Alessandra Nicoletti1, Calogero Edoardo Cicero1, Antonia Mantella2, Loretta Giuliano1, Cristina Rascunà1, Vincenza Paradisi3, Alessandro Bartoloni2, Mario Zappia1 and Vito Sofia1.
1 Department
of Medical and Surgical Sciences and Advanced Technologies “G.F.
Ingrassia”, Section of Neurosciences, University of Catania, Catania,
Italy.
2 Department of Experimental and Clinical
Medicine, Infectious and Tropical Diseases Unit, University of
Florence, Florence, Italy.
3 Italian Society of General Medicine (SIMG), Catania, Italy.
Correspondence to: Alessandra Nicoletti, Department G.F. Ingrassia,
Section of Neurosciences University of Catania, Via Santa Sofia 78,
95123 Catania. Tel. +390953782783. E-mail:
anicolet@unict.it
Published: May 1, 2019
Received: February 6, 2019
Accepted: April 13, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019031 DOI
10.4084/MJHID.2019.031
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
Toxocariasis
is one of the most common helminthiases worldwide. However, there is a
lack of data regarding Southern Italy. We have evaluated the
seroprevalence and associated environmental factors of toxocariasis in
a sample of adults living in the city of Catania.
Presence of anti-Toxocara canis
IgG antibodies was searched using an ELISA test using
excretory/secretory antigens. Environmental risk factors have been
evaluated with a face-to-face questionnaire.
Two hundred eighty-seven subjects (193 [67.3%] women, mean age 48.1±15.6 years) were enrolled, and presence of anti T. canis
antibodies was found in 23 participants, of whom 18 (78.3%) were women
with a mean age of 51.1±14.0 years, giving a seroprevalence of 8.0%
(95%CI 5.4-11.7). At multivariate analysis, a positive association for
subjects with more than three siblings (adjOR 3.17; 95%CI 1.09-9.25)
was recorded.
Our study confirms that exposition to T. canis is frequent also in urban areas of western countries.
|
Introduction
Human toxocariasis is due to the larval stages of the ascarids Toxocara canis and Toxocara cati,
common roundworms of dogs and cats respectively. It is one of the most
prevalent helminthiases worldwide, especially in settings where the
man-soil-dog relationship is particularly close.[1] T. canis
parasites the small intestine of the dog, its main host that can become
infected via the placenta or by contact with contaminated feces. The
female T. canis produces up to 200 000 eggs per day, releasing them to the environment through feces.[2]
Humans can be infected by direct contact with dogs, by the ingestion of
contaminated food or soil or by eating infected meat of paratenic
hosts.[1] While the large majority of infections are
thought to be asymptomatic, visceral larva migrans (VLM) and ocular
larva migrans (OLM) are the most common clinical manifestations,[3]
even if two less severe syndromes have been described: the covert
toxocariasis, which is more common in children, and common toxocariasis
that was reported in adults.[4] However, T. canis
has also been identified in the Central Nervous System, leading to a
wide variety of neurological manifestations collectively termed
neurotoxocariasis.[5] In western countries
toxocariasis is part of the so-called “neglected infections of poverty”
because of its distribution in low income areas of the United States of
America (USA)[6] and, for Europe, among eastern
countries and the southern regions of European countries, both areas
with lower socioeconomic levels, compared with the rest of Europe.[7]
Toxocariasis has a seroprevalence of up to 90% in tropical settings
and, for western countries, ranging from 35% to 42% in rural areas and
between 2% and 5% in urban areas.[8] In Italy, the only two studies have been carried out reporting a seroprevalence of 4.0% in a northern Italian region[9] and a seroprevalence of 1.6% in the Marche region.[10]
The aim of the present study was to describe the seroprevalence of T. canis and the association between demographic and environmental factors in a sample of the adult population in the city of Catania.
Materials and Methods
The
study has been performed in Catania, Italy, a city of the Sicily, which
is located at a mean altitude of about 30 m above sea level and has an
area of 181 km2. Its official population is 293,104 inhabitants.[11]
Participants over 14 years old were selected using a multi-stage
sampling method. The study is part of a larger case-control study aimed
to evaluate the role of both environmental and genetic factors and the
risk of Multiple Sclerosis (MS) in the population of Catania.
Background and methods have been extensively reported elsewhere.[12]
After
enrolment in the study, a blood sample was collected. Samples have been
coded and processed to obtain serum aliquots and then stored at -20°C
in the laboratories of the “Azienda Ospedaliera Policlinico Vittorio
Emanuele.” Serum samples have been shipped in dry ice to the
laboratories of the Infectious Diseases Institute (Malattie Infettive e
Tropicali, AOU Careggi) of the University of Florence and have been
analysed by a biologist blinded to the status of the participants.
Specific T. canis IgG has been detected with a commercial ELISA kit (Ridascreen Toxocara IgG; R-Bio farm, Milan, Italy) using excretory/secretory antigens (Toxocara excretory-secretory antigen [TES-Ag]) from second-stage T. canis larvae.[13]
A face-to-face semi-structured standardized questionnaire about
demographic and environmental factors has been administered to all the
participants.
All the analyses have been conducted with the software STATA 12.0. For the prevalence of anti-T. canis
antibodies the 95% CI have been calculated. Quantitative variables were
described using mean and standard deviation. The difference between
means and the difference between proportions were evaluated by the t-test and the chi-square test, respectively.
Unconditional
logistic regression analysis was performed, and for each study
variable, we calculated OR, 95% CI, and p-value (two-tailed test,
α=0.05). Multivariate analysis was conducted to investigate the
independent effect of risk or protective factor after adjustment for
one or several other factors or to adjust for confounding variables.
Parameters associated with the outcome at the univariate analysis with
a threshold of P = 0.25 were included in the model. The model was
manually constructed using the likelihood ratio test (LRT) to compare
the log-likelihood of the model with and without a specific variable.
Sex, age, and education have been considered a priori confounder
variables. Whenever variables were dichotomized, the cutoffs were
derived from the median value of the pooled distribution. Each
participant was asked to sign an informed consent. The study has been
approved by the Local Ethical Committee (code 64/2018/PO).
Results
At the end of the recruitment process, 300 subjects have been enrolled. After the research of anti-T. canis
antibodies in serum, results of 13 participants were deemed unreliable
due to incorrect storage of the samples, and thus these subjects have
been dropped from the final analysis, with a final sample of 287
participants.Subjects
had a mean age of 48.1±15.6 years, and 193 (67.3%) were women. The
majority of them were professionals (n=103, 36.4%) or housewives (n=79,
28.0%). The demographic characteristics of the population are reported
in Table 1.
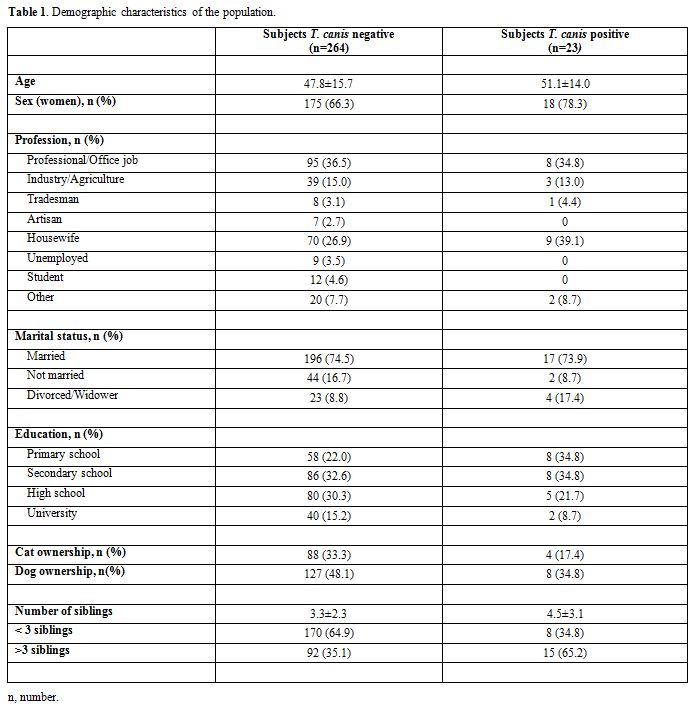 |
Table 1. Demographic characteristics of the population. |
Presence of anti-T. canis
antibodies were found in 23 subjects (18 women, 78.2%; mean age
51.1±14.0 years) resulting in seroprevalence of 8.0% (95%CI 5.4-11.7).
At the univariate analysis, no association has been found with sex,
age, profession or owning pets; a number of siblings were significantly
associated with T. canis
seropositivity with an OR of 3.46 (95%CI 1.41-8.47) for subjects with
more than 3 siblings. A close association was found at multivariate
analysis adjusting by age, sex and education (adj OR 3.17; 95%CI
1.09-9.25) as shown in Table 2.
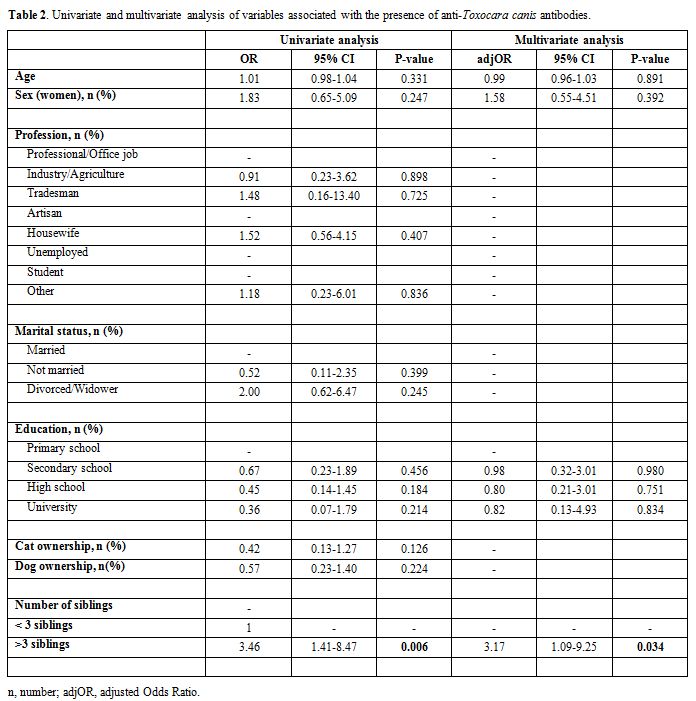 |
Table 2. Univariate and multivariate analysis of variables associated with the presence of anti-Toxocara canis antibodies. |
Discussion
Toxocariasis
is one of the most prevalent helminthiases worldwide and even if the
parasite tends to be more prevalent in tropical settings, in urban
areas of Western countries seroprevalence ranges from 2 to 5%.[8]
This
is the first study carried out to assess the seroprevalence of
toxocariasis in a sample of the adult population (over 14 years) in the
city of Catania, Sicily. We found a seroprevalence of 8.0%, a value
slightly higher with respect to data reported in other urban areas of
European countries,[8] as well as to those previously reported in two earlier studies carried out Italy, 1.6 in Central Italy in 2003[10] and 4.0 in Northern Italy in 1990.[9]
The
higher seroprevalence found in the city of Catania can be explained by
the environmental factors such as the degree of humidity and
temperature that increase the overall survival of T. canis eggs in the soil.[14]
However, another critical aspect of being considered is environmental
contamination due to the presence of infected dog feces that contribute
to the dissemination of T. canis eggs. While we have no data about the prevalence of T. canis
infected dog feces in the urban areas of Catania, a study conducted in
the neighbouring city of Messina, that shares with Catania the same
environmental factors, found a prevalence of T. canis eggs of 3.6% in a random sample of dog feces.[15]
Furthermore, another contribution to the increased seroprevalence is
the increased presence of dogs living in the households, considering
the high level of infestation they bear, up to a prevalence of 76% in
puppies in a recent study conducted in Italy.[16]
Presence of T. canis
IgG was assessed using a TES-ELISA, considered the standard serological
test commonly used in epidemiological surveys to determine the
seroprevalence of T. canis
in a defined population. While we are aware of the limitations of ELISA
test, such as the low specificity, we believe that the lack of a
confirmatory Western Blot, considered the gold standard procedure,[14]
has not influenced our results because the low specificity depends on
the frequent cross-reactions with other nematode infections such as
Ascaris lumbricoides, filariasis or strongyloidiasis, which are rare in
western countries.[14] In our sample T. canis seropositivity was not associated with age , as expected according to recent literature.[17]
Furthermore in our sample pet ownership was not associated with the risk of T. canis
seropositivity, probably because the risk of being infected by the
direct contact with domestic animals has been showed to be low.[18]
On the other hand, the number of siblings (more than 3) was significantly associated with T. canis
seropositivity. Even if we have not a clear explanation for this
association, it is possible to hypothesize that a larger number of
siblings could act as a proxy indicator of a lower socio-economic
level, a risk factor for being infected with T. canis eggs.[17]
The size of the study and the selection of a sample from the general
population using an equal probability selection method (a multi-stage
sampling) represent the main strengths of our study.[12]
However, we are aware that the sex and age distribution of the sampled
population may not be entirely representative of the general
population. It should be underlined in fact that this study is part of
a larger population-based case-control study on MS and to this reason,
healthy subjects were recruited matched by age and sex with the
enrolled MS cases.[12]
Conclusions
Toxocariasis is a neglected disease, but our study confirms that exposition to T. canis
is frequent also in the urban area of western countries. It should be
stressed that even if the majority of infections are asymptomatic, T. canis
can also lead to a wide range of neurological manifestations
(neurotoxocariasis) and that due to the high seroprevalence recorded in
our population, its diagnosis should be taken into account in the
clinical setting.
Acknowledgements
We
are grateful to the Italian Society of General Medicine (SIMG) for its
support, and in particular to Anna Salvo MD, Guglielmo Travaglianti MD,
Gaetano Profeta MD, Carmelo Di Gregorio MD, Antonino Rizzo MD, Nuccia
Spada MD, Giovanni Cappello MD, Salvatore Amato MD, Marco Ciancio MD,
Melchiorre Fidelbo MD, Cettina Persano MD, Valeria Polizzi MD, Giovanni
Marotta MD, Maurizio D’Urso MD for their participation in the study.
Funding
This research was
funded by the Department of Medical and Surgical Sciences and Advanced
Technologies “G.F. Ingrassia”, University of Catania, Italy (Piano
Triennale di Sviluppo delle Attività di Ricerca Scientifica del
Dipartimento 2016-18). References
- Ma G, Holland CV, Wang T, Hofmann A, Fan CK,
Maizels RM, Hotez PJ, Gasser RB. Human toxocariasis. Lancet Infect Dis.
2018 Jan;18(1):e14-e24. https://doi.org/10.1016/S1473-3099(17)30331-6
- Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981; 3:230-250. https://doi.org/10.1093/oxfordjournals.epirev.a036235
- Schantz PM, Glickman LT. Toxocaral visceral larva migrans. N Engl J Med. 1978; 298(8):436-439. https://doi.org/10.1056/NEJM197802232980806 PMid:622118
- Taylor
MR, Keane CT, O'Connor P, Girdwood RW, Smith H. Clinical features of
covert toxocariasis. Scand J Infect Dis. 1987; 19(6):693-696. https://doi.org/10.3109/00365548709117206
- Deshayes
S, Bonhomme J, de La Blanchardière A. Neurotoxocariasis: a systematic
literature review. Infection. 2016; 44(5):565-574. https://doi.org/10.1007/s15010-016-0889-8
- Hotez PJ. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008; 2(6):e256. https://doi.org/10.1371/journal.pntd.0000256
- Hotez PJ, Gurwith M. Europe's neglected infections of poverty. Int J Infect Dis. 2011 15(9):e611-619. https://doi.org/10.1016/j.ijid.2011.05.006 PMid:21763173
- Fan
C-K, Holland CV, Loxton K, Barghouth U. Cerebral Toxocariasis: Silent
Progression to Neurodegenerative Disorders? Clin Microbiol Rev. 2015;
28(3):663-686. https://doi.org/10.1128/CMR.00106-14
- Genchi
C, Di Sacco B, Gatti S, Sangalli G, Scaglia M. Epidemiology of human
toxocariasis in northern Italy. Parassitologia. 1990; 32(3):313-319.
PMid:2132443
- Habluetzel A, Traldi G,
Ruggieri S, Attili AR, Scuppa P, Marchetti R, Menghini G, Esposito F. .
An estimation of Toxocara canis prevalence in dogs, environmental egg
contamination and risk of human infection in the Marche region of
Italy. Vet Parasitol. 2003, 113(3-4):243-252. https://doi.org/10.1016/S0304-4017(03)00082-7
- Istituto Nazionale di Statistica, 2011. 15° Censimento generale della popolazione.
- Nicoletti
A, Messina S, Bruno E, Mostile G, Quattrocchi G, Raciti L, Dibilio V,
Cappellani R, D'Amico E, Sciacca G, Lo Fermo S, Paradisi V, Patti F,
Zappia M. Risk factors in multiple sclerosis: a population-based
case-control study in Sicily. Background and methods. Neurol Sci. 2016
37(12):1931-1937. https://doi.org/10.1007/s10072-016-2685-8
- de
Savigny DH, Voller A, Woodruff AW. Toxocariasis: serological diagnosis
by enzyme immunoassay. J Clin Pathol. 1979; 32(3):284-288. https://doi.org/10.1136/jcp.32.3.284 PMid:372253 PMCid:PMC1145636
- Fillaux J, Magnaval J-F. Laboratory diagnosis of human toxocariasis. Vet Parasitol. 2013; 193(4):327-336. https://doi.org/10.1016/j.vetpar.2012.12.028 PMid:23318165
- Risitano
AL., Brianti E., Gaglio G., Ferlazzo M., Giannetto S. Environmental
contamination by canine feces in the city of Messina: parasitological
aspects and zoonotic hazards. In Proceedings of LXI Congress of the
Italian Society for Veterinary Science (S.I.S.Vet.). Salsomaggiore
Terme, Italy: 135-136.
- Corda A, Tamponi
C, Meloni R, Varcasia A, Parpaglia MLP, Gomez-Ochoa P, Scala A.
Ultrasonography for early diagnosis of Toxocara canis infection in
puppies. Parasitol Res. 2019 Mar;118(3):873-880. https://doi.org/10.1007/s00436-019-06239-4 PMid:30706166
- Berrett
AN, Erickson LD, Gale SD, Stone A, Brown BL, Hedges DW. Toxocara
Seroprevalence and Associated Risk Factors in the United States. Am J
Trop Med Hyg. 2017; 97(6):1846-1850. https://doi.org/10.4269/ajtmh.17-0542 PMid:29016316 PMCid:PMC5805073
- Poeppl
W, Herkner H, Tobudic S, Faas A, Mooseder G, Burgmann H, Auer H.
Exposure to Echinococcus multilocularis, Toxocara canis, and Toxocara
cati in Austria: a nationwide cross-sectional seroprevalence study.
Vector Borne Zoonotic Dis. 2013; 13(11):798-803. https://doi.org/10.1089/vbz.2012.1283 PMid:24107202

