, Chadburn A, Levine AM, Scadden DT. Rituximab does not
improve clinical outcomein a randomized phase 3 trial of CHOP with or
witout rituximab in patients HIV-associated non-Hodgkin lymphoma: AIDS
Malignancies Consortium Trial 010. Blood. 2005;106:1538-43. https://doi.org/10.1182/blood-2005-04-1437 PMid:15914552 PMCid:PMC1895225
- Boue F, Gabarre J, Gisselbrecht C, Reynes J,
Cheret A, Bonnet F, Billaud E, Raphael M, Lancar R, Costagliola D.
Phase II trial of CHOP plus rituximab in patientswith HIV-assoiatd
non-Hodgkin lymphoma. J Clin Oncol. 2006;24:4123-28. https://doi.org/10.1200/JCO.2005.05.4684 PMid:16896005
- Ribera JM, Oriol A, Morgades M, González-Barca E,
Miralles P, López-Guillermo A, Gardella S, López A, Abella E, García M;
PETHEMA, GELTAMO, GELCAB and GESIDA Groups. Safety and efficacy of
cyclophosphamide, adriamycin, vincristine, prednisone and rituximab in
patients with human immunodeficiency virus-associated diffuse large
B-cell lymphoma: results of a phase II trial. Br J Haematol.
2008;140:411-19. https://doi.org/10.1111/j.1365-2141.2007.06943.x PMid:18162120
- Spina M, Jaeger U, Sparano JA, Talamini R,
Simonelli C, Michieli M, Rossi G, Nigra E, Berretta M, Cattaneo C,
Rieger AC, Vaccher E, Tirelli U. Rituximab plus infusional
cyclophosphamide, doxorubicin, and etoposide in HIV-associated
non-Hodgkin lymphoma: pooled results from 3 phase 2 trials. Blood.
2005;105:1891-97. https://doi.org/10.1182/blood-2004-08-3300 PMid:15550484
- Levine AM, Noy A, Lee JY, Tam W, Ramos JC, Henry
DH, Parekh S, Reid EG, Mitsuyasu R, Cooley T, Dezube BJ, Ratner L,
Ceserman E, and Tulpule A. Pegylated liposomal doxorubicin, rituximab,
cyclophosphamide, vincristin e, and prednisone in AIDS-related
lymphoma: AIDS Malignancy Consortium Study 047. J Clin Oncol 2013 Jan
1; 31(1); 58-64 https://doi.org/10.1200/JCO.2012.42.4648 PMid:23169503 PMCid:PMC3530691
- Wyen C1, Jensen B, Hentrich M, Siehl J, Sabranski
M, Esser S, Gillor D, Müller M, Van Lunzen J, Wolf T, Bogner JR,
Wasmuth JC, Christ H, Fätkenheuer G, Hoffmann C. Treatment of
AIDS-related lymphomas: rituximab is beneficial even in severely
immunosuppressed patients. AIDS. 2012;26:457-64. https://doi.org/10.1097/QAD.0b013e32834f30fa PMid:22112600
- Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos
JC, Ambinder RF, Wachsman W, Aboulafia D, Noy A, Henry DH, Von Roenn J,
Dezube BJ, Remick SC, Shah MH, Leichman L, Ratner L, Cesarman E,
Chadburn A, Mitsuyasu R; AIDS Malignancy Consortium. Rituximab plus
concurrent infusional EPOCH chemotherapy is highly effective in
HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008-16. https://doi.org/10.1182/blood-2009-08-231613 PMid:20023215 PMCid:PMC2858478
- Wilson WH, Sin-Ho J, Pitcher BN, His ED, Friedberg
J, Cheson B, Bartlett NL, Smith S, Wagner Johnston N, Kahl BS, Staudt
LM, Blum K, Abramson J, Press OW, Fisher RI, Richards KL, Schoder H,
Cjang JE, Zelenetz AD, Leonard JP: Phase III randomized study of R-CHOP
versus DA-EPOCH-R and molecular analysis of untreated diffuse large
B-cell lymphoma: CALGB/Alliance 50303. Blood. 2016; 130(S1): abstract
n.469.
- Barta SK, Samuel MS, Xue X, Wang D, Lee JY,
Mounier N, Ribera JM, Spina M, Tirelli U, Weiss R, Galicier L, Boue F,
Little RF, Dunleavy K, Wilson WH, Wyen C, Remick SC, Kaplan LD, Ratner
L, Noy A, Sparano JA. Changes in the influence of lymphoma - and
HIV-specific factors on outcome in AIDS-related non-Hodgkin lymphoma.
Ann Oncol. 2015;26:958-66. https://doi.org/10.1093/annonc/mdv036 PMid:25632071 PMCid:PMC4405278
- Navarro JT, Lloveras N, Ribera JM, Oriol A, Mate
JL, Feliu E. The prognosis of HIV-infected patients with diffuse large
B-cell lymphoma treated with chemotherapy and highly active
antiretroviral therapy is similar to that of HIV-negative patients
receiving chemotherapy. Haematologica. 2005;90:704-6. PMid:15921395
- Bayraktar UD, Ramos JC, Petrich A, Gupta N,
Lensing S, Moore PC, Reid EG, Aboulafia DM, Ratner L, Mitsuyasu R,
Cooley T, Henry DH, Barr P, Noy A. Outcome of patients with
relapsed/refractory acquired immune deficiency syndrome-related
lymphoma diagnosed 1999-2008 and treated with curative intent in the
AIDS Maligancy Consortium. Leuk Lymphoma. 2012;53:2383-89. https://doi.org/10.3109/10428194.2012.697559 PMid:22642936 PMCid:PMC3458169
- Re A, Cattaneo C, Michieli M. Casari S, Spina M,
Rupolo M, Allione B, Nosari A, Schiantarelli C, Vigano M, Izzi I,
Ferremi P, Lanfranchi A, Mazzuccato M, Carosi G, Tirelli U, Rossi G.
High-dose therapy and autologous peripheral-blood stem-cell
transplantation as salvage treatment for HIV-associated lymphoma in
patients receiving highly active antiretroviral therapy. J Clin Oncol.
2003;21:4423-27. https://doi.org/10.1200/JCO.2003.06.039 PMid:14581441
- Spina M, Tirelli U, Zagonel V, Gloghini A, Volpe
R, Babare R, Abbruzzese L, Talamini R, Vaccher E, Carbone A. Burkitt's
lymphoma in adults with and without human immunodeficiency virus
infection: a single-institution clinicopathologic study of 75 patients.
Cancer 1998;82:766-74. https://doi.org/10.1002/(SICI)1097-0142(19980215)82:4<766::AID-CNCR21>3.0.CO;2-V
- Lim ST, Karim R, Nathwani BN, Tulpule A, Espina B,
Levine AM. AIDS-related Burkitt's lymphoma versus diffuse large-cell
lymphoma in the pre-highly active antiretroviral therapy (HAART) and
HAART eras: significant differences in survival with standard
chemotherapy. J Clin Oncol. 2005;23:4430-8. https://doi.org/10.1200/JCO.2005.11.973 PMid:15883411
- Cortes J, Thomas D, Rios A, Koller C, O'Brien S,
Jeha S, Faderl S, Kantarjian H. Hyperfractioned cyclophosphamide,
vincristine, doxorubicine, and dexamethasone and highly active
antiretroviral therapy for patients with acquired immunodeficiency
syndrome-related Burkitt lymphoma/leukemia. Cancer. 2002;94:1492-99. https://doi.org/10.1002/cncr.10365 PMid:11920506
- Wang ES, Straus DJ, Teruya-Feldstein J, Qin J,
Portlock C, Moskowitz C, Goy A, Hedrick E, Zelenetz AD, Noy A.
Intensive chemotherapy with cyclophosphamide, doxorubicine, high-dose
methotrexate/ifosfamide, etoposide, and high-dose cytarabine
(CODOX-M/IVAC) for human immunodeficiency virus-associated Burkitt
lymphoma. Cancer. 2003; 98:1196-205. https://doi.org/10.1002/cncr.11628 PMid:12973843
- Galicier L, Fieschi C, Borie R, Meignin V, Daniel
MT, Gerard L, Oksanhendler E. Intensive chemotherapy regimen (LMB86)
for St Jude stage IV AIDS-related Burkitt lymphoma/leukemia: a
prospective study. Blood. 2007;110:2846-54. https://doi.org/10.1182/blood-2006-10-051771 PMid:17609431
- Xicoy B, Ribera JM, Muller M, Garcia O, Hoffmann
C, Oriol A, Hentrich M, Grande C, Wasmuth JC, Esteve J, van Lunzen J,
Del Potro E, Knechten H, Brunet S, Mayr C, Escoda L, Schommers P,
Alonso N, Vall-Llovera F, Perez M, Morgades M, Gonzalez J, Fernandez A,
Thoden J, Gokbuget N, Hoelzer D, Fatkenheuer G, Wyen C; PETHEMA Group
and German HIV Lymphoma Cohort. Dose-intensive chemotherapy including
rituximab is highly effective but toxic in human immunodeficiency
virus-infected patients with Burkitt lymphoma/leukemia: parallel study
of 81 patients. Leuk Lymphoma. 2014;55:2341-48.
https://doi.org/10.3109/10428194.2013.878933 PMid:24397614
- Oriol A, Ribera JM, Bergua J, Gimenez Mesa E,
Grande C, Esteve J, Brunet S, Moreno MJ, Escoda L, Hernandez-Rivas JM,
Hoelzer D. High-dose chemotherapy and immunotherapy in adult Burkitt
lymphoma: comparison of results in human immunodeficiency
virus-infected and noninfected patients. Cancer. 2008;113:117-25. https://doi.org/10.1002/cncr.23522 PMid:18457327
- Ribera JM, Garcia O, Grande C, Esteve J, Oriol A,
Bergua J, González-Campos J, Vall-Llovera F, Tormo M, Hernández-Rivas
JM, García D, Brunet S, Alonso N, Barba P, Miralles P, Llorente A,
Montesinos P, Moreno MJ, Hernández-Rivas JÁ, Bernal T. Dose-intensive
chemotherapy including rituximab in Burkitt's leukemia or lymphoma
regardless of human immunodeficiency virus infection status: final
results of a phase 2 study (Burkimab). Cancer. 2013;119:1660-68. https://doi.org/10.1002/cncr.27918 PMid:23361927
- Noy A, Lee JY, Ceserman E, Ambinder R, Baiocchi R,
Reid E, Ratner L, Wagner-Johnston N, Kaplan L; AIDS Malignancy
Consortium. AMC 048: modified CODOX-M/IVAC-rituximab is safe and
effective for HIV-associated Burkitt lymphoma. Blood. 2015;126:160-66. https://doi.org/10.1182/blood-2015-01-623900 PMid:25957391 PMCid:PMC4497960
- Dunleavy K, Pittaluga S, Shovlin M, Steinberg SM,
Cole D, Grant C, Widermann B, Staudt LM, Jaffe ES, Little RF, Wilson
WH, et al. Low-intensity therapy in adults with Burkitt's lymphoma. N
Engl J Med. 2013;369:1915-25. https://doi.org/10.1056/NEJMoa1308392 PMid:24224624 PMCid:PMC3901044
- Ferreri AJM, Spina M, Cattaneo C, Verga L, Allione
B, Ferrari D, Rigacci L, Fumagalli L, Donadoni G, Lleshi A, Sassone M,
Rossi G, and Re A. Safety and activity of a dose-dense short-term
chemoimmunotherapy in HIV-positive patients with Burkitt lymphoma
(HIV-BL pts): Final results of the Carmen phase II trial. Blood.
2017;130(S1):abstract n.2828.
- Delecluse HJ, Anagnostopoulos I, Dallenbach F,
Hummel M, Marafioti T, Schneider U, Huhn D, Schmidt-Westhausen A,
Reichart PA, Gross U, Stein H. Plasmablastic lymphomas of the oral
cavity: a new entity associated with the human immunodeficiency virus
infection. Blood. 1997;89:1413-20. PMid:9028965
- Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood. 2015;125:2323-30. https://doi.org/10.1182/blood-2014-10-567479 PMid:25636338
- Taddesse-Heath L, Meloni-Ehrig A, Scheerle J,
Kelly JC,Jaffe ES. Plasmablastic lymphoma with MYC translocation:
evidence for a common pathway in the generation of plasmablastic
features. Mod Pathol. 2010;23:991-99. https://doi.org/10.1038/modpathol.2010.72 PMid:20348882
- Castillo J, Pantanowitz L, Dezube BJ.
HIV-associated plasmablastic lymphoma: lessons learned from 112
published cases. Am J Hematol. 2008;83:804-9. https://doi.org/10.1002/ajh.21250 PMid:18756521
- Castillo JJ, Furman M, Beltran BE, Bibas M,Bower
M, Chen W, et al. Human immunodeficiency virus-associated plasmablastic
lymphoma: poor prognosis in the era of highly active antiretroviral
therapy. Cancer. 2012; 118:5270-7. https://doi.org/10.1002/cncr.27551 PMid:22510767
- Schommers P, Wyen C, Hentrich M, Gillor D, Zoufaly
A, Jensen B, Bogner JR, Thoden J, Wasmuth JC, Fätkenheuer G, Hoffmann
C. Poor outcome of HIV-infected patients with plasmablastic lymphoma:
results from the German AIDS-related lymphoma cohort study. AIDS.
2013;27:842-5. https://doi.org/10.1097/QAD.0b013e32835e069d PMid:23574794
- Ibrahim IF, Shapiro GA, Naina HVK. Treatment of
HIV-associated plasmablastic lymphoma: a single-center experience with
25 patients. J Clin Oncol. 2014;32: abstr 8583.
- Noy A, Lensing SY, Moore PC, Gupta N, Aboulafia D,
Ambinder R, Baiocchi R, Dezube BJ, Henry D, Kaplan L, Levine AM,
Mitsuyasu R, Ratner L, Reid E, Remick S, Sparano J, Tzachanis D,
Wachsman W, and Chadburn A. Plasmablastic Lymphoma is Treatable in the
HAART Era. A 10 year Retrospective by the AIDS Malignancy Consortium
(AMC). Leuk Lymphoma. 2016; 57:1731–4. https://doi.org/10.3109/10428194.2015.1113281 PMid:26674561 PMCid:PMC4899288
- Cattaneo C, Re A, Ungari M, Peli A, Casari S,
Castelnuovo F, Fisogni S, Lonardi S, Pellegrini V, Petullà M, Facchetti
F, Rossi G. Plasmablastic lymphoma among human immunodeficiency
virus-positive patients: results of a single center's experience. Leuk
Lymphoma. 2015;56:267–9. https://doi.org/10.3109/10428194.2014.911867 PMid:24712980
- Al-Malki MM, Castillo JJ, Sloan JM, Re A.
Hematopoietic cell transplantation for plasmablastic lymphoma: a
review. Biol Blood Marrow Transplant. 2014;20:1877-84. https://doi.org/10.1016/j.bbmt.2014.06.009 PMid:24946718
- Castillo JJ, Guerrero-Garcia T, Baldini F,
Tchernonog E, Cartron G, Ninkovic S, Cwynarski K, Dierickx D, Tousseyn
T, Lansigan F, Linnik Y, Mogollon R, Navarro JT, Olszewski AJ, Reagan
JL, Fedele P, Gilbertson M, Grigoriadis G, Bibas M. Bortezomib plus
EPOCH is effective as frontline treatment in patients with
plasmablastic lymphoma. Br J Haematol. 2018;12: [Epub ahead of print] https://doi.org/10.1111/bjh.15156
- M Schmit JM, DeLaune J, Norkin M, Grosbach A. A
Case of Plasmablastic Lymphoma Achieving Complete Response and Durable
Remission after Lenalidomide-Based Therapy. Oncol Res Treat.
2017;40:46–48. https://doi.org/10.1159/000455146 PMid:28095384
- Nador RG, Cesarman E, Chadburn A, Dawson DB,
Ansari MQ, Sald J, Knowles DM. Primary effusion lymphoma: a distinct
clinicopathologic entity associated with the Kaposi's
sarcoma-associated herpes virus. Blood. 1996;88:645-56 PMid:8695812
- Boulanger E, Gérard L, Gabarre J, Molina JM, Rapp
C, Abino JF, Cadranel J, Chevret S, Oksenhendler E. Prognostic factors
and outcome of human herpesvirus 8-associated primary effusion lymphoma
in patients with AIDS. J Clin Oncol. 2005;23:4372-80. https://doi.org/10.1200/JCO.2005.07.084 PMid:15994147
- Simonelli C, Spina M, Cinelli R, Talamini R,
Tedeschi R, Gloghini A, Vaccher E, Carbone A, Tirelli U. Clinical
features and outcome of primary effusion lymphoma in HIV-infected
patients: a single-institution study. J Clin Oncol. 2003;21:3948-54
https://doi.org/10.1200/JCO.2003.06.013 PMid:14581418
- Chen YB, Rahemtullah A, Hochberg E. Primary effusion lymphoma. Oncologist. 2007;12:569-76. https://doi.org/10.1634/theoncologist.12-5-569 PMid:17522245
- Boulanger E, Daniel MT, Agbalika F, Oksenhendler
E. Combined chemotherapy including high-dose methotrexate in
KSHV/HHV8-associated primary effusion lymphoma. Am J Hematol.
2003;73:143-8. https://doi.org/10.1002/ajh.10341 PMid:12827649
- Luppi M, Trovato R, Barozzi P, Vallisa D, Rossi G,
Re A, Ravazzini L, Potenza L, Riva G, Morselli M, Longo G, Cavanna L,
Roncaglia R, Torelli G. Treatment of herpesvirus associated primary
effusion lymphoma with intracavity cidofovir. Leukemia. 2005;19:473-6. https://doi.org/10.1038/sj.leu.2403646 PMid:15674353
- Gupta A, Sen S, Marley E, Chen W, Naina HV.
Management and outcomes of HIV-associated primary effusion lymphoma: a
single center experience. Clin Lymphoma Myeloma Leuk. 2016; 16
(Suppl):S175–S180.
- Antar A, El Hajj H, Jabbour M, Khalifeh I,
El-Merhi F, Mahfouz R, Bazarbachi A. Primary effusion lymphoma in an
elderly patient effectively treated by lenalidomide: case report and
review of literature. Blood Cancer J. 2014; 4:e190.
- Shah NN, Singavi AK, and Harrington A. Daratumumab in Primary Effusion Lymphoma. N Engl J Med. 2018;379:689-90 https://doi.org/10.1056/NEJMc1806295 PMid:30110586
- Leitch HA and Oksenhendler. HIV-associated primary
effusion lymphoma. In: M.Hentrich, S.K. Barta, eds. HIV-associated
Hematological Malignancies. Springer international Publishing
Switzerland. 2016;pag.83-94.
- Remick SC, Diamond C, Migliozzi JA, Solis O,
Wagner H Jr, Haase RF, Ruckdeschel JC. Primary central nervous system
lymphoma in patients with and without the acquired immune deficiency
syndrome: a retrospective analysis and review of the literature.
Medicine (Baltimore). 1990;69:345-60. https://doi.org/10.1097/00005792-199011000-00003
- MacMahon EM, Glass JD, Hayward SD, Mann RB, Becker
PS, Charache P, McArthur JC, Ambinder RF. Epstein-Barr virus in
AIDS-related primary central nervous system lymphoma. Lancet.
1991;338:969-73 https://doi.org/10.1016/0140-6736(91)91837-K
- Ferreri AJ, Reni M, Foppoli M, Martelli M,
Pangalis GA, Frezzato M, Cabras MG, Fabbri A, Corazzelli G, Ilariucci
F, Rossi G, Soffietti R, Stelitano C, Vallisa D, Zaja F, Zoppegno L,
Aondio GM, Avvisati G, Balzarotti M, Brandes AA, Fajardo J, Gomez H,
Guarini A, Pinotti G, Rigacci L, Uhlmann C, Picozzi P, Vezzulli P,
Ponzoni M, Zucca E, Caligaris-Cappio F, Cavalli F; International
Extranodal Lymphoma Study Group (IELSG). High dose cytarabine plus
high-dose methotrexate versus high-dose methotrexate alone in patients
with primary CNS lymphoma: a randomised phase 2 trial. Lancet.
2009;374:1512-20. https://doi.org/10.1016/S0140-6736(09)61416-1
- Baumgartner JE, Rachlin JR, Beckstead JH, Meeker
TC, Levy RM, Wara WM, Rosenblum ML. Primary central nervous system
lymphomas: natural history and response to radiation therapy in 55
patients with acquired immunodeficiency syndrome. J Neurosurg.
1990;73:206-11. https://doi.org/10.3171/jns.1990.73.2.0206 PMid:2366078
- Skiest DJ, Crosby C. Survival is prolonged by
highly active antiretroviral therapy in AIDS patients with primary
central nervous system lymphoma. AIDS. 2003;17:1787-93.
https://doi.org/10.1097/00002030-200308150-00007
- Nagai H, Odawara T, Ajisawa A, Tanuma J, Hagiwara
S, Watanabe T, Kambe T, Konishi M, Saito S, Takahama S, Tateyama M,
Okada S.Whole brain radiation alone produces favourably outcomes for
AIDS-related primary central nervous system lymphoma in the HAART era.
Eur J Haematol. 2010; 84:499-505. https://doi.org/10.1111/j.1600-0609.2010.01424.x PMid:20132301
- Uldrick TS, Pipkin S, Scheer S, Hessol NA: Factors
associated with survival among patients with AIDS-related primary
central nervous system lymphoma. AIDS. 2014;28:397-405. https://doi.org/10.1097/QAD.0000000000000030 PMid:24076659 PMCid:PMC3966974
- Travi G, Ferreri AJ, Cinque P, Gerevini S,
Ponzoni M. Long-term remission of HIV-associated primary CNS lymphoma
achieved with highly active antiretroviral therapy alone. J Clin Oncol.
2012;30:119-21. https://doi.org/10.1200/JCO.2011.39.9642 PMid:22355047
- Gupta NK, Nolan A, Omuro A, Reid EG, Wang C-C,
Mannis G, Jaglal M, Chavez JC, Rubinstein PG, Griffin A, Abrams DI,
Hwang J, Kaplan LD, Luce JA, Volberding P, Treseler PA, and Rubenstein
JL. Long-term survival in AIDS-related primary central nervous system
lymphoma. Neuro-oncology. 2017;19:99-108. https://doi.org/10.1093/neuonc/now155 PMid:27576871 PMCid:PMC5193026
- Moulignier A, Lamirel C, Picard H, Lebrette MG,
Amiel C, Hamidi M, Polivka M, Mikol J, Cochereau I, Pialoux G.
Long-term AIDS-related PCNSL outcomes with HD-MTX and combined
antiretroviral therapy. Neurology. 2017;89:1-9. https://doi.org/10.1212/WNL.0000000000004265 PMid:28747447
- O'Neill A, Mikesch K, Fritsch K, Kasenda B,
Banerjee L, Burns F, Zakout G, Johnston R, Illerhaus G, Cwynarski K.
Outcomes for HIV-positive patients with primary central nervous system
lymphoma after high-dose chemotherapy and auto-SCT. Bone marrow
Transplant. 2015;50:999-1000. https://doi.org/10.1038/bmt.2015.18 PMid:25867650
- Fajgenbaum DC, Ruth JR, Kelleher D, Rubenstein
AH. The collaborative network approach: a new framework to accelerate
Castleman's disease and other rare disease research. Lancet Haematol.
2016;3:150-2. https://doi.org/10.1016/S2352-3026(16)00007-7
- Oksenhendler E, Boutboul D, Fajgenbaum D, Mirouse
A, Fieschi C, Malphettes M, Vercellino L, Meignin V, Gérard L, Galicier
L. The full spectrum of Castleman disease: 273 patients studied over 20
years. Br J Haematol. 2018;180:206-16. https://doi.org/10.1111/bjh.15019 PMid:29143319
- Oksenhendler E, Carcelain G, Aoki Y, Boulanger E,
Maillard A, Clauvel JP, Agbalika F. High levels of human herpesvirus 8
viral load, human interleukin-6, interleukin-10, and C reactive protein
correlate with exacerbation of multicentric Castleman disease in
HIV-infected patients. Blood. 2000;96:2069-73. PMid:10979949
- Hoffmann C, Schmid H, Müller M, Teutsch C, van
Lunzen J, Esser S, Wolf T, Wyen C, Sabranski M, Horst HA, Reuter S,
Vogel M, Jäger H, Bogner J, Arasteh K. Improved outcome with rituximab
in patients with HIV-associated multicentric Castleman disease. Blood.
2011;118:3499-503. https://doi.org/10.1182/blood-2011-02-333633 PMid:21778341
- Bower M, Powles T, Williams S, Davis TN, Atkins
M, Montoto S, Orkin C, Webb A, Fisher M, Nelson M, Gazzard B, Stebbing
J, Kelleher P. Brief communication: rituximab in HIV-associated
multicentric Castleman disease. Ann Intern Med. 2007;147:836-9. https://doi.org/10.7326/0003-4819-147-12-200712180-00003 PMid:18087054
- Gérard L, Bérezné A, Galicier L, Meignin V,
Obadia M, De Castro N, Jacomet C, Verdon R, Madelaine-Chambrin I,
Boulanger E, Chevret S, Agbalika F, Oksenhendler E. Prospective study
of rituximab in chemotherapy-dependent human immunodeficiency virus
associated multicentric Castleman's disease: ANRS 117 CastlemaB Trial.
J Clin Oncol. 2007;25:3350-6. https://doi.org/10.1200/JCO.2007.10.6732 PMid:17664482
- Gérard L, Michot JM, Burcheri S, Fieschi C,
Longuet P, Delcey V, Meignin V, Agbalika F, Chevret S, Oksenhendler E,
Galicier L. Rituximab decreases the risk of lymphoma in patients with
HIV-associated multicentric Castleman disease. Blood. 2012;119:2228-33.
https://doi.org/10.1182/blood-2011-08-376012 PMid:22223822
- Bower M. How I treat HIV-associated multicentric Castleman disease. Blood. 2010;116:4415-21. https://doi.org/10.1182/blood-2010-07-290213 PMid:20688959
- Nagao A, Nakazawa S, Hanabusa H. Short-term
efficacy of the IL6 receptor antibody tocilizumab in patients with
HIV-associated multicentric Castleman disease: report of two cases. J
Hematol Oncol. 2014;17:10. https://doi.org/10.1186/1756-8722-7-10 PMid:24438824 PMCid:PMC3896700
- Errante D, Gabarre J, Ridolfo AL, Rossi G, Nosari
AM, Gisselbrecht C, Kerneis Y, Mazzetti F, Vaccher E, Talamini R,
Carbone A, Tirelli U. Hodgkin's disease in 35 patients with HIV
infection: an experience with epirubicin, bleomycin, vinblastine and
prednisone chemotherapy in combination with antiretroviral therapy and
primary use of G-CSF. Ann Oncol. 1999;10:189-95.
https://doi.org/10.1023/A:1008338915945 PMid:10093688
- Hentrich M, Maretta L, Chow KU, Bogner JR,
Schürmann D, Neuhoff P, Jäger H, Reichelt D, Vogel M, Ruhnke M, Oette
M, Weiss R, Rockstroh J, Arasteh K, Mitrou P. Highly active
antiretroviral therapy (HAART) improves survival in HIV-associated
Hodgkin's disease: results of a multicenter study. Ann Oncol.
2006;17:914-9. https://doi.org/10.1093/annonc/mdl063 PMid:16565210
- Castillo JJ, Bower M, Brühlmann J, Novak U,
Furrer H, Tanaka PY, Besson C, Montoto S, Cwynarski K, Abramson JS,
Dalia S, Bibas M, Connors JM, Furman M, Nguyen ML, Cooley TP, Beltran
BE, Collins JA, Vose JM, Xicoy B, Ribera JM. HIV-Associated Hodgkin
Lymphoma in the cART Era Study Group. Prognostic factors for
advanced-stage human immunodeficiency virus-associated classical
Hodgkin lymphoma treated with doxorubicin, bleomycin, vinblastine, and
dacarbazine plus combined antiretroviral therapy: a multi-institutional
retrospective study. Cancer. 2015;121:423-31. https://doi.org/10.1002/cncr.29066 PMid:25251326
- Spina M, Antinori A, Bibas M, Mancuso S, Re A,
Schiantarelli C, Talamini R,Vaccher E,Tirelli U. VEBEP regimen in
patients with HD and HIV infection (HIV-HD): final results of a phase
II study of the italian cooperative group on AIDS and Tumors (GICAT).
Haematologica. 2011;96(s2):abstract n.773.
- Spina M, Gabarre J, Rossi G, Fasan M,
Schiantarelli C, Nigra E, Mena M, Antinori A, Ammassari A, Talamini R,
Vaccher E, di Gennaro G, Tirelli U. Stanford five regimen and
concomtant HAART in 59 patients with Hodgkin disease and HIV infection.
Blood. 2002; 100:1984-8. https://doi.org/10.1182/blood-2002-03-0989 PMid:12200356
- Xicoy B, Ribera JM, Miralles P, Berenguer J,
Rubio R, Mahillo B, Valencia ME, Abella E, López-Guillermo A, Sureda A,
Morgades M, Navarro JT, Esteban H; GESIDA Group; GELCAB Group. Results
of treatment with doxorubicin, bleomycin, vinblastine and dacarbazine
and highly active antiretroviral therapy in advanced stage, human
immunodeficiency virus-related Hodgkin's lymphoma. Haematologica.
2007;92:191-8. https://doi.org/10.3324/haematol.10479 PMid:17296568
- Montoto S, Shaw K, Okosun J, Gandhi S, Fields P,
Wilson A, Shanyinde M, Cwynarski K, Marcus R, de Vos J, Young AM,
Tenant-Flowers M, Orkin C, Johnson M, Chilton D, Gribben JG, Bower M.
HIV status does not influence outcome in patients with classical
Hodgkin lymphoma treated with chemotherapy using doxorubicin,
bleomycin, vinblastine, and dacarbazine in the highly active
antiretroviral therapy era. J Clin Oncol. 2012;3:4111-6. https://doi.org/10.1200/JCO.2011.41.4193 PMid:23045581 PMCid:PMC5320889
- Besson C, Lancar R, Prevot S, Brice P, Meyohas
MC, Marchou B, Gabarre J, Bonnet F, Goujard C, Lambotte O, Boué F,
Mounier N, Partisani M, Raffi F, Costello R, Hendel-Chavez H,
Algarte-Genin M, Trabelsi S, Marchand L, Raphael M, Taoufik Y,
Costagliola D. High Risk Features Contrast With Favorable Outcomes in
HIV-associated Hodgkin Lymphoma in the Modern cART Era, ANRS CO16
LYMPHOVIR Cohort. Clin Infect Dis. 2015;61:1469-75. https://doi.org/10.1093/cid/civ627 PMid:26223997
- Hentrich M, Berger M, Wyen C, Siehl J, Rockstroh
JK, Müller M, Fätkenheuer G, Seidel E, Nickelsen M, Wolf T, Rieke A,
Schürmann D, Schmidmaier R, Planker M, Alt J, Mosthaf F, Engert A,
Arasteh K, Hoffmann C. Stage-adapted treatment of HIV-associated
Hodgkin lymphoma: results of a prospective multicenter study J Clin
Oncol. 2012;30:4117-23. https://doi.org/10.1200/JCO.2012.41.8137 PMid:23045592
- Hartmann P, Rehwald U, Salzberger B, Franzen C,
Sieber M, Wöhrmann A, Diehl V. BEACOPP therapeutic regimen for patients
with Hodgkin's disease and HIV infection. Ann Oncol. 2003;14:1562-9. https://doi.org/10.1093/annonc/mdg408 PMid:14504059
- Danilov AV, Li H, Press OW, Shapira I, Swinnen
LJ, Noy A, Reid E, Smith SM, Friedberg JW. Feasibility of interim
positron emission tomography (PET)-adapted therapy in HIV-positive
patients with advanced Hodgkin lymphoma (HL): a sub-analysis of SWOG
S0816 Phase 2 trial. Leuk Lymphoma. 2017;58:461-65. https://doi.org/10.1080/10428194.2016.1201573 PMid:27386786 PMCid:PMC5130311
- Gandhi M, Petrich A. Brentuximab vedotin in patients with relapsed HIV-related lymphoma. J Natl Compr Canc Netw. 2014;12:16-9. https://doi.org/10.6004/jnccn.2014.0003 PMid:24453289
- Rubinstein PG, Moore PC, Rudek MA, Henry DH,
Ramos JC, Ratner L, Reid E, Sharon E, Noy A; AIDS Malignancy Consortium
(AMC). Brentuximab vedotin with AVD shows safety, in the absence of
strong CYP3A4 inhibitors, in newly diagnosed HIV-associated Hodgkin
lymphoma. AIDS. 2018;32:605-11. PMid:29280762
- Chang E, Rivero G, Patel NR, Chiao EY, Lai S,
Bajaj K, Mbue JE, Yellapragada SV. HIV-related Refractory Hodgkin
Lymphoma: A Case Report of Complete Response to Nivolumab. Clin
Lymphoma Myeloma Leuk. 2018 Feb;18:143-46. https://doi.org/10.1016/j.clml.2017.12.008 PMid:29342442
- Desai J, Mitnick RJ, Henry DH, Llena J, Sparano
JA. Patterns of central nervous system recurrence in patients with
systemic human immunodeficiency virus-associated non-hodgkin lymphoma.
Cancer. 1999;86:1840-7. https://doi.org/10.1002/(SICI)1097-0142(19991101)86:9<1840::AID-CNCR28>3.0.CO;2-C
- Michele Spina, Emanuela Chimienti, Ferdinando
Martellotta, Emanuela Vaccher, Massimiliano Berretta, Ernesto Zanet,
Arben Lleshi, Vincenzo Canzonieri, Pietro Bulian, and Umberto Tirelli.
Phase 2 Study of Intrathecal, Long-Acting Liposomal Cytarabine in the
Prophylaxis of Lymphomatous Meningitis in Human Immunodeficiency
Virus-Related Non-Hodgkin Lymphoma. Cancer. 2010;116:1495-501. https://doi.org/10.1002/cncr.24922 PMid:20108270
- José-Tomás Navarro, Ferran Vall-Llovera,
José-Luis Mate, Mireia Morgades, Evarist Feliu, Josep-Maria Ribera.
Decrease in the frequency of meningeal involvement in AIDS-related
systemic lymphoma in patients receiving HAART. Haematologica.
2008;93:149-50. https://doi.org/10.3324/haematol.11767 PMid:18166804
- Ribera JM, Oriol A, Morgades M, González-Barca E,
Miralles P, López-Guillermo A, Gardella S, López A, Abella E, García M;
PETHEMA, GELTAMO, GELCAB and GESIDA Groups. Safety and efficacy of
cyclophosphamide, adriamycin, vincristine, prednisone and rituximab in
patients with human immunodeficiency virus-associated diffuse large
B-cell lymphoma: results of a phase II trial. Br J Haematol.
2008;140:411-9. https://doi.org/10.1111/j.1365-2141.2007.06943.x PMid:18162120
- Stefan K. Barta, Jitesh Joshi, Nicolas Mounier,
Xiaonan Xue, Dan Wang, Josep-Maria Ribera, Jose-Tomas Navarro,
Christian Hoffmann, Kieron Dunleavy, Richard F. Little, Wyndham H.
Wilson, Michele Spina, Lionel Galicier, Ariela Noy, and Joseph A.
Sparano. Central nervous system involvement in AIDS-related lymphomas.
Br J Haematol. 2016; 173:857–66. https://doi.org/10.1111/bjh.13998 PMid:27062389 PMCid:PMC4900917
- Gabarre J, Azar N, Autran B, Katlama C, Leblond
V. High-dose therapy and autologous haematopoietic stem-cell
transplantation for HIV-1-associated lymphoma. Lancet 2000;355:1071-2.
https://doi.org/10.1016/S0140-6736(00)02041-9
- Krishnan A, Molina A, Zaia J, Nademanee A, Kogut
N, Rosenthal J, Woo D, Forman SJ. Autologous stem cell transplantation
for HIV associated lymphoma. Blood 2001;98:3857-9.
https://doi.org/10.1182/blood.V98.13.3857 PMid:11739198
- Serrano D, Carrion R, Balsalobre P, Miralles P,
Berenguer J, Bu-o I, Gómez-Pineda A, Ribera JM, Conde E, Díez-Martín
JL; Spanish Cooperative Groups GELTAMO and GESIDA. HIV-associated
lymphoma successfully treated with peripheral blood stem cell
transplantation. Experimental Hematology. 2005;3:487-94. https://doi.org/10.1016/j.exphem.2004.12.008 PMid:15781340
- Balsalobre P, Diez-Martin JL, Re A, Michieli M,
Ribera JM, Canals C, Rosselet A, Conde E, Varela R, Cwynarski K,
Gabriel I, Genet P, Guillerm G, Allione B, Ferrant A, Biron P, Espigado
I, Serrano D, Sureda A. Autologous stem-cell transplantation in
patients with HIV-related lymphoma. J Clin Oncol 2009;27:2192-8. https://doi.org/10.1200/JCO.2008.18.2683 PMid:19332732
- Krishnan A, Molina A, Zaia J,Smith D, Vasquez D,
Kogut N, Falk PM, Rosenthal J, Alvarnas J, Forman SJ. Durable
remissions with autologous stem cell transplantation for high risk
HIV-associated lymphomas. Blood 2005;105:874-8. https://doi.org/10.1182/blood-2004-04-1532 PMid:15388574
- Gabarre J, Marcelin AG, Azar N, Choquet S, Levy
V, Levy Y, Tubiana R, Charlotte F, Norol F, Calvez V, Spina M, Vernant
JP, Autran B, Leblond V. High dose therapy plus autologous
hematopoietic stem cell transplantation for human immunodeficiency
virus (HIV)-related lymphoma: results and impact on HIV disease.
Haematologica. 2004;89:1100-8. PMid:15377471
- Re A, Michieli M, Casari S, Allione B, Cattaneo C, Rupolo M, Spina
M, Manuele R, Vaccher E, Mazzucato M, Abbruzzese L, Ferremi P, Carosi
G, Tirelli U, Rossi G. High-dose therapy and autologous peripheral
blood stem cell transplantation as salvage treatment for AIDS-related
lymphoma: long-term results of the Italian Cooperative Group on AIDS
and Tumors (GICAT) study with analysis of prognostic factors. Blood.
2009;114:1306-13. https://doi.org/10.1182/blood-2009-02-202762 PMid:19451551
- Spitzer TR, Ambinder RF, Lee JY, Kaplan LD,
Wachsman W, Straus DJ, Aboulafia DM, Scadden DT. Dose-reduced busulfan,
cyclophosphamide, and autologous stem cell transplantation for human
immunodeficiency virus-associated lymphoma: AIDS Malignancy Consortium
study 020. Biol Blood Marrow Transplant 2008;14:59-66. https://doi.org/10.1016/j.bbmt.2007.03.014 PMid:18158962 PMCid:PMC4524737
- Alvarnas JC, Le Rademacher J, Wang Y, Little RF,
Akpek G, Ayala E, Devine S, Baiocchi R, Lozanski G, Kaplan L, Noy A,
Popat U, Hsu J, Morris LE Jr, Thompson J, Horowitz MM, Mendizabal A,
Levine A, Krishnan A, Forman SJ, Navarro WH, Ambinder R. Autologous
hematopoietic cell transplantation for HIV-related lymphoma: results of
the BMT CTN 0803/AMC 071 trial. Blood. 2016;128:1050-8. https://doi.org/10.1182/blood-2015-08-664706 PMid:27297790 PMCid:PMC5000843
- Serrano D, Miralles P, Carrion R, Berenguer J,
Balsalobre P, Anguita J, Ribera JM, Varela R, Loscertales J, Conde E,
Arranz R, Escoda L, I. Espigado I, Rodriguez G, Diez-Martin JL on
behalf of cooperative groups GELTAMO/GESIDA. Long-term follow-up of
autologous stem cell transplant in AIDS-related Lymphoma patients.
Results of Spanish Cooperative Registry GELTAMO/GESIDA. Bone Marrow
Transplantation. 2010;45:abstract n.822.
- Diez-Martin JL, Balsalobre P, Re A, Michieli M,
Ribera JM, Canals C, Conde E, Rosselet A, Gabriel I, Varela R, Allione
B, Cwynarski K, Genet P, Espigado I, Biron P, Schmitz N, Hunter AE,
Ferrant A, Guillerm G, Hentrich M, Jurado M, Fernández P, Serrano D,
Rossi G, Sureda A; European Group for Blood and Marrow Transplantation
Lymphoma Working Party. Comparable survival between HIV+ and HIV-
non-Hodgkin and Hodgkin lymphoma patients undergoing autologous
peripheral blood stem cell transplantation. Blood. 2009;113:6011-4. https://doi.org/10.1182/blood-2008-12-195388 PMid:19307667
- Krishnan A, Palmer JM, Zaia JA, Tsai NC, Alvarnas
J. HIV status does not affect the outcome of autologous stem cell
transplantation (ASCT) for non-Hodgkin lymphoma (NHL). Biol Blood
Marrow Transplant. 2010;16:1302-8. https://doi.org/10.1016/j.bbmt.2010.03.019 PMid:20353830 PMCid:PMC2916976
- Re A, Gini G, Rupolo M, Levis A, Bandera A,
Liberati AM, Tozzi P, Cattaneo C, Casari S, Skert C, Bocci C, Spina M,
Allione B, Verga L, Michieli M, Almici C, Leali PF, Tirelli U, Rossi G.
Early consolidation with high-dose therapy and autologous stem cell
transplantation is a feasible and effective treatment option in
HIV-associated non-Hodgkin lymphoma at high risk. Bone Marrow
Transplant. 2018;53:228-30. https://doi.org/10.1038/bmt.2017.230 PMid:28991244
- Bryant A, Milliken S. Successful
reduced-intensity conditioning allogeneic HSCT for HIV-related primary
effusion lymphoma. Biol Blood Marrow Transplant. 2008;14:601-2.
https://doi.org/10.1016/j.bbmt.2008.01.010 PMid:18410904
- Gupta V, Tomblyn M, Pedersen TL, Atkins HL,
Battiwalla M, Gress RE, Pollack MS, Storek J, Thompson JC, Tiberghien
P, Young JA, Ribaud P, Horowitz MM, Keating A. Allogeneic hematopoietic
cell transplantation in human immunodeficiency virus-positive patients
with hematologic disorders: a report from the center for international
blood and marrow transplant research. Biol Blood Marrow Transplant.
2009;15:864-71. https://doi.org/10.1016/j.bbmt.2009.03.023 PMid:19539219 PMCid:PMC2881828
- Ambinder RF, Wu J, Logan B, et al. Allogeneic
hematopoietic cell transplant (alloHCT) for hematologic malignancies in
human immunodeficiency virus infected (HIV) patients (pts): Blood and
Marrow Transplant Clinical Trials Network (BMT CTN 0903/AIDS Malignancy
Consortium (AMC-080) trial. J Clin Oncol. 2017; 35:abstract n.7006
[TOP]






