Anke Verlinden1,4, Veronique De Vroey2, Herman Goossens2,4, Ella Roelant3,5, Ann L Van De Velde1,4, Zwi N Berneman1,4, Wilfried A Schroyens1,4 and Alain P Gadisseur1,4.
1 Department of Haematology, Antwerp University Hospital, Edegem, Belgium.
2 Department of Clinical Biology, Antwerp University Hospital, Edegem, Belgium.
3
Clinical Trial Center (CTC), Clinical Research Center (CRC) Antwerp,
Antwerp University Hospital, University of Antwerp, Edegem, Belgium.
4 Vaccine & Infectious Disease Institute, Faculty of Medicine & Health Sciences, University of Antwerp, Antwerp, Belgium.
5 StatUa, Center for Statistics, University of Antwerp, Edegem, Belgium.
Correspondence to: Anke Verlinden, UZA, Wilrijkstraat 10, 2650 Edegem,
Belgium. Telephone: +32 3 821 39 19,Fax: +32 3 821 42 86. E-mail:
anke.verlinden@uza.be
Published: March 1, 2019
Received: November 19, 2018
Accepted: February 1, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019023 DOI
10.4084/MJHID.2019.023
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Management
of fever in prolonged, profound neutropenia remains challenging with
many possible infectious and non-infectious causes. We investigated
whether procalcitonin (PCT) is superior to C-reactive protein (CRP) in
discriminating between different aetiologies of fever in this setting.
CRP
and PCT were tested daily during 93 neutropenic episodes in 66
patients. During this study period, 121 febrile episodes occurred and
were classified into four categories based on clinical and
microbiological findings: microbiologically documented infection (MDI);
clinically documented infection (CDI); proven or probable invasive
fungal disease (IFD); fever of unknown origin (FUO). Values of PCT and
CRP at fever onset as well as two days later were considered for
analysis of their performance in distinguishing aetiologies of fever.
At
fever onset, no significant difference in PCT values was observed
between different aetiologies of fever, whereas median CRP values were
significantly higher in case of IFD (median 98.8 mg/L vs 28.8 mg/L,
p=0.027). Both PCT and CRP reached their peak at a median of 2 days
after fever onset. Median PCT values on day 2 showed no significant
difference between the aetiologies of fever. Median CRP values on day 2
were significantly higher in IFD (median 172 mg/L versus 78.4 mg/L,
p=0.002). In MDI median CRP values rose > 100 mg/L, whereas they did
not in CDI or FUO.
PCT has no added value over CRP for clinical
management of fever in prolonged, profound neutropenia. When performing
reassessment 2 days after fever onset, CRP has better discriminatory
power between aetiologies of fever.
|
Introduction
Febrile
neutropenia occurs very frequently in patients with prolonged, profound
neutropenia caused by treatment with intensive myelosuppressive
chemotherapy for haematological malignancies, exceeding 80% of cases.[1-3]
Management remains challenging as the presence of fever in this patient
population is neither specific for infection nor is it pathognomonic of
any type of infection. It may also be caused by reactions to drugs and
blood products, non-infectious inflammatory responses secondary to the
malignancy, administration of chemotherapy, antithymocyte globulin
(ATG) or engraftment and graft versus host disease (GvHD) after
allogeneic hematopoietic stem cell transplantation (HSCT). In more than
60% of cases, there is no documented infectious aetiology and
unresolved febrile neutropenia often results in multiple empirical
modifications of antibacterial therapy and/or addition of antifungal
therapy. Unfortunately, indiscriminate use of broad-spectrum
antibiotics can lead to important collateral damage including toxicity,
selection of multidrug-resistant pathogens and an increased
predisposition to other infections such as Clostridium difficile or
yeasts/fungi.[4] In case of invasive fungal disease, prompt diagnosis and early initiation of antifungal therapy is known to improve survival.[5]
In
clinical practice, C-reactive protein (CRP) is currently used in the
decision-making process when treating patients with febrile
neutropenia. However, it is part of the nonspecific acute-phase
response to most forms of tissue damage, infection, inflammation and
malignant neoplasia.[6] Procalcitonin (PCT) is a 116-aminoacid precursor peptide for the hormone calcitonin expressed by the CALC1-gene.[7]
During infection, the combination of microbial products (e.g.
lipopolysaccharides) and pro-inflammatory cytokines results in an
up-regulation of the gene expression of the CALC1-gene and PCT is
released from nearly all tissues and cell types in the body.[8]
PCT promptly increases within 6 to 12 hours upon stimulation and
circulating PCT levels halve daily when the infection is controlled by
the host immune system or antibiotic therapy.[9]
Procalcitonin
(PCT) has demonstrated superior diagnostic accuracy when compared to
CRP as a biomarker of infection in non-haematological populations.[10-13]
It has been used successfully in algorithms for antimicrobial therapy
in acute respiratory infections and management of sepsis in intensive
care units. In 2008 Sakr et al. reviewed the available literature on
the use of PCT in febrile neutropenia, concluding that this biomarker
may be helpful in differentiating infection and sepsis from
non-infectious causes of fever.[14] However, due to
the heterogeneity of study populations, the specific value of PCT
assessment in adults with prolonged, profound neutropenia following
intensive chemotherapy remains uncertain.
With this study, we
wanted to compare the evolution of CRP and PCT with daily measurements
in a large cohort of patients with prolonged, profound neutropenia.
This differs from older studies where PCT values were often tested at
intervals of 3 to 5 days or daily only for a few days around a febrile
episode. With this design, we hoped to find medications or clinical
situations that affect CRP and PCT values differently. Furthermore, we
aimed to clarify whether PCT has superior reliability in comparison to
CRP in the clinical management of febrile patients in the setting of
prolonged profound neutropenia.
Material and Methods
Study design.
This single-centre prospective observational study was carried out at
the adult haematology ward of the Antwerp University Hospital, which is
equipped with high-efficiency particulate air (HEPA) filtration.
Throughout 18 months (March 2015 until September 2016), consecutive
patients admitted for induction/consolidation chemotherapy for acute
leukaemia, intensive chemotherapy followed by autologous stem cell
rescue or allogeneic HSCT for diverse haematological malignancies were
enrolled. Patients could be included several times in the study for
different admission periods. After written informed consent, CRP and
PCT were measured daily on standard blood draws during the entire
hospitalisation period. All patients received standard care, and no
clinical decisions were based on PCT results as those were not
available to treating physicians. The protocol was reviewed and
approved by the local ethics committee. This study was conducted in
agreement with the Declaration of Helsinki as well as the laws and
regulations of the Belgian government, whichever provides the greatest
protection for the patient.
Data collection.
Results of daily CRP and PCT measurements were recorded, as well as
administration of drugs that could potentially influence their values
such as corticosteroids, cytarabine, antithymocyte globulin and
immunosuppressive therapy in case of allogeneic HSCT. CRP was measured
daily by nephelometry using the Dimension Vista® 1500 System (Siemens
Healthcare, Munich, Germany). PCT was measured daily using the Elecsys®
BRAHMS PCT Assay on the Modular E170 instrument (Roche Diagnostics,
Rotkreuz, Switzerland). This automated test is performed in human serum
using the ECLIA (ElectroChemiLuminiscence ImmunoAssay) technique with a
detection limit of 0.02 µg/L and an upper limit of normal (ULN) of 0.5
µg/L.
Definitions and infectious work-up.
Febrile neutropenia was defined as an axillary temperature of ≥38.3°C
on a single occasion or ≥38.0°C sustained over a 2 hour period during
neutropenia defined as an absolute neutrophil count < 500/µL.[15] A new febrile episode was defined as a relapsing fever after more than 72 hours of apyrexia (<38.0°C).
For
each febrile episode, the initial diagnostic workup consisted of a
thorough physical examination, one set of aerobic and anaerobic blood
cultures drawn by phlebotomy and one set via each lumen of the central
venous line, urine culture and chest X-ray. Blood cultures were
obtained repeatedly during the first three fever spikes, galactomannan
antigenemia was measured twice weekly, and additional specific
investigations were performed according to the clinical presentation.
When fever persisted for more than four days, the diagnostic
reassessment included a thoraco-abdominal CT-scan and bronchoscopy with
BAL in the presence of a lung infiltrate.
Febrile episodes were
classified into four categories based on clinical and microbiological
findings without any knowledge of the analysed PCT values: 1)
microbiologically documented infection (MDI, i.e. proven microbial
pathogen with or without microbiologically defined site of infection);
2) clinically documented infection (CDI, i.e. diagnosed site of
infection without proven microbiologic pathogenesis); 3) proven or
probable invasive fungal disease (IFD); 4) fever of unknown origin
(FUO).[16,17]
Statistical analysis.
All data were analysed using a statistical software package (IBM SPSS
Statistics 23, Chicago, IL). Continuous variables were compared using
the Mann-Whitney (2-group comparison) or Kruskall Wallis
(multiple-group comparison) non-parametric tests. Categorical variables
were compared with the X2
or Fischer’s exact test, as appropriate. A linear mixed model using R
was performed to evaluate the effects of different variables on the
values of CRP and PCT over time. A two-sided p-value of less than 0.05
was considered as statistically significant. The peak values of PCT and
CRP were considered for analysis of their performance in distinguishing
aetiologies of fever in receiver operating characteristic (ROC) curves
and by calculation of sensitivity, specificity, positive & negative
predictive values and efficiency. The best cut-off was defined on the
basis of the highest calculated efficiency.
Results
Patient inclusion.
During the 18-month study period, 66 patients were enrolled in this
study and data was collected for 93 admissions. The median duration of
each hospitalisation was 26 days, with a median duration of profound
neutropenia of 12 days. During these 93 neutropenic periods, a total of
121 febrile episodes (FE) occurred. A total amount of 2535 patient days
was evaluated for PCT and CRP values. Patient demographics and
characteristics of neutropenic & febrile episodes can be found in Table 1.
As an interim analysis performed after 40 admissions showed that ATG
had an important impact on the PCT value, FE classified as fever of
unknown origin that occurred within 48 hours after administration of
ATG were separated for further analysis.
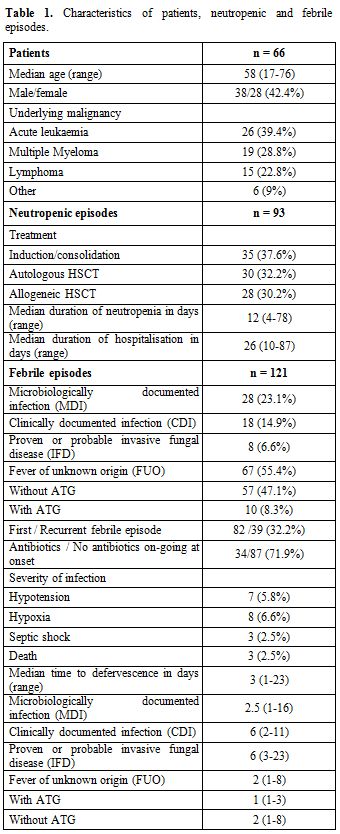 |
Table
1. Characteristics of patients, neutropenic and febrile episodes. |
Comparison of PCT and CRP evolution during prolonged, profound neutropenia.
In a first analysis, the individual curves of CRP and PCT evolution
during the 93 hospitalisation periods were reviewed visually. In 31 out
of 93 neutropenic episodes (33.3%) their pattern of evolution appeared
similar, whereas in 61 neutropenic episodes (66.7%) their evolution was
clearly different. CRP seemed to be a more volatile parameter, rising
above its ULN (3 mg/L) during every single neutropenic episode. Its
reactivity to stimuli was also quite pronounced with a median of 2
surges above 100 mg/L per neutropenic episode. In contrast, PCT did not
rise above its ULN of 0.5 ng/mL in 50 out of 93 neutropenic episodes
(53.8%).
The present study, to evaluate the influence of
confounding factors on the evolution of CRP and PCT, utilises a
linear mixed model fitting with the neutropenic episode as a random
effect. The logarithm of the outcomes was modelled as residual
assumptions are better met in this way. This model included the
following parameters: temperature, white blood cell count (WBC),
absolute neutrophil count (ANC), administration of ATG,
corticosteroids, cytarabine, cyclosporine A, mycophenolate &
methotrexate and presence of cytarabine-induced dermatitis or
engraftment syndrome. We found that many of these factors had a
statistically significant effect on both the CRP and PCT values.
However, the only one with a clinically relevant large effect size was
ATG: administration of ATG resulted in an 11-fold increase [95% CI
(8.7, 15.1)] of the PCT value one day later versus only a 2-fold
increase [95% CI (1.4, 2.9)] of the CRP value.
Comparison of PCT and CRP evolution during febrile episodes. Figure 1 and Table 2 show the kinetics of PCT & CRP for different aetiologies of febrile neutropenia.
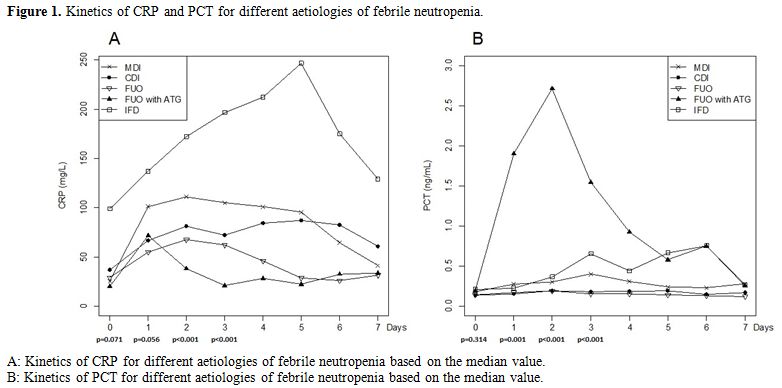 |
Figure 1. Kinetics of CRP and PCT for different aetiologies of febrile neutropenia. |
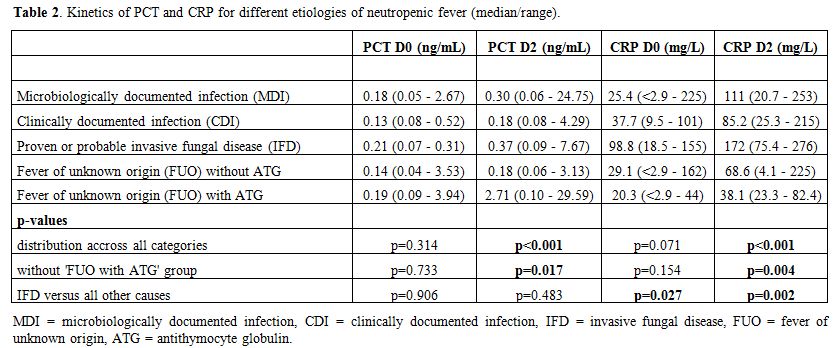 |
Table 2. Kinetics of PCT and CRP for different etiologies of neutropenic fever (median/range). |
Initial diagnostic assessment.
On the day of fever onset, no significant difference in PCT values was
observed between the different categories (p=0.314). Nine FE presented
with a PCT value ≥ 0.5 ng/mL: 2 MDI, 1 CDI, 4 FUO and 2 FUO with ATG.
None of these FE was associated with severe clinical signs such as
hypotension, hypoxia and the need for transfer to intensive care
facilities. Thirteen FE, which were complicated by a severe clinical
course, showed a median PCT value of 0.15 ng/mL (range 0.09 - 0.34
ng/mL) on the day of fever onset.
CRP values were significantly
higher on the day of fever onset in patients suffering from IFD versus
all other aetiologies (median 98,75 mg/L versus 28.8 mg/L, p=0.027).
Thirteen FE presented with a CRP value ≥ 100 mg/L: 3 MDI, 1 CDI, 5 FUO
and 4 IFD. Three of these FE ran a severe clinical course. Median CRP
on the day of fever onset was 36.6 mg/L (range < 2.9 - 137 mg/L) in
the thirteen FE complicated by a severe clinical course.
Diagnostic reassessment.
Both PCT and CRP reached their peak value at a median of 2 days [95% CI
(1,10) for PCT & 95% CI (1,7) for CRP respectively]. PCT values on
day 2 were significantly higher in FUO after ATG versus all other
aetiologies (median 2.72 ng/mL versus 0.21 ng/mL, p<0.001). In cases
of MDI and IFD, median PCT values rose > 0.25 ng/mL on day 2. In
contrast, in cases of CDI or FUO without ATG, they stayed lower.
Thirteen FE that were complicated by a severe clinical course showed a
median PCT value on day 2 of 0.35 ng/mL (range 0.09 - 7.67 ng/mL)
versus 0.22 ng/mL (range 0.06 - 29.59 ng/mL) in all other uncomplicated
cases (p=0.139).
CRP values on day 2 were significantly higher in
IFD versus all other aetiologies (median 172 mg/L versus 78.4 mg/L,
p=0.002). In cases of MDI, median CRP values rose > 100 mg/L on day
2. In contrast, in cases of CDI or FUO (with/without ATG), they stayed
lower. Thirteen FE that were complicated by a severe clinical course
showed a median CRP value on day 2 after onset of fever of 182 mg/L
(range 75.4 - 276 mg/L) versus 59.5 mg/L (range 4.1 – 259 mg/L) in all
other uncomplicated cases (p<0.001).
When looking at the 28
episodes of MDI, 15 were caused by gram-positive bacteraemia, eight by
gram-negative bacteraemia and the remaining five by urinary tract
infection and viral or bacterial pneumonia. The median values of CRP
and PCT did not differ depending on the underlying cause of MDI or
specific bacterial isolate.
Differential diagnosis between FUO and IFD. ROC curves were computed to see whether PCT and/or CRP were able to discriminate between FUO and IFD. Figure 2 demonstrates that the discriminatory power of CRP on day two after the onset of fever was superior to that of PCT. Table 3
shows the predictive value of CRP for IFD at different cut-offs. With
the cut-off set at 200 mg/L, CRP on day 2 has a positive predictive
value of 66.7% and a negative predictive value of 94.2% for the
diagnosis of IFD versus FUO. This leads to an efficiency of 92%.
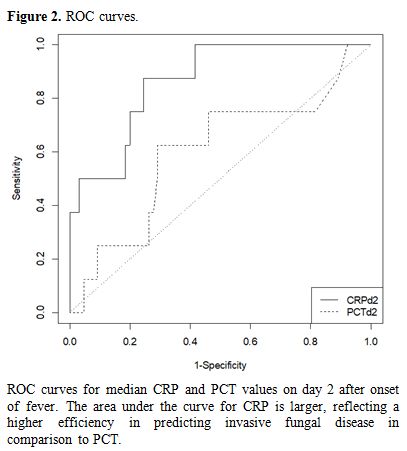 |
Figure 2. ROC curves. |
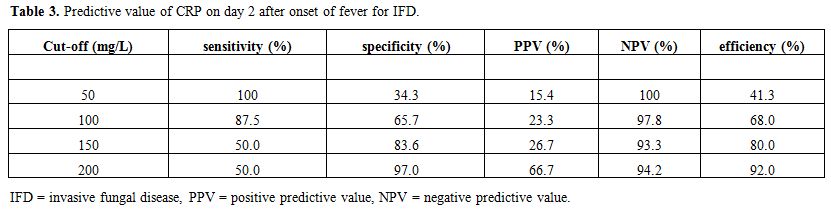 |
Table 3. Predictive value of CRP on day 2 after onset of fever for IFD. |
Discussion
The
management of febrile neutropenia in patients with prolonged, profound
neutropenia remains challenging as there are many possible infectious
and non-infectious causes for fever. The possible risk of a fatal
outcome from bacterial infection warrants immediate administration of
broad-spectrum antibiotics. However, in many cases such antibiotic
therapy might not be necessary and long periods of treatment with
broad-spectrum antibiotics can result in toxicity, selection of
multidrug-resistant pathogens and increased predisposition to
infections by Clostridium difficile and yeasts/fungi. While the
clinical condition of the patient is the most important element in the
decision-making process leading to the initiation, reassessment and
(dis)continuation of broad-spectrum antibiotics, laboratory parameters
denoting infection/inflammation are also included. CRP is a very basic
and widespread test to reflect inflammation but is not specific for
infection. In this study, we investigated whether PCT could provide
superior reliability in comparison to CRP in the clinical management of
fever in patients with prolonged, profound neutropenia. To achieve
this, we performed daily determinations of CRP and PCT whereas several
other studies in the field have limited the number of PCT measurements
and might have only provided a partial picture.
On the day of
fever onset, both CRP and PCT were not able to differentiate between
the aetiologies of febrile episodes nor were they able to predict the
severity of the clinical picture (including hypotension, hypoxia and
the need for transfer to intensive care facilities). As such, the
decision whether or not to start antibiotics when febrile neutropenia
occurs cannot be delayed even with low values of CRP and/or PCT.
However, after two days of febrile neutropenia, reassessment needs to
be performed to decide on (dis)continuation of broad-spectrum
antibiotics. In our study, median CRP values at this time were
significantly higher in the case of IFD as well as MDI in contrast to
CDI and FUO where they stayed below 100 mg/L. The CRP value on day 2
was significantly higher in episodes of febrile neutropenia running a
severe clinical course, whereas this was not the case for PCT. Median
PCT values at this point were especially high in case of FUO with ATG,
which confirms previous findings by Brodska et al. & Hambach et al.[18-19]
In IFD and MDI they rose above 0.25 ng/mL, whereas they did not in case
of CDI and FUO without ATG. However, these differences were not
statistically significant, and PCT surpassed the threshold of 0.5 ng/mL
only in 9 out of 23 FE (39.1%) caused by bacteraemia on day two after
fever onset.
These findings contrast the results of three prior
studies discussing the value and/or dynamics of PCT in this specific
patient population. Gac et al. prospectively studied 29 patients with
39 instances of chemotherapy and found that all neutropenic episodes
with bacteraemia reached a PCT value of 0.5 ng/mL at 15 days after the
onset of chemotherapy.[20] Robinson et al.
prospectively studied 194 consecutive febrile episodes during 125
neutropenic episodes in 90 patients. They observed that a PCT threshold
of 0.5 ng/mL on day two after the onset of fever allowed the best
discrimination of severe infections from infections due to
coagulase-negative staphylococci (CoNS), superficial infections or
fever of unknown origin.[21] Koivula et al. analysed
90 episodes of febrile neutropenia in 66 patients and concluded that an
elevated level of PCT above 0.5 ng/mL within 24 hours after onset of
fever was able to predict bacteraemia and Gram-negative bacteraemia
with a sensitivity of 57% & 70% and a specificity of 81% & 77%
respectively.[22]
Contrasting results have also been reported in the setting of allogeneic HSCT, where studies by Pihush et al.[23] and Koya et al.[24]
concluded that PCT has a superior discriminatory power for detection of
systemic infection and can differentiate infection from other
transplant-related complications such as GvHD despite steroid therapy.
However, these results contradict older studies by Blijlevens et al.,
Hambach et al. and Ortega et al.[19,25-26]
All three studies concluded that the diagnostic value of PCT was not
superior to that of CRP in the detection of infections after allogeneic
HSCT and did not facilitate the differential diagnosis of febrile
episodes.
A possible explanation for these conflicting results
could be the presence of severe neutropenia whereas peripheral blood
mononuclear cells have been described as a major source for PCT release
in sepsis.[27] Some authors reported low sensitivity of PCT levels in patients with a WBC count < 1x109/L and previous studies confirmed a correlation of PCT with low neutrophil count.[26,28]
However, we could not confirm this correlation in our dataset. Another
possible confounding factor could be the fact that in many studies PCT
samples were frozen and analysed in batch at a later time. In our
study, we performed daily measurements on fresh samples as this would
be the way one would eventually implement it into real life daily
practice and decision making.
From a practical point of view,
MDI and CDI are usually already diagnosed by day two based on clinical
examination, chest X-ray and microbiological cultures. As such the most
important differential diagnosis at this point concerns FUO versus IFD,
coupled with the decision to discontinue antibiotics and/or initiate
antifungal treatment. Current guidelines suggest diagnostics for IFD to
be performed after four days of persistent febrile neutropenia.
However, in our study, a CRP value above 200 mg/L showed a positive
predictive value of 66.7% and a negative predictive value of 94.2% for
the diagnosis of IFD versus FUO (when MDI and CDI were ruled out by
diagnostic workup). A high CRP in the absence of a clear focus of
infection might prompt earlier investigation for IFD, leading to
earlier treatment initiation and lower mortality. Given the low numbers
of IFD in our study, these findings should be confirmed in a larger
patient population.
Conclusions
In
haematological patients with prolonged, profound neutropenia, PCT has
no added value over CRP for clinical management of febrile neutropenia.
Both CRP and PCT are not able to predict either aetiology or severity
of infection at the onset of fever. When performing a reassessment of
antibiotic therapy two days after the onset of fever, CRP has the
better discriminatory power between aetiologies of fever and shows
higher peak values in clinically severe infections. As such, there
seems to be no reason to introduce PCT in the daily clinical
decision-making process on antibiotic and antifungal therapy in
prolonged, profound neutropenic patients suffering from febrile
neutropenia.
Acknowledgements
This
study was supported by a collaborative grant from MSD-Merck as well as
a research grant from the Multidisciplinary Oncology Centre Antwerp
(MOCA – Antwerp University Hospital / University of Antwerp). The
authors have no competing interests to declare. All authors contributed
significantly to the presented research and read/approved the final
paper. The corresponding author would like to thank Erick van der Bos
for his help with designing the dataset and lay-out of figures
References
- Klastersky J. Management of fever in neutropenic
patients with different risks of complications. Clin Infect Dis.
2004;39:S32-7. https://doi.org/10.1086/383050 PMid:15250018
- Bucaneve
G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al.
Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) Infection
Program. Levofloxacin to prevent bacterial infection in patients with
cancer and neutropenia. N Engl J Med. 2005;353:977-87. https://doi.org/10.1056/NEJMoa044097 PMid:16148283
- Verlinden
A, Jansens H, Goossens H, van de Velde AL, Schroyens WA, Berneman ZN,
et al. Clinical and microbiological impact of discontinuation of
fluoroquinolone prophylaxis in patients with prolonged profound
neutropenia. Eur J Haematol. 2014;93:302-8. https://doi.org/10.1111/ejh.12345 PMid:24750350
- Gyssens
I, Kern W, Livermore D on behalf of ECIL-4, a joint venture of EBMT,
EORTC, ICHS and ESGICH of ESCMID. The role of antibiotic stewardship in
limiting antibacterial resistance in haematology patients.
Haematologica. 2013;98:1821-5. https://doi.org/10.3324/haematol.2013.091769 PMid:24323982 PMCid:PMC3856956
- Bhatt V, Viola G, Ferrajoli A. Invasive Fungal Infections in Acute Leukemia. Ther Adv Haematol. 2011;2:231-47. https://doi.org/10.1177/2040620711410098 PMid:23556092 PMCid:PMC3573411
- Pepys
MB, Baltz ML. Acute phase proteins with special reference to C-reactive
protein and related proteins (pentaxins) and serum amyloid A protein.
Adv Immunol. 1983;34:141-212. https://doi.org/10.1016/S0065-2776(08)60379-X
- Le
Moullec JM, Jullienne A, Chenais J, Lasmoles F, Guliana JM, Milhaud G,
et al. The complete sequence of human preprocalcitonin. FEBS Lett.
1984;167:93-7. https://doi.org/10.1016/0014-5793(84)80839-X
- Müller
B, White JC, Nylén ES, Snider RH, Becker KL, Habener JF. Ubiquitous
expression of the calcitonin I gene in multiple tissues in response to
sepsis. J Clin Endocrinol Metab. 2001;86:396-404 https://doi.org/10.1210/jc.86.1.396
- Becker
KL, Nylén ES, White JC, Müller B, Snider RH Jr. Procalcitonin and the
calcitonin gene family of peptides in inflammation, infection, and
sepsis: a journey from calcitonin back to its precursors. J Clin
Endocrinol Metab. 2004;89:1512-25. https://doi.org/10.1210/jc.2002-021444 PMid:15070906
- Simon
L, Gauvin F, Amre DK, Saint-Louis P, Lacroix J. Serum procalcitonin and
C-reactive protein levels as markers of bacterial infection: a
systematic review and meta-analysis. Clin Infect Dis. 2004;39:206-17. https://doi.org/10.1086/421997 PMid:15307030
- Limper
M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. The diagnostic role
of procalcitonin and other biomarkers in discriminating infectious from
noninfectious fever. J Infect. 2010;60:409-16. https://doi.org/10.1016/j.jinf.2010.03.016 PMid:20347867
- Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010;48:2325-9. https://doi.org/10.1128/JCM.00655-10 PMid:20421436 PMCid:PMC2897488
- Reinhart K, Meisner M. Biomarkers in the critically ill patient: procalcitonin. Crit Care Clin. 2011;27:253-63. https://doi.org/10.1016/j.ccc.2011.01.002 PMid:21440200
- Sakr
Y, Sponholz C, Tuche F, Brunkhorst F, Reinhart K. The role of
procalcitonin in febrile neutropenic patients: review of the
literature. Infection. 2008;36:396-407. https://doi.org/10.1007/s15010-008-7374-y PMid:18759057
- Klastersky
J, de Naurois J, Rolston K, Rapoport B, Maschmeyer G, Aapro M, et al.
On behalf of the ESMO Guidelines Committee. Management of febrile
neutropaenia: ESMO Clinical Practice Guidelines. Annals of Oncology.
2016; 27:v111-8. https://doi.org/10.1093/annonc/mdw325 PMid:27664247
- Anonymous.
From the Immunocompromised Host Society. The design, analysis, and
reporting of clinical trials on the empirical antibiotic management of
the neutropenic patient. Report of a consensus panel. J Infect Dis.
1990;161:397-401. https://doi.org/10.1093/infdis/161.3.397 PMid:2179421
- De
Pauw B, Walsh T, Donnelly P, Stevens DA, Edwards JE, Calandra T, et al.
Revised Definitions of Invasive Fungal Disease from the European
Organization for Research and Treatment of Cancer/Invasive Fungal
Infections Cooperative Group and the National Institute of Allergy and
Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.
Clin Infect Dis. 2008;46:1813-21. https://doi.org/10.1086/588660 PMid:18462102 PMCid:PMC2671227
- Brodska
H, Drabek T, Malickova K, Kazda A, Vitek A, Zima T, Markova M. Marked
increase of procalcitonin after the administration of anti-thymocyte
globulin in patients before hematopoietic stem cell transplantation
does not indicate sepsis: a prospective study. Crit Care
2009;13(2):R37. https://doi.org/10.1186/cc7749 PMid:19291300 PMCid:PMC2689473
- Hambach
L, Eder M, Dammann E, Schrauder A, Sykora KW, Dieterich C, et al.
Diagnostic value of procalcitonin serum levels in comparison with
C-reactive protein in allogeneic stem cell transplantation.
Haematologica. 2002;87:643-51. PMid:12031922
- Gac
AC, Parienti JJ, Chantepie S, Fradin S, Le Coutour X, Leclercq R, et
al. Dynamics of procalcitonin and bacteremia in neutropenic adults with
acute myeloid leukemia. Leuk Res. 2011;35:1294-6. https://doi.org/10.1016/j.leukres.2011.05.035 PMid:21831426
- Robinson
JO, Lamoth F, Bally F, Knaup M, Calandra T, Marchetti O. Monitoring
procalcitonin in febrile neutropenia: what is its utility for initial
diagnosis of infection and reassessment in persistent fever? PLoS One.
2011;6:e18886. https://doi.org/10.1371/journal.pone.0018886 PMid:21541027 PMCid:PMC3081821
- Koivula
I, Hämäläinen S, Jantunen E, Pulkki K, Kuittinen T, Nousiainen T, et
al. Elevated procalcitonin predicts Gram-negative sepsis in
haematological patients with febrile neutropenia. Scand J Infect Dis.
2011;43:471-8. https://doi.org/10.3109/00365548.2011.554855 PMid:21299364
- Pihusch
M, Pihusch R, Fraunberger P, Pihusch V, Andreesen R, Kolb HJ, et al.
Evaluation of C-reactive protein, interleukin-6, and procalcitonin
levels in allogeneic hematopoietic stem cell recipients. Eur J
Haematol. 2006;76:93-101. https://doi.org/10.1111/j.0902-4441.2005.00568.x PMid:16405429
- Koya
J, Nannya Y, Ichikawa M, Kurokawa M. The clinical role of procalcitonin
in hematopoietic SCT. Bone Marrow Transplant. 2012;47:1326-31. https://doi.org/10.1038/bmt.2012.18 PMid:22343672
- Blijlevens
NM, Donnelly JP, Meis JF, De Keizer MH, De Pauw BE. Procalcitonin does
not discriminate infection from inflammation after allogeneic bone
marrow transplantation. Clin Diagn Lab Immunol. 2000;7:889-92. https://doi.org/10.1128/CDLI.7.6.889-892.2000
- Ortega
M, Rovira M, Filella X, Almela M, Puig de la Bellacasa J, Carreras E,
et al. Prospective evaluation of procalcitonin in adults with febrile
neutropenia after haematopoietic stem cell transplantation. Br J
Haematol. 2004;126:372-6. https://doi.org/10.1111/j.1365-2141.2004.05053.x PMid:15257709
- Oberhoffer
M, Stonans I, Russwurm S, Stonane E, Vogelsang H, Junker U, et al.
Procalcitonin expression in human peripheral blood mononuclear cells
and its modulation by lipopolysaccharides and sepsis-related cytokines
in vitro. J Lab Clin Med 1999; 134:49-55. https://doi.org/10.1016/S0022-2143(99)90053-7
- Svaldi
M, Hirber J, Lanthaler AI, Mayr O, Faes S, Peer E & Mitterer M.
Procalcitonin-reduced sensitivity and specificity in heavily leucopenic
and immunosuppressed patients. British Journal of Haematology 2001;
115:53-57. https://doi.org/10.1046/j.1365-2141.2001.03083.x PMid:11722409
[TOP]




