A.A. Bukar1, M.M. Sulaiman2, A.I. Ladu1, A.M. Abba1, M.K. Ahmed1, G.T. Marama1 and U.M. Abjah1.
1 Department of Haematology, University of Maiduguri,.P.M.B 1069, Maiduguri Nigeria.
2 Department of Medicine (Nephrology Unit), University of Maiduguri. P.M.B 1069, Maiduguri Nigeria.
Correspondence to: Dr A.I. Ladu Department of Haematology, College
of Medical Sciences, University of Maiduguri, P.M.B D1069. Maiduguri,
Nigeria. Email:
adamaisahladu@gmail.com
Published: January 1, 2019
Received: June 5, 2018
Accepted: November 26, 2018
Mediterr J Hematol Infect Dis 2019, 11(1): e2019010 DOI
10.4084/MJHID.2019.010
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Background:
Involvement of the kidneys in patients with sickle cell anaemia is a
well recognised chronic complication. This study seeks to determine the
prevalence of chronic kidney disease in patients with homozygous sickle
cell disease (HbSS) and to identify risk factors associated with its
development.
Methodology:
The subjects consisted of adolescents and adults with HbSS recruited
sequentially from the adult haematology outpatient clinic and Daycare
ward of the unit. Clinical variables including age at diagnosis of SCA,
the frequency of vaso-occlusive crisis and transfusion therapy, as well
as laboratory data including haematological profile and renal function
tests were obtained. The glomerular filtration rate was estimated
(eGFR) using the ‘modification of diet in renal disease’ (MDRD) formula.
Results:
Two hundred and eighty-four HbSS patients were recruited. The
prevalence of CKD amongst them was 38.9%. Further stratification of the
patients based on eGFR showed that sixty- nine (26.8%) had
hyperfiltration; 35 (13.6%) stage 1 CKD; 53 (20.6%) stage 2 CKD; 33
(12.8%)
stage 3a CKD; 28 (10.9%) stage 3b CKD; 30 (11.7%) stage 4
CKD and 9 (3.5%) had end stage renal disease. There was significant
association between eGFR and clinical parameters such as age (r -0.353,
p=0.000), SBP (r -0.148, p= 0.021), DBP (r -0.213, p=0.001) and total
number of blood received (r -0.276, p=0.000); and laboratory parameters
such as PCV (r 0.371, p=0.000); urea ( r 0.527, p=000 ); creatinine (r
0.625, p=0.000) and uric acid ( r -0.419, p=0.000).
Conclusions:
The present study has revealed a high prevalence of CKD amongst
patients with SCA in our region. Clinical and laboratory predictors of
CKD using eGFR were identified to include age, SBP, number of units of
blood transfusion, PCV, urea, creatinine and uric acid levels.
|
Introduction
Homozygous
sickle cell disease (HbSS) is an inherited disorder of haemoglobin
resulting from single nucleotide change i.e. substitution of thymine
for adenine in the sixth codon of the beta globin-chain gene, this
causes coding of amino acid valine instead of glutamate in position 6
of the beta-globin chain resulting in formation of an abnormal
haemoglobin termed haemoglobin S (HbS).[1] The haemoglobin S derived from this mutation forms polymers if it undergoes deoxygenation;[2] this polymerisation is the primary though not the exclusive cause of the clinical manifestation of sickle cell anaemia (SCA).[1,2,3] The net effects of polymerized haemoglobin are membrane damage,[4] cell dehydration[5,6] and altered rheology[7] with resulting chronic haemolysis and vaso-occlusion;[8]
hence the dominant clinical features of haemolytic anaemia and painful
crisis. The clinical manifestation of SCA varies, ranging from mild
clinical disease diagnosed incidentally to very severe debilitating
disease.[1] Individuals with HbSS disease can be in
relatively good health termed ‘steady state’ or present with acute
exacerbation of symptoms termed ‘crisis’ or can present with chronic
complications manifesting in virtually all systems in the body such as
the brain, skeletal system and the kidneys.[1,3,9]
Involvement
of the kidneys by sickle cell anaemia is termed sickle cell nephropathy
and is a well-recognised entity in sickle cell disease.[10-15]
The renal manifestations of sickle cell disease include hyposthenuria,
haematuria due to papillary necrosis, proteinuria which can vary from
microalbuminuria to nephrotic range, focal glomerulosclerosis that can
lead to end-stage renal disease and renal medullary carcinoma.[10,13-15] Sickle cell nephropathy (SCN) progresses steadily with different manifestations in various decades of life.[10,11,14,15]
This heterogeneity in presentation manifests in the first decade of
life as decreased medullary blood flow, nocturia, enuresis,
hyposthenuria and glomerular hyperfiltration;[11] these proceed to microscopic haematuria due to renal papillary necrosis and loss of vasa recta in the second decade of life.[11,14]
The findings in patients in the third decade may include gross
haematuria secondary to renal papillary necrosis,
interstitial nephritis, membranoproliferative
glomerulonephritis, decreased renal blood flow and glomerular
filtration rate. Pyelonephritis, decreased uric acid clearance and
hypertension starts from the fourth decade of life[11,14,15] with eventual progression to chronic kidney disease (CKD)/ end-stage renal disease.[14,15]
The
index study is a single centre study aimed at finding the prevalence of
chronic kidney disease and at identifying its risk factors among adult
patients with HbSS. The previous studies[12,13] have
revealed the enormous burden of CKD and SCN with varying reports of
prevalence of SCN ranging from 5-18% of the total SCA population
worldwide.[15,16,17,18] Studies by Arogundade[16] et al. and later by Bolarinwa et al[17]
identified the risk factors and clinical course of SCN in South-Western
Nigerian. But such study does not exist in our centre which may be
different being located in Northeastern Nigeria, a region having one of
the worst health care indices in the country[19] and peculiar geographical characteristics which have been shown to affect the morbidity of SCA,[20] which further buttress the justification for this study.
Patients and Methods
This
was a single centre cross- sectional study carried out at University of
Maiduguri teaching hospital, Borno state, northeastern Nigeria. The
subjects consisted of adolescents and adults with SCA diagnosed using
Hb electrophoresis at pH 8.6. Patients were recruited sequentially from
the adult haematology outpatient clinic and Daycare ward of the unit
from January 2013 to April 2018 (5 years period). Socio- demographic
(age, sex), anthropometric (height, weight), systolic and diastolic
blood pressure were recorded during a routine physical examination.
Clinical variables including age at diagnosis of SCA, the frequency of
vaso-occlusive crises, number of hospitalisations per annum and
transfusion therapy were collected. Laboratory data including
haematological profile, renal function test were also obtained. The
glomerular filtration rate was estimated (eGFR) using the ‘modification
of diet in renal disease’ (MDRD) formula 21. Proteinuria was reported
as trace or 1+–4+ from the dipstick. The staging of kidney disease was
based on the Kidney Disease Outcome Quality Initiative (K/DOQI)22
recommended classification system as follows: G1: GFR>90 ml/min/1.73
m2; G2: GFR 60–89 ml/min/1.73 m2; G3a: GFR 45–59 ml/min/1.73 m2; G3b: GFR30-44, G4: GFR 15–29 ml/min/1.73 m2 , G5: GFR < 15 ml/ min/1.73 m2.
Albuminuria level A1: < 30mg/g, A2: 30-300mg/g, A3: >
300mg/g. Patients who have GFR < 60ml/min/1.73 m2
and/or albuminuria >30mg/g are considered to have chronic kidney
disease. Hyperfiltration is defined as GFR >120ml/min/1.73 m2 and albuminuria < 30mg/g in females and >130ml/min/1.73 m2 and albuminuria < 30mg/g in males. In the current study, CKD was classified based on EGFR only.
Statistical analysis.
Data collected were transferred into Excel (Microsoft, Seattle, WA,
USA) and SPSS version 20 (IB Corp. Armonk, NY, USA). Variables were log
transformed where appropriate to obtain a normal distribution. The
student t-test was used for continuous variables and chi-square for
categorical variable for comparisons of means and proportions amongst
subgroups. Associations between continuous variables were assessed
using Pearson’s correlation. For all analysis, a p-value of <0.05
was considered significant.
Results
Clinical and laboratory parameters of study participants.
Two hundred and eighty-four patients were recruited during the study
period, 27 (9.5%) patients were excluded from the study due to
incomplete data. There were a roughly equal number of males 129 (50.2%)
and females 128 (49.8%).
The clinical and laboratory parameters of the participants are shown in Table 1.
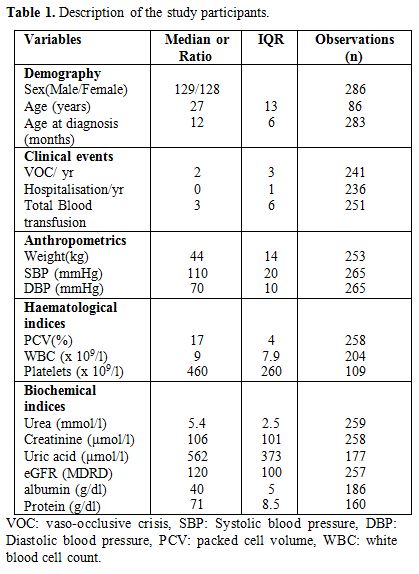 |
Table 1. Description of the study participants. |
Prevalence and various stages of CKD.
A total of 257 HbSS patients had glomerular filtration estimates
available. The median serum creatinine was 106 µmol/l (IQR 101) and
eGFR was 120 ml/min/1.73m2 (IQR 100) (Table 1).
The results showed that one hundred patients (38.9%) had CKD (GFR<60
ml/minute) whereas 157 (61.1%) patients had normal renal parameters.
Further stratification of subjects based on eGFR showed that sixty-nine
(26.8%) had hyperfiltration and 9 (3.5%) had end-stage renal disease (Table 2). The prevalence of CKD was slightly higher amongst the male patients (55%) compared to the female population (45%)
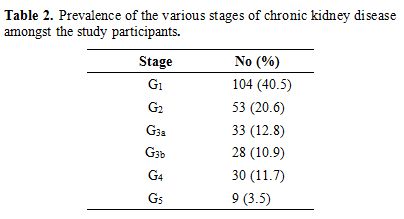 |
Table
2. Prevalence of the various stages of chronic kidney disease amongst the study participants. |
Association of clinical and laboratory parameters amongst patients with and without CKD.
Patients with CKD were found to be older than patients with normal
kidney function (p=0.000). There was no gender variation between
patients with CKD compared with those with normal kidney function
(p=0.219). Also, there was no significant difference between the age of
diagnosis of SCA (p=0.847), a number of vaso- occlusive crises (p=
0.536) and the total number of admissions per annum (p=0.425) amongst
patients with and without CKD. Similarly, the weight (p=0.742), SBP
(p=0.179) and DBP (p=0.071) showed no comparable difference. In
contrast to these clinical parameters, there was a significant
difference in the median PCV (p=0.000), urea (p=0.000), creatinine
(p=0.000) and uric acid (p=0.000) of patients with CKD compared to
those without (Table 3).
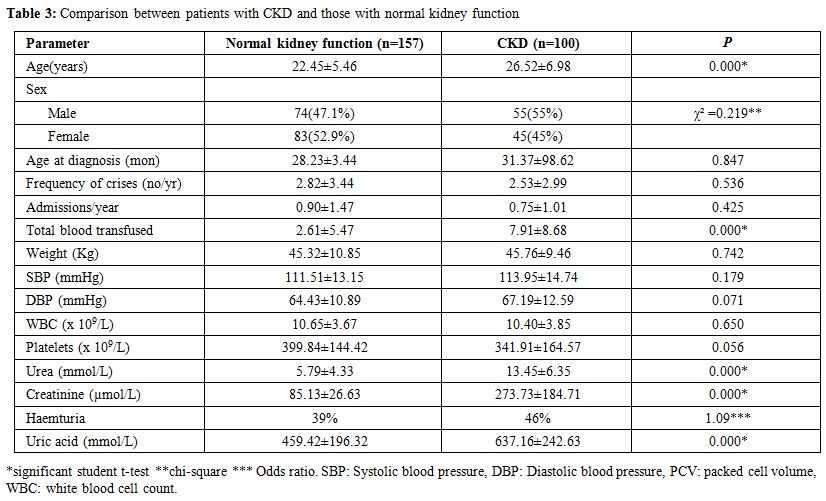 |
Table 3. Comparison between patients with CKD and those with normal kidney function |
Clinical and laboratory factors affecting eGFR. The
eGFR was significantly and negatively associated with the median values
for age (r -0.353, p=0.000), SBP (r - 0.148, p= 0.021), DBP (r -0.213,
p=0.001) and total number of blood units received (r -0.276, p=0.000).
The median PCV (r 0.371, p=0.000), urea (r 0.527, p=000), creatinine (r
0.625, p=0.000) were all positive predictors of eGFR. There was a
negative correlation between eGFR and uric acid level (r -0.419,
p=0.000). There was no significant association between the eGFR and
clinical parameters such as patient sex, number of vaso- occlusive
crises per annum and age at diagnosis of SCA; or laboratory indices
such as WBC and platelets count (Table 4).
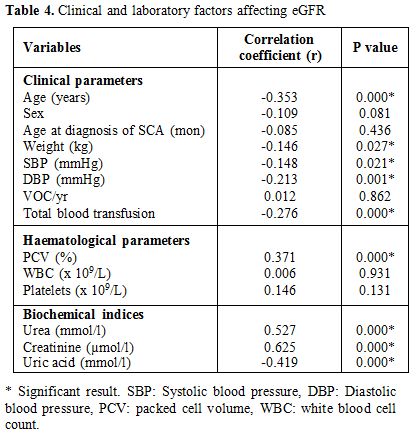 |
Table 4. Clinical and laboratory factors affecting eGFR. |
Out
of 257 patients studied, 85 (33.1%) had haematuria whereas 172 (66.9%)
had no haematuria. Haematuria was noted in 36 (35.0%) of the patients
with GFR < 60ml/min/1.73 m2 and 49 (32.0%) of those with GFR > 60ml/min/1.73 m2 (Table 5).
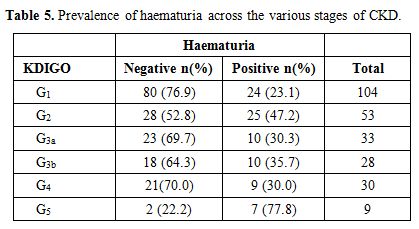 |
Table 5. Prevalence of haematuria across the various stages of CKD. |
Discussion
Advances
in the medical care of patients with SCA has made it possible for this
individual to live longer and as such, they are confronted with
long-term complications of the condition such as chronic kidney
disease. This study is the first in Northeastern Nigeria to determine
the prevalence of CKD amongst patients with SCA and risk factors
contributing to its development. The median age of the patients in this
study was similar to that found in several similar studies by
Arogundade et al.[16] and Bolarinwa et al.[17] in South-West Nigeria as well as Powars et al.[18]
The results indicate a high prevalence of CKD (38.9%) amongst the
sickle cell population. This finding is similar to that obtained in the
study by Arogundade et al;[16] however, this is higher than the prevalence of CKD in the non-sickle cell population in Nigeria,[23,24] indicating an additional impact of HbSS on the prevalence of kidney disease.
The
prevalence of glomerular hyperfiltration of 26.8% was comparable
to the previous study by Bolarinwa from South Western Nigeria; but
differ with previous occurrence rates in reports from other parts of
the world.[25,26,27] Hyperfiltration is an early marker of renal dysfunction, with an increased risk of progression into CKD and ESRD.[11,28] Vazquez et al[26]
have found increased levels of nephrin in the urine of HbSS patients
with persistent hyperfiltration suggests that glomerular damage is
caused by hyperfiltration. Future research on this group of patients
could lead to the discovery of useful interventions that can prevent
the progression into sickle cell nephropathy. About one-third of our
patient's population belonged to the category of early stage renal
disease (34% combined rates for stages 1 and 2 CKD), in contrast to
66.7%,[17] 88.8%[28] and 53%[30]
rates previously observed. The British guideline on the classification
of CKD recognises this group to be associated with the tendency for
worsening kidney function and cardiovascular complications.[31] The K/DOQI also recognises individuals in this category of renal disease to be at risk of progressive disease.[22]
The prevalence rates for stage 3 renal disease was 23.7%, in contrast,
Bolarinwa et al. and Yusuf et al. reported lower rates of 2.7% and 9.5%
respectively, and Aneke et al.[30] reported a higher
rate of 42% from their study. Stage 3 renal disease marks a critical
point in the spectrum of CKD because it represents the beginning of
established kidney disease.[22] Identifying patients
in this category is important in ensuring measures can be taken to slow
the progression to more advanced stages.
Furthermore, our result
showed a combined prevalence rate of 15.2% for stage 4 CKD and ESRD, in
contrast to previously reported rates of 1.4% and 5% from earlier
studies.[29,30] However, these other studies
investigated a a small number of subjects compared to our large cohort.
Overall, our result shows that more than two third of our patients
present with advanced stage CKD (stage 3 and above). It is possible
that other yet to be identified factors may be explaining these
differences, such as environmental and social factors.
The current
study identified some important risk factors associated with the
development of renal dysfunction. Our results replicate the previously
reported positive association of eGFR with clinical parameters
including age,[23,24,25] DBP[25] and total blood transfusion received;[30] and with laboratory parameters, such as haematocrit, serum levels of urea, creatinine and uric acid.[25] Recent studies by Drawz et al.[26]
has shown that age is inversely related to GFR among HbSS patients.
Both age and DBP have been associated as risk factors for the
development of microalbuminuria, a marker of early renal impairment.[11,17] Several earlier studies have shown that anaemia correlated with GFR.[16,17,18] An elaborate study by Saraf et al[33]
showed that haemoglobinuria was associated with progression of CKD in
SCA. However, the index study did not assess for haemoglobinuria, but
the prevalence of haematuria was assessed and noted to be more in
patients with CKD. Chronic kidney disease patients have reduced
response to erythropoietin. Thus their anaemia tends to worsen with the
progression of the disease process. In this study, there was a slight
male preponderance among patients who developed kidney disease which is
consistent with male sex being a risk factor for kidney disease and its
progression.[25] However, markers of severity of
sickle cell disease, such as the frequency of painful crisis and age at
diagnosis of SCA were not associated with the development of renal
dysfunction in contrast with previous reports.[16,17,18]
It is possible that a large proportion of patients who were diagnosed
early in this study may have received care that reduced their risk of
developing kidney disease.
Conclusions
The
present study has revealed a high prevalence of CKD amongst patients
with SCA in this region (38.9%; N=257). About a quarter of these
patients had hyperfiltration, while more than two-third had advanced
stage CKD (stage 3 and above). Age, SBP and DBP, total blood
transfusion were important clinical predictors of eGFR; while
haematocrit, urea, creatinine and uric acid levels significantly
predicted eGFR.
Future direction and way forward.
Monitoring and detection of early stages will allow for interventions
which may delay progression into advanced stages and ESRD. Further
studies to correlate such parameters as following up those with
hyperfiltration and also studies to elucidate yet to be identified
environmental, genetic or epigenetic risk factors for CKD in SCA in our
region will be pursued.
References
- Weatherall DJ. Genetic disorders of haemoglobin.
In: Hoffbrand AV, Lewis SM, Tuddenham EGD (eds). Postgraduate
Haematology 4th edition. Great Britain. Butterworth- Heinemann, 1999:
91-119.
- Eaton WA, Hofrichter J. Sickle haemoglobin polymerization. Adv Protein Chem. 1990; 40:63. https://doi.org/10.1016/S0065- 3233(08)60287-9
- Beutler
E. The sickle cell diseases and related problems. In: Williams JW,
Beutler E, Erslev AJ, Lichtman M (eds). Haematology 4th edition. New
York: McGraw- Hill, 1990: 613- 644.
- Noguchi
CT, Schechter AN. Sickle haemoglobin polymerization in solution and in
cells. Annual Review of Biophysics and Biophysical chemistry. 1985; 14:
239-263 https://doi.org/10.1146/annurev.bb.14.060185.001323 PMid:3890882
- Mohandas
N, Ross ME, Clark MR. Association between morphologic distortion of
sickle cells and deoxygenation- induced cation permeability increase.
Blood. 1986; 68 (2): 450-454 PMid:3730609
- Lew
VL, Etzion Z, Bookchin RM. Dehydration response of sickle cells to
sickling- induced Ca++ permeabilization. Blood. 2002; 99(7): 2578-
2585. https://doi.org/10.1182/blood.V99.7.2578 PMid:11895796
- Akinola
NO, Stevens SME, Franklin IM, Nash GB, Stuart J. Rheological changes in
the prodromal and established phases of sickle cell vaso- occlusive
crisis. British Journal of Haematology, 1992, 81: 598-602. https://doi.org/10.1111/j.1365-2141.1992.tb02998.x PMid:1390248
- Bookchin
RM, Lew VL. Pathophysiology of sickle cell anaemia. Haematology/
Oncology Clinic of North America. 1996; 10 (6): 1241- 1254. https://doi.org/10.1016/S0889-8588(05)70397-X
- Flemming
AF. The presentation, management and prevention of crises in
sickle-cell disease in Africa. Blood rev. 1989; 31: 18–28. https://doi.org/10.1016/0268-960X(89)90022-2n
- Bainbridge
R, Higgs DR, Maude GH, Serjeant GR. Clinical presentation of homozygous
sickle cell disease. J pedtr. 1985; 106: 881. https://doi.org/10.1016/S0022-3476(85)80230-4
- Serjeant GR. Sickle cell disease. Oxford. Oxford University Press. 1992; 261.
- Akinsola
W, Odesanmi WO, Ogunniyi JO, and Ladipo GOA. Diseases causing chronic
renal failure in Nigerians- a prospective study of 100 cases. Afr J Med
Sci. 1989; 18(2): 131–137.
- Ulasi II,
Ijoma CK. The enormity of chronic kidney disease in Nigeria: The
situation in a teaching hospital in south east Nigeria. Journal of
Tropical Medicine. 2010 https://doi.org/10.1155/2010/501957 PMid:20613945 PMCid:PMC2896838
- Pham
PT, Pham PC, Wilkinson AH, Lew SQ. Renal abnormalities in sickle cell
disease. Kidney Int. 2000; 57: 1.
https://doi.org/10.1046/j.1523-1755.2000.00806.x PMid:10620181
- Saborio P, Scheinman JI. Sickle cell Nephropathy. J Am Soc Nephrol. 1999; 10: 187- 192. PMid:9890326
- Arogundade
FA, Sanusi AA, Hassan MO, Salawu L, Durosinmi MA, Akinsola A (2010). An
appraisal of renal dysfunction and its risk factors in patients with
sickle cell disease. Nephron. Clin. Pract. 118: 225- 231. https://doi.org/10.1159/000321138 PMid:21196767
- Bolarinwa
RA, Akinlade KS, Kuti M et al: Renal disease in adult Nigerians with
sickle cell anaemia: a report of prevalence, clinical features and risk
factors. Saudi J Kidney Dis. Transpl 2012, 23:171- 175. PMid:22237246
- Powars
DR, Elliot-Mills DD, Chan L, Hiti AL, Opas LM, Johnson C: chronic renal
failure in sickle cell disease: Risk factors, clinical course and
mortality. Ann Intern med 115: 614-620. https://doi.org/10.7326/0003-4819-115-8-614 PMid:1892333
- National
Population Commission Federal republic of Nigeria. Nigeria Demographic
and Health survey preliminary report. Calverton, Maryland, USA. Measure
DHS, ICF macro, 2008; 17-24.
- Ahmed SG,
Kagu MB, Abjah UA, Bukar AA. (2012) Seasonal variations in the
frequencies of acute vaso- occlusive Morbidities among sickle cell
anaemia and patients in Northern Nigeria. Journal of Blood disorders
and Transfusion. 01/2012; 3(120)
- Levey
AS, Bosch J, Lewis J, Greene T, Rogers N and Roth D. A more accurate
ethod to estimate glomerular filtration rate from serum creatinine: a
new prediction equation. Modification of Diet in Renal disease study
group. Annals of internal medicine, 1999, 130, 461-470 https://doi.org/10.7326/0003-4819-130-6-199903160-00002 PMid:10075613
- K/DOQI:
Clinical practice guidelines for chronic kidney disease: evaluation,
classification, and stratification. Am J Kidney Dis 2002; 39:S1–S266.
PMid:11904577
- Wachukwu CM, Emem-Chioma
PC, Wokoma FS, Oko-Jaja RI. Prevalence of risk factors for chronic
kidney disease among adults in a university community in southern
Nigeria. Pan African Medical Journal. 2015; 21:120
doi:10.11604/pamj.2015.21.120.7079 https://doi.org/10.11604/pamj.2015.21.120.7079
- Egbi
OG, Okafor UH, Miebodei KE, Kasia BE, Kunle-Olowu OE, Unuigbe EI.
Prevalence and correlates of chronic kidney disease among civil
servants in Bayelsa state, Nigeria. Niger J Clin Pract 2014;17:602- 7. https://doi.org/10.4103/1119-3077.141426 PMid:25244271
- Geard
A, Pule GD, Chemegni BC, Bitoungui VJN, Kengne AP, Chimusa ER et al.
Clinical and genetic predictors of renal dysfunction in sickle cell
anaemia in Cameroon. British Journal of Haematology, 2017; 178 :
629-639 https://doi.org/10.1111/bjh.14724 PMid:28466968 PMCid:PMC5660286
- Vazquez
B, Shah B, Zhang X, Lash J P, Gordeuk V R, Saraf S L. Hyperfiltration
is Associated with Microalbuminuria in Patients with Sickle Cell
Anemia. Am J Hematol. 2014 December; 89(12): 1156-
1157. https://doi.org/10.1002/ajh.23817 PMid:25132221 PMCid:PMC4237621
- Aloni
MN, Ngiyulu RM, Gini-Ehungu JL, Nsibu CN, Ekila MB and Lepira FB. Renal
function in children suffering from sickle cell disease: challenge of
early detection in highly resource-scarce settings. PLoS ONE,
2014,9,:1-5. https://doi.org/10.1371/journal.pone.0096561 PMid:24810610 PMCid:PMC4014510
- AI
Ladu, AM Abba; 2017. A Review of Prevalence, Risk Factors and Clinical
Significance of Microalbuminuria in Patients with Sickle Cell Anaemia.
Sokoto Journal of Medical Laboratory Science, 2(2): 141 – 145
- Yusuf
R, Hassan A, Ibrahim IN, Babadoko AA, Ibinaiye PO. Assessment of kidney
function in sickle cell anemia patients in Zaria, Nigeria. Sahel Med J
2017; 20:21-5.
- Aneke JC, Adegoke AO,
Oyekunle AA, Osho PO, Sanusi AA and Okocha EC et al. Degrees of Kidney
Disease in Nigerian Adults with Sickle-Cell Disease. Med Princ Pract
2014;23:271–274 https://doi.org/10.1159/000361029 PMid:24751459 PMCid:PMC5586881
- Clinical guideline 73: Chronic Kidney Disease. London, National Institute for Health and Clinical Excellence, 2008, pp 4.
- Drawz
P, Ayyappan S, Nouraie M, Saraf S, Gordeuk V, Hostetter T, Gladwin M T,
Little J. Kidney Disease among Patients with Sickle Cell Disease,
Hemoglobin SS and SC. Clin J Am Soc Neph; 2015 Feb 5;
11(2): 207-215. https://doi.org/10.2215/CJN.03940415 PMid:26672090 PMCid:PMC4741034
- Saraf
SL, Zhang X, Kanias T, Lash JP et al. Haemoglobinuria is associated
with chronic kidney disease and its progression in patients with sickle
cell anaemia. (2014): 165 (5): 729- 739.
[TOP]




