Caterina Giovanna Valentini,
Eugenia Rosa Nuzzolo, Maria Bianchi, Nicoletta Orlando, Maria Grazia
Iachininoto, Priscilla Pinci and Luciana Teofili.
Fondazione Policlinico Universitario A. Gemelli IRCCS - Università Cattolica del Sacro Cuore, UNICATT Cord Blood Bank, Roma
Correspondence to: Luciana Teofili, MD. Fondazione Policlinico
Universitario A. Gemelli IRCCS, Università cattolica del Sacro Cuore,
UNICATT Cord Blood Bank. Largo Gemelli, 8, I-00168 Rome, Italy. Tel:
+39-0630154373, Fax +39-063055153. E-mail:
luciana.teofili@unicatt.it
Published: January 1, 2019
Received: September 19, 2018
Accepted: November 8, 2018
Mediterr J Hematol Infect Dis 2019, 11(1): e2019021 DOI
10.4084/MJHID.2019.021
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Platelet-rich plasma (PRP) is an inexpensive and safe substitute of recombinant growth factors in vitro and in vivo.
Due to its putative effect on tissue repair, the use of autologous PRP
has become largely popular. Recently, a jellified PRP derivative
obtained from umbilical cord blood (CB) has been utilized in vivo
to treat mucosal and cutaneous lesions. Nevertheless, whether PRP
derived from CB and adult blood display different potency in promoting
cell growth in vitro has been
rarely investigated. In this study, we compared cytokine profile and
mesenchymal cell growth supporting the ability of platelet lysate
obtained from adult and cord blood. Our in vitro results strongly back the utilization of CB platelet lysate in vivo,
as an efficacious, safe and inexpensive alternative to promote damaged
tissue healing when the autologous PRP is contraindicated. Moreover,
the policy of manufacturing CB platelet lysate can limit the current
disposal of many collected CB units not suitable for transplant due to
their low nucleated cell count.
|
Introduction
Platelets
are a natural reservoir of growth factors for several cell lineages.
Their activation at sites of tissue injury promotes cell proliferation
and tissue repair, including revascularization. For this reason, human
platelet lysate (PL) is considered a less expensive and safer
substitute for recombinant growth factors or animal-derived products.[1,2]
PL obtained from platelet-enriched-plasma (PRP) contains additional
factors, such as fibrinogen and other extracellular matrix precursors,
which further promote cell adhesion and tissue repair.[3] Due to its putative effect on tissue repair, the use of autologous PRP has become largely popular.[4]
In fact PRP and derivatives are currently used in the context of
musculoskeletal disease such as soft tissue injuries, bone fractures,
orthopedic surgery, osteoarthritis, joint degeneration, and wound care,[5] but also in ophthalmology to promote epithelial healing,[6,7] or in cosmetology and dermatology.[8]
Recently, the standardized production of a jellified PRP derivative
obtained from umbilical cord blood (CB) has been reported.[9] The CB platelet gel has shown in vivo positive results in epidermolysis bullosa,[10,11] in diabetic foot ulcer healing,[12] and in mucositis caused by chemotherapy.[13]
It
is widely acknowledged that cord blood contains a high amount of growth
factors. Nevertheless, there are very few studies investigating whether
cord and adult PLs display different potency in promoting cell growth in vitro.[14] Similarly, the types of cytokines and growth factors released by fetal platelets have been only partially investigated.[14,15]
Materials and Methods
Platelet lysate manufacture. CB
PL was manufactured from CB units collected for solidary donation
purpose at the UNICATT Cord Blood Bank, according to the Bank standard
procedures and national regulation. CB units were collected in
citrate-phosphate-dextrose. The units collected in the previous 24
hours, not suitable for transplant due to a total nucleated cell
content <1.5 x109, were utilized
in the study. All units displayed cell blood count within the normal
ranges, no signs of clots or hemolysis and negative direct antiglobulin
test, Adult PL was manufactured from blood collected in sodium citrate
in four healthy female volunteers, after informed consent. Samples were
maintained at 2-6°C until processing. PL was obtained according to a
two-step procedure. In the first step, a PRP was obtained by soft
centrifugation (246 g for 25 min) without a break. The PLT
concentration of PRP was then assessed using the ADVIA 2120 (Siemens
Healthcare Diagnostics, IL, USA). A final PLT concentration of 1.5 x 106/µl
was obtained in all PRP samples through further centrifugation at 2218
g for 10 min and the subsequent removal of a plasma aliquot, which was
calculated according to the following formula: PRP volume - (PRP volume x [PLT]/1.5).
The standardized PRP so obtained, together with the aliquots of the
relative plasma previously removed, were frozen at -80°C. Before the
use, PL was thawed at 37°C, then again frozen at −80° to favor the
platelet lysis, and finally thawed and subjected to centrifugation at
2218 g for 10 min.
Mesenchymal cell cultures.
Mesenchymal cells (MSCs) were obtained from bone marrow (BM) samples of
a healthy BM donor using residual nucleated cells recovered from
filters utilized for the BM graft filtration, after repeated washed
with PBS. After a density gradient centrifugation (Lympholyte-H,
Cedarlane Lab, Canada), mononuclear cells were then collected and
seeded at 10x106 /ml in αMEM (Lonza,
Italy) containing 20% FBS (Life Technologies, USA). After three days,
non-adherent cells were discharged, and adherent cells were left to
grow until confluence. Cultures were maintained at 37°C in humidified
5% CO2 atmosphere, and the medium was
changed twice a week. When 80% of confluence was achieved, MSCs were
detached by Trypsin 1mM EDTA (Sigma Aldrich, Italy), counted and
sub-cultured in the same medium containing 10% FBS. The cell population
doubling (PD) rate was calculated in MSC cultures after the passage 3
as previously reported,[14] according to the formula PD = log10(N)/log10(2),
where N was the ratio between harvested and seeded cells. The colony
forming unit-fibroblast (CFU-F) assay was performed by seeding MSCs
(from passage 5 or higher), in 60 mm dishes at a concentration of 1-2
cell/cm2 in αMEM and 20% FBS.[14]
After two weeks of culture, colonies were counted, fixed and visualized
by crystal violet staining. In order to compare the effect of CB and
adult blood PLs, FBS was substituted by 20% of PL obtained either
pooling the eight samples of CB PLs or the four samples of adult blood
PLs.
Cytokine assay.
The cytokine and growth factors profile were evaluated in adult and CB
starting plasma samples and in final adult and CB PL products. The
Bio-Plex Human Cytokine 27-Plex Assay and the Bio-Plex MAGPIX™
Multiplex Reader (Bio-Rad, CA, USA) were utilized. The following
molecules were dosed: growth factors (G-CSF, GM-CSF, VEGF, FGF, PDGF),
chemokines (MCP-1/CCL2, MIP-1a/CCL3, MIP-1b/CCL4, RANTES/CCL5,
Eotaxin/CCL11, IP-10/CXCL10), interleukins (IL-1β, IL-1RA, IL-2,IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL10, IL-12, IL-13,IL-15, IL-17), and pro-inflammatory mediators (TNF-α, INF-γ). All samples were evaluated in duplicate, according to the manufacturer’s instructions.
Statistics.
Continuous variables were expressed as mean values + standard error
(SEM), and they were analyzed through the Mann-Whitney U test or the
Kruskal-Wallis test. The GraphPad PRISM 5.0 software and the IBM SPSS
Statistics 21.0 software were utilized. The p-value <0.05 were regarded as statistically significant.
Results and Discussion
Cord and adult blood platelet lysates display similar profiles of bioactive molecules. Figure 1 shows
results obtained in cord and adult plasma samples and in their
corresponding PLs. Relative to adult PLs, our findings are in agreement
with those previously reported by Mussano et. al, demonstrating
detectable amounts of all investigated analytes using the same
methodology.[15] In particular, PLs from either adult
or cord blood exhibited a higher concentration of all investigated
mediators than the corresponding plasma samples, including pro- and
anti-inflammatory cytokines (Figure 1 A and Figure 1 B, respectively), chemokines (Figure 2 A) and growth factors (Figure 2 B).
Notably, the only significant difference emerging from the comparison
between adult and cord blood PLs was the higher concentration of IL17
and RANTES in adult PLs (Figure 1 A and Figure 2 A). This finding is not surprising, considering that both molecules are main players in the innate immunity response.[16,17]
In fact, cord blood samples used in this study were collected
from healthy term babies, whose innate immune system was totally
inexperienced. A previous study carried out in CB platelet gel
demonstrated that this product underactivation releases VEGF, PDGF-BB,
FGF, hepatocyte growth factor and transforming growth factor-β1.[18]
These findings were subsequently confirmed at the proteomic analysis of
cord blood plasma and platelet gel releasate, showing higher levels of
angiogenic factors in comparison with the peripheral blood
counterparts.[14] The authors also found that products obtained from adult blood were much richer in inflammatory factors.[14]
The discrepancies between these data and our results might have various
explanations, including the use of different methodologies (proteomic
array and Bioplex analysis) and product characteristics (platelet gel
release and platelet lysate). Moreover, a large interindividual
variability has been reported among adults regarding the PRP content of
bioactive molecules.[15]
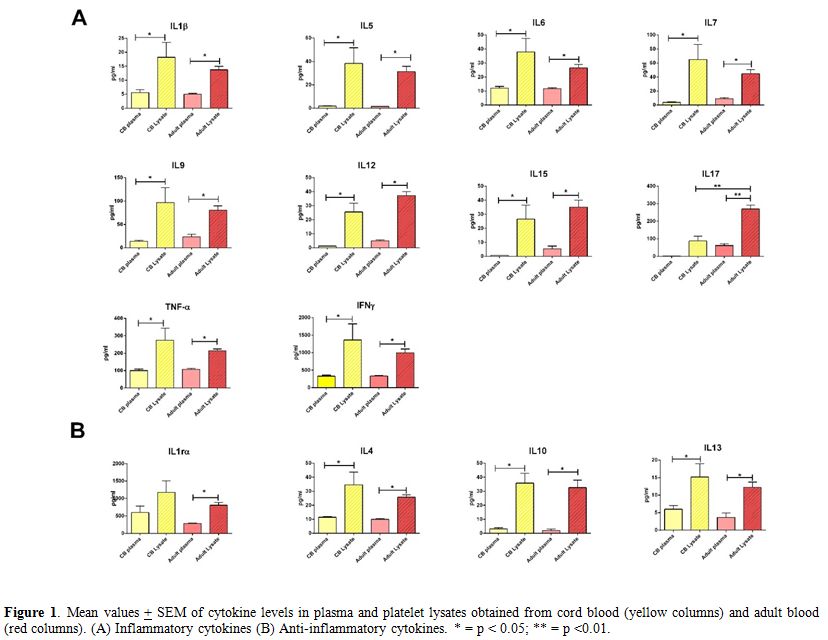 |
Figure 1.
Mean values + SEM of cytokine levels in plasma and platelet
lysates obtained from cord blood (yellow columns) and adult blood (red
columns). (A) Inflammatory cytokines (B) Anti-inflammatory cytokines. *
= p < 0.05; ** = p <0.01. |
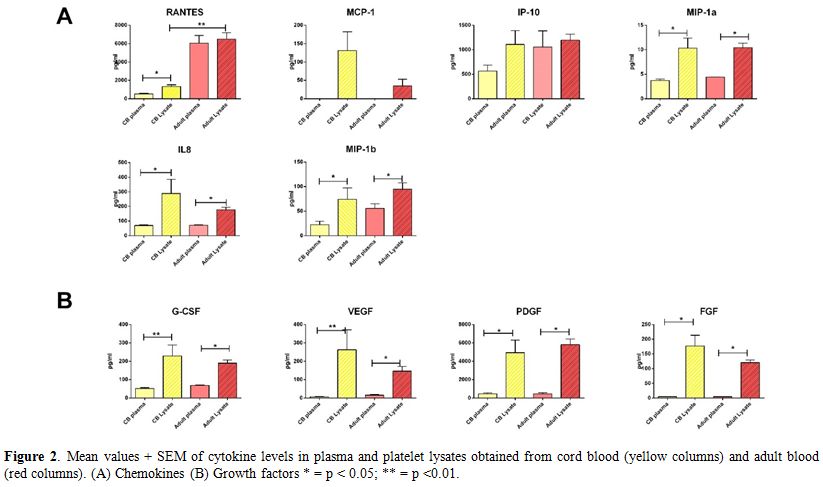 |
Figure 2. Mean values +
SEM of cytokine levels in plasma and platelet lysates obtained from
cord blood (yellow columns) and adult blood (red columns). (A)
Chemokines (B) Growth factors * = p < 0.05; ** = p <0.01. |
Cord platelet lysates more efficiently promote cell proliferation than their adult blood counterparts. The kinetic of MSC expansion in cultures supplemented with FBS, adult PLs or CB PLs is shown in Figure 3A.
As shown by the cumulative PD of three single experiments, CB PLs
(obtained by mixing 8 single PLs samples in 3 different pools) were
significantly more active in supporting MSC growth over the whole time
of culture (p < 0.001 at every passage). Similarly, fibroblast
colony yield in the presence of CB PLs was significantly higher than in
culture supplemented with FBS or adult PLs (p = 0.003; Figure 3 B). On the whole, our data are in partial agreement with those reported by Parazzi et al. in adipose tissue-derived MSCs.[14]
In fact, the authors found no difference between adult and cord blood
platelet releasate in supporting cell proliferation, whereas both were
much more active than FBS.[14] The use of MSCs from
different sources (BM in our study and adipose tissue in Parazzi’
study), in addition to the use of different PRP derivatives (platelet
releasate and platelet lysate), may account for these differences.
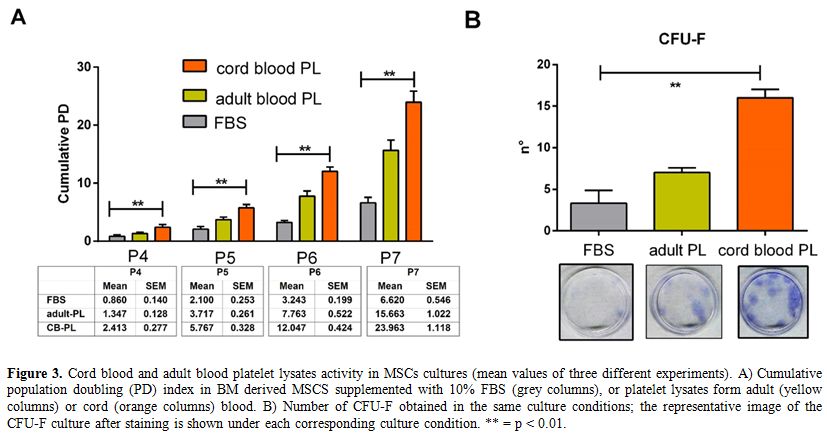 |
Figure 3. Cord blood and
adult blood platelet lysates activity in MSCs cultures (mean values of
three different experiments). A) Cumulative population doubling (PD)
index in BM derived MSCS supplemented with 10% FBS (grey columns), or
platelet lysates form adult (yellow columns) or cord (orange columns)
blood. B) Number of CFU-F obtained in the same culture conditions; the
representative image of the CFU-F culture after staining is shown under
each corresponding culture condition. ** = p < 0.01. |
Conclusions
This
study investigated the biologic characteristic of a PRP derivative of
cord blood, expanding the findings previously gathered in a similar
product. Although we found no prominent differences regarding the
cytokine and growth factor profiles between adult and cord blood
samples, cord blood PLs were much more effective in supporting cell
proliferation in vitro,
suggesting that additional factors, not included in our cytokine array,
might be implicated. This hypothesis is supported by previous
observations highlighting a differential content of proteins between
adult and cord blood-derived platelet gels.[19,20] On the whole, these findings may help to identify future appropriate clinical applications better.
In conclusion, our data strongly support the exploitation of cord blood PLs in vitro and prompt its utilization in vivo,
to promote damaged tissue healing. Notably, this could be a more
efficacious and safer alternative to the autologous PRP. Indeed, cord
blood platelet lysate could be a valid alternative in case of
contraindication to the autologous PRP for patient refusal or
discomfort, unsuitability of venous accesses, or coexistence of
inflammatory, autoimmune or hematological diseases. In the
meanwhile, the manufacture of CB derivative as medical products for
local use, could limit the current disposal of several CB units
collected for solidary donation, and not suitable for transplant due to
their low nucleated cell count.
Acknowledgments
This
work was supported by Fondi d’Ateneo, Progetti D1 2017 Università
Cattolica to Luciana Teofili. The authors are in debt to the staff of
the Unicatt Cord Blood Bank for their indefatigable work.
References
- Hofbauer, P. et al. Human platelet lysate is a
feasible candidate to replace fetal calf serum as medium supplement for
blood vascular and lymphatic endothelial cells. Cytotherapy 2014; 16:
1238–1244. https://doi.org/10.1016/j.jcyt.2014.04.009 PMid:24927718
- Kim,
H. et al. Human platelet lysate improves human cord blood derived ECFC
survival and vasculogenesis in three dimensional (3D) collagen
matrices. Microvasc. Res. 2015; 101: 72–81. https://doi.org/10.1016/j.mvr.2015.06.006 PMid:26122935 PMCid:PMC4537830
- Seiffert,
D. & Schleef, R. R. Two functionally distinct pools of vitronectin
(Vn) in the blood circulation: identification of a heparin binding
competent population of Vn within platelet alpha-granules. Blood 1996;
88:552–560. PMid:8695803
- Harm SK, Fung MK. Platelet-rich plasma injections: out of control and on the loose? Transfusion. 2015;55(7):1596–8. https://doi.org/10.1111/trf.13160 PMid:26172145
- Platelet-rich
plasma injections for wound healing and tissue rejuvenation: a review
of clinical effectiveness, cost-effectiveness and guidelines. Ottawa:
CADTH; 2017 Jun (CADTH rapid response report: summary with critical
appraisal). Available at https://www.cadth.ca/sites/default/files/pdf/htis/2017/RC0885%20PRP%20Injections%20Final.pdf. (Last accessed November 5, 2018)
- Alio
JL, Rodriguez AE, WróbelDudzińska D. Eye platelet-rich plasma in the
treatment of ocular surface disorders. Curr Opin Ophthalmol.
2015;26(4):325-32. https://doi.org/10.1097/ICU.0000000000000169 PMid:26058033
- Valentini
CG, Nuzzolo ER, Orlando N, Metafuni E, Bianchi M, Chiusolo P, Zini G,
Teofili L. Cytokine profile of autologous platelet-derived eye drops in
patients with ocular chronic graft-versus-host disease. Vox Sang.
2016;110(2):189-92. https://doi.org/10.1111/vox.12325 PMid:26383050
- Zhang
M, Park G, Zhou B, Luo D. Applications and efficacy of platelet-rich
plasma in dermatology: A clinical review. J Cosmet Dermatol.
2018;17:660-665. https://doi.org/10.1111/jocd.12673 PMid:30047234
- Rebulla
P, Pupella S, Santodirocco M, Greppi N, Villanova I, Buzzi M, De Fazio
N, Grazzini G; Italian Cord Blood Platelet Gel Study Group (see
Appendix 1). Multicentre standardisation of a clinical grade procedure
for the preparation of allogeneic platelet concentrates from umbilical
cord blood. Blood Transfus. 2016;14(1):73-9. PMid:26509822
PMCid:PMC4731342
- Gelmetti A, Greppi N,
Guez S, Grassi F, Rebulla P, Tadini G. Cord blood platelet gel for the
treatment of inherited epidermolysis bullosa. Transfus Apher Sci.
2018;57:370-373. https://doi.org/10.1016/j.transci.2018.05.021 PMid:29933907
- Tadini
G, Pezzani L, Ghirardello S, Rebulla P, Esposito S, Mosca F. Cord blood
platelet gel treatment of dystrophic recessive epidermolysis bullosa.
BMJ Case Rep. 2015 Jan 8;2015.
- Volpe P,
Marcuccio D, Stilo G, Alberti A, Foti G, Volpe A, Princi D, Surace R,
Pucci G, Massara M. Efficacy of cord blood platelet gel application for
enhancing diabetic foot ulcer healing after lower limb
revascularization. Semin Vasc Surg. 2017; 30:106-112. https://doi.org/10.1053/j.semvascsurg.2017.12.001 PMid:29793677
- Piccin
A, Rebulla P, Pupella S, Tagnin M, Marano G, Di Pierro AM, Santodirocco
M, Di Mauro L, Beqiri L, Kob M, Primerano M, Casini M, Billio A,
Eisendle K, Fontanella F. Impressive tissue regeneration of severe oral
mucositis post stem cell transplantation using cord blood platelet gel.
Transfusion. 2017;57(9):2220-2224. https://doi.org/10.1111/trf.14205 PMid:28656652
- Parazzi
V, Lavazza C, Boldrin V, Montelatici E, Pallotti F, Marconi M, Lazzari
L. Extensive Characterization of Platelet Gel Releasate From Cord Blood
in Regenerative Medicine. Cell Transplant. 2015;24(12):2573-84. https://doi.org/10.3727/096368915X687471 PMid:25695232
- Mussano
F, Genova T, Munaron L, Petrillo S, Erovigni F, Carossa S. Cytokine,
chemokine, and growth factor profile of platelet-rich plasma.
Platelets. 2016 Jul;27(5):467-71. https://doi.org/10.3109/09537104.2016.1143922 PMid:26950533
- Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008 Sep;43(3):402-7. https://doi.org/10.1016/j.cyto.2008.07.017 PMid:18701318 PMCid:PMC2582446
- Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 2009 Apr 1;182(7):3945-6. https://doi.org/10.4049/jimmunol.0990015 PMid:19299688
- Parazzi
V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for
tissue engineering. Platelets. 2010;21(7):549-54. https://doi.org/10.3109/09537104.2010.514626 PMid:20873963
- Longo
V, Rebulla P, Pupella S, Zolla L, Rinalducci S. Proteomic
characterization of platelet gel releasate from adult peripheral and
cord blood. Proteomics Clin Appl. 2016 Aug; 10(8): 870-82 https://doi.org/10.1002/prca.201500126 PMid:27377258
- Stokhuijzen
E, Koornneef JM, Nota B, van den Eshof BL, van Alphen FPJ, van den
Biggelaar M, van der Zwaan C, Kuijk C, Mertens K, Fijnvandraat K,
Meijer AB. Differences between Platelets Derived from Neonatal Cord
Blood and Adult Peripheral Blood Assessed by Mass Spectrometry. J
Proteome Res. 2017 Oct 6;16(10):3567-3575. https://doi.org/10.1021/acs.jproteome.7b00298 PMid:28823163
[TOP]