Mingyue Sun1, Chunguang Chen2, Weiqiang Xiao1, Yanmin Chang1, Cailin Liu3 and Qingxia Xu1.
1 Department
of Clinical Laboratory, Affiliated Cancer Hospital of Zhengzhou
University & Henan Cancer Hospital, Zhengzhou, Henan, People's
Republic of China
2 Department of Clinical Laboratory, The Sixth People's Hospital of Zhengzhou City, Zhengzhou, People's Republic of China.
3
Department of Clinical Laboratory, The First Affiliated Hospital of
Zhengzhou University, Zhengzhou, People's Republic of China.
Correspondence to: Qingxia Xu. Department of Clinical Laboratory,
Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer
Hospital. Dongming Road No.127, Zhengzhou, Henan province, China. Tel
+0371-65587147, Fax +0371- 65587147. E-mail:
qxiaxu@126.com
Published: January 1, 2019
Received: July 20, 2018
Accepted: November 26, 2018
Mediterr J Hematol Infect Dis 2019, 11(1): e2019012 DOI
10.4084/MJHID.2019.012
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
This study aimed to identify the risk factors of candidemia and asses possible clinically significant differences between Candida parapsilosis and other Candida species
in a Chinese tertiary cancer center over six years. A total of 323
cancer patients were enrolled and analyzed from 2012 to 2018. Among the
isolates, the species most frequently isolated was C. parapsilosis (37.15%, 120/323), and C. albicans only accounted for 34.37%. Based on statistical analysis, when candidemia patients who had C. parapsilosis were compared with other Candida spp., the following factors were found to be significantly associated with C. parapsilosis
fungemia: parenteral nutrition (p < 0.001), neutropenia (p <
0.001), receipt of chemotherapy (p = 0.002), and previous antifungal
use (p < 0.001). Parenteral nutrition was a factor that
independently predicted C. parapsilosis candidemia (OR, 0.183; 95% CI, 0.098–0.340; p < 0.001).In short, C. parapsilosis as the leading non-albicans Candida spp.
isolates in candidemia are posing a major threat for cancer patients.
The study highlights the urgent need to evaluate the possibility of
development of C. parapsilosis
candidemia in cancer patients exposed to these risk factors effective
and prevention strategies against this causative agent transmitted
through nosocomial route should be implemented.
|
Introduction
Candida species are among the most important causes of nosocomial bloodstream infection (BSI).[1]
Candidemia was cited as the fourth most prevalent nosocomial BSI in the
United States and seventh to tenth in population-based studies with
mortality of around 40%.[2-5] It is, therefore, a public health concern worldwide.[6]
Numerous
surveillance programs have focused on candidemia and have documented
the prevalence of different Candida species. Until recently, C. albicans
was the predominant Candida spp. isolated from patients with nosocomial
candidemia. However, in recent years, there has been an increase in the
proportion of non-albicans Candida spp. (NAC) isolates, and in some
European and Latin American centers, it has overtaken C. albicans as the predominant cause of nosocomial candidemia.[7-9]
Considering the different worldwide distribution of Candida spp., some
researchers have recommended that the epidemiology of Candida
infections should be studied at local levels rather than on a worldwide
scale.[10]
There is a consensus that antifungal therapy should be initiated before candidemia ensues to avoid mortality,[8] considering that the incubation time has a statistically significant impact on in-hospital mortality,[1] and delaying empirical treatment for more than 12 h is associated with high mortality.[11] Duration of therapy is an important point.[12] What’s more, NAC is associated with stronger biofilm production than C. albicans spp.[13-15]
Thus, eradication of NAC candidemia is likely to require high doses of
fluconazole or other effective agents (e.g., echinocandin or
amphotericin B).[8,16] Epidemiological data that can help differentiate NAC from C. albicans infections may, therefore, be important in selecting the appropriate antifungal treatment.
Although
studies to date have sought to identify specific risk factors for
nosocomial NAC candidemia, available data mostly come from Western
countries.[8] Even though several studies had reported
the epidemiology of Candida infections in China, they mainly focused on
adults or special groups, such as neonates.[17,18] In China, investigations on C. parapsilosis compared with Candida non-parapsilosis and C. albicans
compared with NAC candidemia in malignancy groups are limited. We
performed this retrospective study to investigate the epidemiology of
candidemia among cancer patients in central China. Our findings may
facilitate the application of antifungal prophylaxis to patients
at greatest risk and contribute to prognosis improvement.[5]
Material and Methods
This
retrospective study was carried out at Henan Cancer Hospital, a
2,991-bed special hospital located in Henan, China. From 1 March 2012
to 28 February 2018, all patients with positive blood culture for
Candida species were identified.
Candidemia was defined as at
least one positive blood culture for Candida spp. in patients
hospitalized for more than 48 h. Those without complete case files were
excluded. When a case of candidemia was identified, the following data
were collected in a standardized case report form: demographics,
underlying medical conditions, exposure to invasive medical procedures,
immunosuppressive therapy, use of antibiotics and prophylaxis
antifungal agent (fluconazole), and antifungal therapeutic duration
(including the prophylaxis use of antifungal agent prior to the
occurrence of candidemia and treatment during candidemia), use of H2
blockers and 30-day survival, presence of central venous catheter (CVC)
and subsequent removal, the CVC was considered to be removed if this
procedure was performed during the first 3 days following the first
blood culture positive for Candida infection.
Catheter-related
bloodstream infections were defined as 1) a colony count of blood
obtained through the catheter hub that was >5-fold higher than that
in blood obtained from a peripheral vein or 2) a catheter tip culture
that was positive for Candida spp.[19] Delayed
treatment was defined when treatment was started >2 days from blood
culture or when treatment was not started because the patient was dead
when the diagnosis was established. All clinical data were
collected within 30 days prior to the first positive blood culture, and
crude mortality referred to the ratio of death within 30 days after the
first positive blood culture. This study obtained permissions from the
Bioethics Committee of Affiliated Cancer Hospital of Zhengzhou
University & Henan Cancer Hospital and participants (consent to
participate was obtained from participants) to review patient records
and use the data. Types of cancer were differentially diagnosed by
pathological examination. Recurrent BSI was defined as an episode of
infection occurring at least one month after the initial diagnosis.
Neutropenia was defined as an absolute neutrophil count of <1.5×109/L.
Blood
samples were cultured in the BACTEC-FX system (BD, USA). All positive
cultures were manually sampled and inoculated on CHROMagar
Candida medium (Autobio, Zhengzhou, China) to ensure viability and
purity. An aliquot was Gram- stained for preliminary identification of
the microorganism. All species were identified using the API 20C AuX
system (Biomérieux, France). Antifungal susceptibility tests were
performed using the broth microdilution assay according to the Clinical
and Laboratory Standards Institute (CLSI, formerly NCCLS), M27-A2
document.[20] Statistical analysis was performed
using the SPSS 22 software (SPSS Inc., Chicago, IL, USA). Univariate
analysis was performed using Fisher exact test or Chi-squared test (as
appropriate) for categorical variables. All tests were two-tailed, and
a level of significance of p < 0.05 was considered statistically
significant. Parameters related to C. parapsilosis candidemia and C. albicans candidemia were analyzed by multivariate logistic regression.
Results
During
the study period, 323 episodes of candidemia occurred in 323 patients,
58 with hematological malignancies (17.95%), and 265 (82.04%) with
solid tumors (STs). The overall incidence rate was 1.3 episodes/1000
hospital admissions. The overall incidence rate of hematological
malignancies was higher than STs (1.6 episodes/1000 hospital admissions
vs. 0.6 episodes/1000 hospital admissions). C. parapsilosis was the most frequently isolated from blood cultures (37.15%, 120/323), followed by C. albicans (34.37%, 111/323), C. tropicalis (16.10%, 52/323), and C. glabrata (8.98%, 29/323). Other less common species included C. krusei, C. guilliermondii, C. dubliniensis, and C. lusitaniae.
There
were 186 males and 137 females. The average age was 52.81 ± 18.38
years. The median time from admission to the first positive blood
specimen was 19 days. There were 155 patients from surgical wards
(47.99%), 141 patients from medical wards (43.65%), and 27 patients
from the ICU (8.36%). Common underlying diseases and risk factors 30
days prior to the first positive blood culture are listed in Table 1.
Most of the cases patients with candidemia had received antibiotic
therapy (91%) and had an indwelling CVC (83.3%) at the time of
infection. CVCs were removed within 72hours from the onset of
candidaemia in 96 patients (29.7%). CVC-related candidaemia was more
likely to occur in non-albicans Candida spp. isolates. Advanced age,
STs, abdominal surgery, and ICU stay at diagnosis were related with C. albicans candidemia.
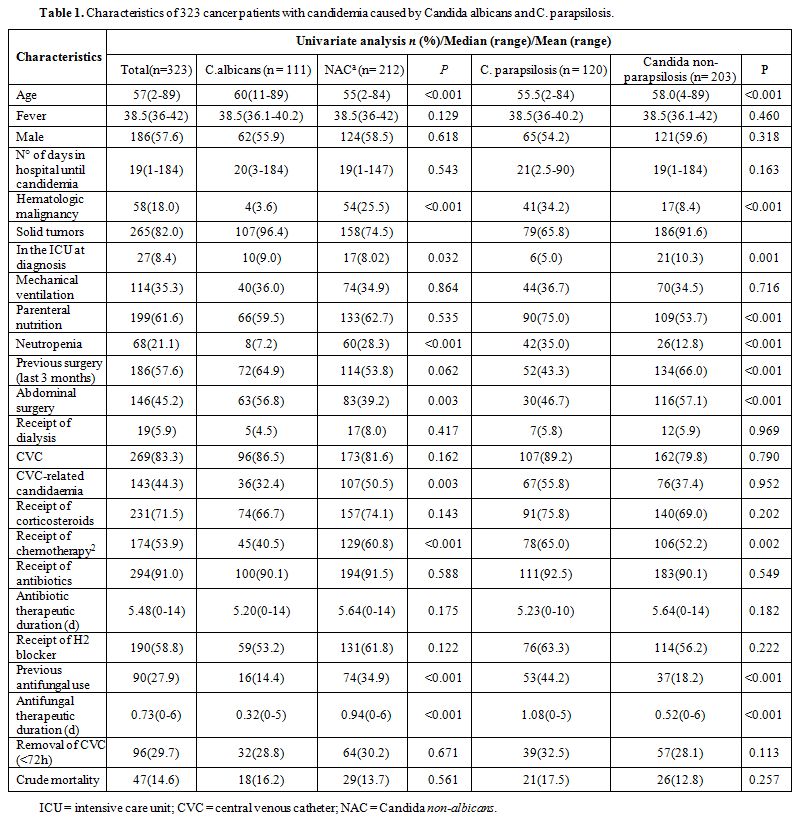 |
Table
1. Characteristics of 323 cancer patients with candidemia caused by Candida albicans and C. parapsilosis. |
When C. parapsilosis was compared with Candida non-parapsilosis candidemia (Table 2), the cases of C. parapsilosis
BSI were exposed more frequently to parenteral nutrition and CVC and
less frequently to surgery. As regards the underlying diseases, both
neutropenia and previous antifungal use were associated with C. parapsilosis candidemia, whereas STs and ICU stay at diagnosis were related to non-C. parapsilosis candidemia. Moreover, parenteral nutrition and receipt of chemotherapy were associated with C. parapsilosis candidemia. However, advanced age and surgery were correlated with non-C. parapsilosis candidemia. In a model of multivariate independently predicting C. parapsilosis candidemia (OR, 0.183; 95% CI, 0.098–0.340; p < 0.001). Another factor that predicted C. albicans
candidemia was type of cancer (OR,
0.164; 95% CI, 0.030–0.899; p = 0.036). In other
words, solid malignancy is a factor independently predicting C. albicans, and hematologic malignancy occurs more frequently with C. parapsilosis candidemia (Table 3).
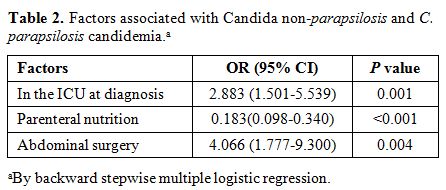 |
Table
2. Factors associated with Candida non-parapsilosis and C. parapsilosis candidemia.a |
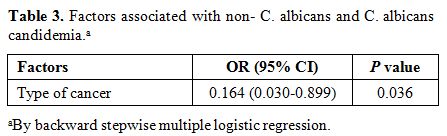 |
Table 3. Factors associated with non- C. albicans and C. albicans candidemia.a |
As shown in Table 4,
the susceptibility test of antifungal drugs was performed for four
mainly isolates of Candida species. Concern need be addressed on C. albicans, C. tropicals and C. glabrata which had higher MICs to fluconazole than C.parapsilosis.
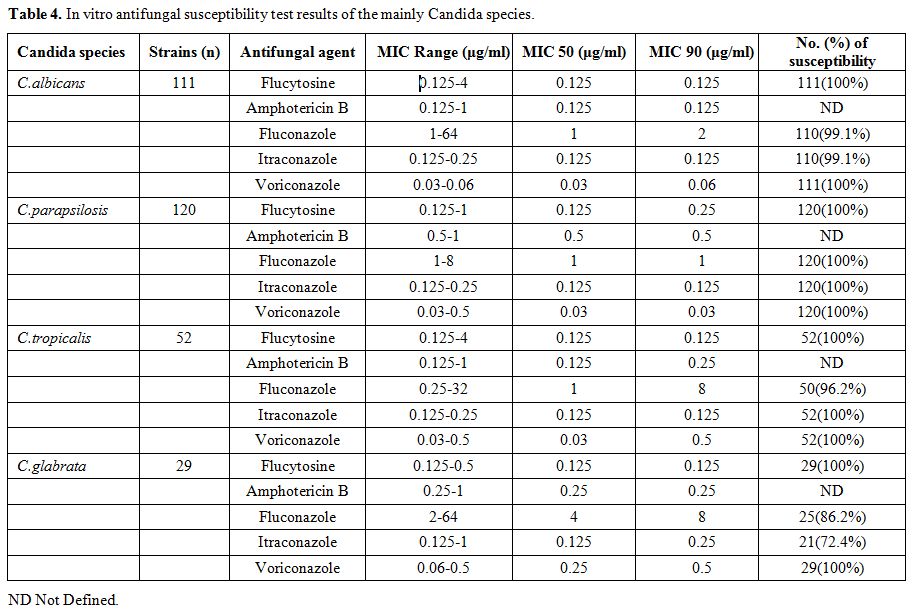 |
Table 4. In vitro antifungal susceptibility test results of the mainly Candida species. |
The overall mortality among affected patients was 14.6%. C. albicans and C. parapsilosis
were associated with a mortality rate of 16.2% and 17.5%, respectively.
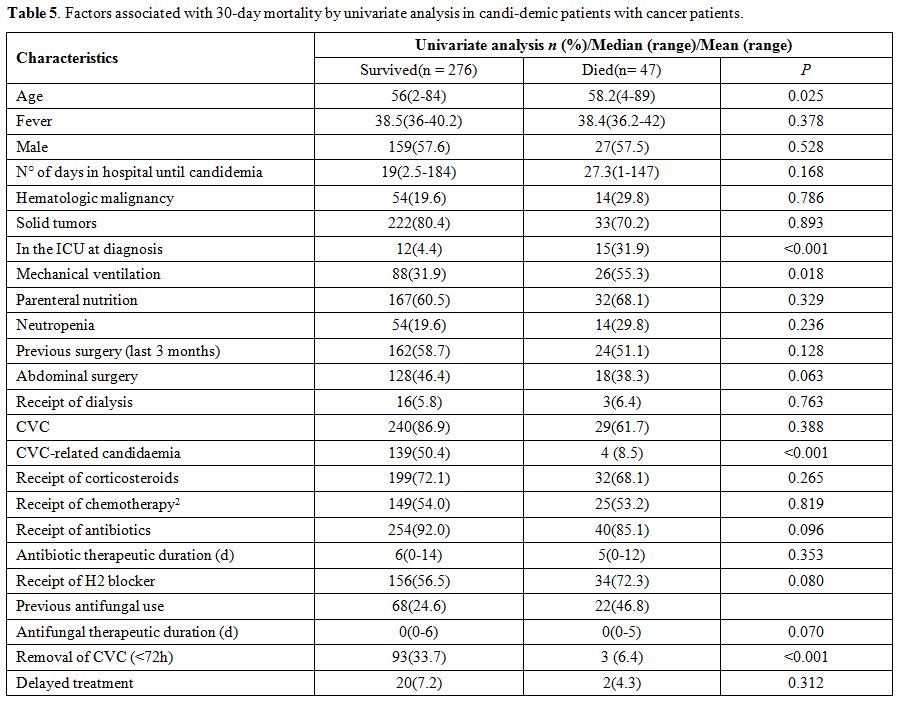
There was no significance between the two groups, C. albicans and non-albicans Candida (p = 0.561) and C. parapsilosis and non-C. parapsilosis (p = 0.257). Univariate predictors of poor outcome in candidemia of cancer patients are shown in Table 5.
The variables associated with 30-day mortality were as follows: older
age, in the ICU at diagnosis and mechanical ventilation. Factors
associated with 30-day survival were as follows: CVC-related
candidaemia and removal of CVC (<72h). As shown in Table 6,
factors associated with 30-day mortality by multivariate
analysis among candidemia with cancer patients was
in the ICU at diagnosis (OR 5.487; 95% CI 1.139- 6.441), whereas
candidemia due to removal of CVC (<72h) (OR 0.248; 95% CI
0.067-0.915) was associated with 30-day survival.
 |
Table
5. Factors associated with 30-day mortality by univariate analysis in candi-demic patients with cancer patients. |
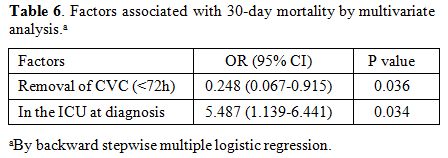 |
Table 6. Factors associated with 30-day mortality by multivariate analysis.a |
Discussion
The percentage of NAC isolates varies considerably from region to region.[21,22]
In our study there was an increase in cases of candidemia caused by C. parapsilosis, consistent with the results of studies from Spain, Italy, and Turkey.[23,24] However, to our knowledge, many studies in China indicated that candidemia is mainly caused by C. albicans.[5,8,17,18] In this report, we found that C. parapsilosis is the most common cause for the occurrence of candidemia.
C. parapsilosis
is an emerging major human pathogen that has dramatically
increased in significance and prevalence over the past two
decades. It causes invasive candidal disease in patients at high risk
of severe infection, especially ICU patients.[25] C. parapsilosis
is frequently linked to an exogenous source, such as the hands of
healthcare providers, or can be part of the normal flora of the human
skin, appearing to be directly introduced into the bloodstream.[26,27] High rates of candidemia due to C. parapsilosis can be attributed to nosocomial transmission. In addition, infections due to C. parapsilosis are especially associated with parenteral nutrition and indwelling catheters.[25-28] Our findings are in agreement with previous epidemiological studies showing that C. parapsilosis infections are more frequent in patients with parenteral nutrition.
Girmenia et al. showed an overall decrease in isolation of C. albicans with a concomitant increase in isolation of C. parapsilosis among adult patients with cancer,[29] which is accord with this report. In other studies, C. albicans
was more frequently associated with STs of the gastrointestinal and
genitourinary tracts and breast, whereas NAC was most frequently recovered from hematologic patients.[30] The results of our study were consistent with previous studies, wherein 12.7% of patients with C. parapsilosis and 16.7% non-albicans candidemia had a hematologic malignity. In solid cancer patients, C. albicans
candidemia accounted for 32.8%. Moreover, in the present study, there
was a significant difference in age between the patients with C. parapsilosis candidemia and those with other Candida spp.
The
crude mortality of candidemia shows slight differences when it comes to
species and not consistent in different studies. Our data show lower
overall mortality in candidemia. The possible reasons are as follows:
firstly, the majority of isolates were fluconazole susceptible,
therefore, this antifungal drug is a reasonable alternative for the
treatment of candidemia; furthermore, our study introduces an important
observation of a relatively high proportion (44.3%) of CVC-related
candidaemia episodes, however, the rate of removal CVC within 72h was
higher than another study;[31] what’s more, it is
known that a delay in the treatment start has a negative impact on
survival, but the incidence of delayed treatment was particularly low
in the report. Finally, different studyperiod and underlying diseases might contribute to the conflicting conclusions.
Conclusions
The emergence of C. parapsilosis
as the leading NAC species is posing a major threat for cancer
patients. Similarly, studies reported an increase in cases of
candidemia due to C. parapsilosis. Given the incidence of disease and the unacceptably high morbidity and mortality associated with C. parapsilosis, the study highlights the urgent need to evaluate the possibility of development of C. parapsilosis
candidemia in cancer patients exposed to these risk factors. Much
emphasis must also be given on the early implementation of a medical
intervention to reduce the incidences of candidemia in malignancy. In
light of the results of this study, it can be suggested that effective
prevention strategies against this causative agent transmitted through
nosocomial route should be implemented. However, Candida species may
vary with geographic regions, and local risk factors in cancer patients
can be different. Therefore, local risk factors and epidemiological
trends specific to cancer patients should be investigated.
References
- Morii
D, Seki M, Binongo JN, et al. Distribution of Candida species isolated
from blood cultures in hospitals in Osaka, Japan. Journal of Infection
and Chemotherapy 2014; 20(9): 558-562. https://doi.org/10.1016/j.jiac.2014.05.009 PMid:25009091
- Kullberg BJ, Campion EW, Arendrup MC. Invasive Candidiasis. New England Journal of Medicine 2015; 373(15): 1445-1456. https://doi.org/10.1056/NEJMra1315399 PMid:26444731
- Magill
SS, Edwards JR, Bamberg W, et al. Multistate Point- Prevalence Survey
of Health Care–Associated Infections. New England Journal of Medicine
2014; 370(13): 1198-1208. https://doi.org/10.1056/NEJMoa1306801 PMid:24670166 PMCid:PMC4648343
- Chowdhary
A, Cleveland AA, Harrison LH, et al. Declining Incidence of Candidemia
and the Shifting Epidemiology of Candida Resistance in Two US
Metropolitan Areas, 2008–2013: Results from Population- Based
Surveillance. Plos One 2015; 10(3): e0120452 https://doi.org/10.1371/journal.pone.0120452 PMid:25822249 PMCid:PMC4378850
- Li
D, Xia R, Zhang Q, et al. Evaluation of candidemia in epidemiology and
risk factors among cancer patients in a cancer center of China: an
8-year case-control study. BMC Infectious Diseases 2017; 17(1). https://doi.org/10.1186/s12879-017-2636-x
- Pfaller
MA, Diekema DJ. Epidemiology of Invasive Candidiasis: a Persistent
Public Health Problem. Clinical Microbiology Reviews 2007; 20(1):
133-163. https://doi.org/10.1128/CMR.00029-06 PMid:17223626 PMCid:PMC1797637
- Pappas
PG, Rex JH, Lee J, et al. A Prospective Observational Study of
Candidemia: Epidemiology, Therapy, and Influences on Mortality in
Hospitalized Adult and Pediatric Patients. Clinical Infectious Diseases
2003; 37(5): 634-643. https://doi.org/10.1086/376906 PMid:12942393
- Ding
X, Yan D, Sun W, et al. Epidemiology and risk factors for nosocomial
Non-Candida albicans candidemia in adult patients at a tertiary care
hospital in North China. Medical Mycology 2015; 53(7): 684-690. https://doi.org/10.1093/mmy/myv060 PMid:26229153
- Montagna
MT, Caggiano G, Lovero G, et al. Epidemiology of invasive fungal
infections in the intensive care unit: results of a multicenter Italian
survey (AURORA Project). Infection 2013; 41(3): 645-653. https://doi.org/10.1007/s15010-013-0432-0 PMid:23463186 PMCid:PMC3671106
- Mikulska
M, Bassetti M, Ratto S, et al. Invasive Candidiasis in
Non-Hematological Patients. Mediterranean Journal of Hematology and
Infectious Diseases 2011; 3(1): e2011007. https://doi.org/10.4084/mjhid.2011.007 PMid:21625311 PMCid:PMC3103237
- Morrell
M, Fraser VJ, Kollef MH. Delaying the Empiric Treatment of Candida
Bloodstream Infection until Positive Blood Culture Results Are
Obtained: a Potential Risk Factor for Hospital Mortality. Antimicrobial
Agents and Chemotherapy 2005; 49(9): 3640-3645 https://doi.org/10.1128/AAC.49.9.3640-3645.2005 PMid:16127033 PMCid:PMC1195428
- Wilson
Dib, R., Hachem, R., Chaftari, A.-M., & Raad, I. (2018).
Appropriate duration of intravenous treatment of candidemia and timing
of step down to oral therapy in non-neutropenic patients. Mediterranean
Journal of Hematology and Infectious Diseases, 10(1), e2018028. https://doi.org/10.4084/mjhid.2018.028
- Hajjeh
RA, Sofair AN, Harrison LH, et al. Incidence of Bloodstream Infections
Due to Candida Species and In Vitro Susceptibilities of Isolates
Collected from 1998 to 2000 in a Population-Based Active Surveillance
Program. Journal of Clinical Microbiology 2004; 42(4): 1519-1527. https://doi.org/10.1128/JCM.42.4.1519-1527.2004 PMid:15070998 PMCid:PMC387610
- Tumbarello
M, Posteraro B, Trecarichi EM, et al. Biofilm Production by Candida
Species and Inadequate Antifungal Therapy as Predictors of Mortality
for Patients with Candidemia. Journal of Clinical Microbiology 2007;
45(6): 1843-1850. https://doi.org/10.1128/JCM.00131-07 PMid:17460052 PMCid:PMC1933062
- Melo
AS, Bizerra FC, Freymüller E, et al. Biofilm production and evaluation
of antifungal susceptibility amongst clinicalCandidaspp. isolates,
including strains of the Candida parapsilosis complex. Medical Mycology
2011; 49(3): 253-262. https://doi.org/10.3109/13693786.2010.530032 PMid:21039308
- Serefhanoglu
K, Timurkaynak F, Can F, et al. Risk factors for candidemia with
non-albicans Candida spp. in intensive care unit patients with
end-stage renal disease on chronic hemodialysis. Journal of the
Formosan Medical Association 2012; 111(6): 325-332 https://doi.org/10.1016/j.jfma.2011.03.004 PMid:22748623
- Fu
J, Ding Y, Wei B, et al. Epidemiology of Candida albicans and
non-C.albicans of neonatal candidemia at a tertiary care hospital in
western China. BMC Infectious Diseases 2017; 17(1). https://doi.org/10.1186/s12879-017-2423-8
- Li
C, Wang H, Yin M, et al. The Differences in the Epidemiology and
Predictors of Death between Candidemia Acquired in Intensive Care Units
and Other Hospital Settings. Internal Medicine 2015; 54(23): 3009-3016.
https://doi.org/10.2169/internalmedicine.54.3744 PMid:26631884
- Jung
DS, Farmakiotis D, Jiang Y, et al. Uncommon CandidaSpecies Fungemia
among Cancer Patients, Houston, Texas, USA. Emerging Infectious
Diseases 2015; 21(11) https://doi.org/10.3201/eid2111.150404
- Bergamasco
MD, Garnica M, Colombo AL, et al. Epidemiology of candidemia in
patients with hematologic malignancies and solid tumours in Brazil.
Mycoses 2013; 56(3): 256-263 https://doi.org/10.1111/myc.12013 PMid:23043234
- Pfaller
MA, Boyken L, Hollis RJ, et al. In Vitro Susceptibilities of Candida
spp. to Caspofungin: Four Years of Global Surveillance. Journal of
Clinical Microbiology 2006; 44(3): 760-763. https://doi.org/10.1128/JCM.44.3.760-763.2006 PMid:16517851 PMCid:PMC1393154
- Pfaller
MA, Boyken L, Hollis RJ, et al. Global Surveillance of In Vitro
Activity of Micafungin against Candida: a Comparison with Caspofungin
by CLSI-Recommended Methods. Journal of Clinical Microbiology 2006;
44(10): 3533-3538. https://doi.org/10.1128/JCM.00872-06 PMid:17021079 PMCid:PMC1594802
- Horasan
EŞ, Ersöz G, Göksu M, et al. Increase in Candida parapsilosis Fungemia
in Critical Care Units: A 6-Years Study. Mycopathologia 2010; 170(4):
263-268. https://doi.org/10.1007/s11046-010-9322-5 PMid:20524154
- Almirante
B, Rodriguez D, Cuenca-Estrella M, et al. Epidemiology, Risk Factors,
and Prognosis of Candida parapsilosis Bloodstream Infections:
Case-Control Population-Based Surveillance Study of Patients in
Barcelona, Spain, from 2002 to 2003. Journal of Clinical Microbiology
2006; 44(5): 1681-1685. https://doi.org/10.1128/JCM.44.5.1681-1685.2006 PMid:16672393 PMCid:PMC1479182
- Trofa
D, Gacser A, Nosanchuk JD. Candida parapsilosis, an Emerging Fungal
Pathogen. Clinical Microbiology Reviews 2008; 21(4): 606- 625. https://doi.org/10.1128/CMR.00013-08 PMid:18854483 PMCid:PMC2570155
- Bonassoli
LA, Bertoli M, Svidzinski TIE. High frequency of Candida parapsilosis
on the hands of healthy hosts. Journal of Hospital Infection 2005;
59(2): 159-162. https://doi.org/10.1016/j.jhin.2004.06.033 PMid:15620452
- Clark
TA, Slavinski SA, Morgan J, et al. Epidemiologic and Molecular
Characterization of an Outbreak of Candida parapsilosis Bloodstream
Infections in a Community Hospital. Journal of Clinical Microbiology
2004; 42(10): 4468-4472. https://doi.org/10.1128/JCM.42.10.4468-4472.2004 PMid:15472295 PMCid:PMC522355
- Bassetti
M, Righi E, Costa A, et al. Epidemiological trends in nosocomial
candidemia in intensive care. BMC Infectious Diseases 2006; 6(1). https://doi.org/10.1186/1471-2334-6-21 PMid:16472387 PMCid:PMC1379648
- Girmenia
C, Martino P, De Bernardis F, et al. Rising Incidence of Candida
parapsilosis Fungemia in Patients with Hematologic Malignancies:
Clinical Aspects, Predisposing Factors, and Differential Pathogenicity
of the Causative Strains. Clinical Infectious Diseases 1996; 23(3):
506-514. https://doi.org/10.1093/clinids/23.3.506 PMid:8879773
- Sabino
R, Verissimo C, Brandao J, et al. Epidemiology of candidemia in
oncology patients: a 6-year survey in a Portuguese central hospital.
Medical Mycology 2009: 1-10. https://doi.org/10.1080/13693780903161216 PMid:19657956
- Gamaletsou
MN, Walsh TJ, Zaoutis T, et al. A prospective, cohort, multicentre
study of candidaemia in hospitalized adult patients with haematological
malignancies. Clinical microbiology and infection : the official
publication of the European Society of Clinical Microbiology and
Infectious Diseases 2014; 20(1): O50-7.
[TOP]





