Isacco Ferrarini1, Antonella Rigo1, Alberto Gandini2 and Fabrizio Vinante1.
1 Section of
Hematology, Cancer Research and Cell Biology Laboratory, Department of
Medicine, University of Verona, Verona, Italy.
2 Pediatrics
Section, Department of Surgical, Odontostomatological and
Maternal-Infantile Sciences, University of Verona, Verona, Italy.
Correspondence to: Dr. Isacco Ferrarini, Section of Hematology, Cancer
Research and Cell Biology Laboratory, Department of Medicine,
University of Verona, Verona, Italy. Email:
isacco.ferrarini@univr.it
Published: May 1, 2019
Received: January 29, 2019
Accepted: April 3, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019033 DOI
10.4084/MJHID.2019.033
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
West
Nile virus is a zoonotic agent causing life-threatening encephalitis in
a proportion of infected patients. Older age, immunosuppression, and
mutations in specific host genes (e.g., CCR5 delta-32 mutation)
predispose to neuroinvasive infection. We report on two cases of severe
West Nile encephalitis in recently-treated, different-aged, chronic
lymphocytic leukemia patients. Both patients developed high-grade fever
associated with severe neurological impairment. The younger one
harboured germ-line CCR5 delta-32 mutation, which might have played a
role in the pathogenesis of its neuroinvasive manifestations.
|
Introduction
West Nile virus (WNV) infection is a zoonotic disease first recognized in Europe in the 1960s.[1] Although the virus can also be transmitted through blood transfusion or organ transplantation, humans are mostly infected by Culex mosquitos in the transmission season, typically lasting from July to October.[2] As compared to the previous 4 years, WNV infections have sharply increased in Europe in 2018,[3]
probably due to circumstances favouring mosquito breeding and
propagation. While 80% of infected patients are asymptomatic, some of
them experience symptoms ranging from low-grade fever to severe
neurological illness.[2] Several risk factors for central nervous system involvement have been identified, among which older age,[4] impaired adaptive immunity, and reduced chemokine receptor 5 (CCR5) expression appear pivotal.[5]
At least the first two conditions are frequently shared among patients
affected by chronic lymphocytic leukemia (CLL), an indolent B-cell
lymphoproliferative disorder characterized by slowly progressive clonal
expansion in primary and secondary lymphoid organs, and associated with
an abnormal T helper cell profile.[6]
Here we
describe two cases of severe WNV encephalitis occurring in
recently-treated CLL patients in August 2018. Both cases have been
recorded during the infection spike, taking place in Eastern Italy in
the same period.
Case Report
(a)
A 53-year-old man was diagnosed with Rai II/Binet B CLL in September
2014. Immunoglobulin variable region heavy chain genes (IGHV) were
unmutated, fluorescent in situ hybridization (FISH) for common
cytogenetic abnormalities was unremarkable and TP53 status was wild
type. Because of massive symptomatic splenomegaly, he underwent
Rituximab-Fludarabine-Cyclofosfamide treatment in November 2017 through
April 2018, achieving partial remission. In August 2018, he was
hospitalized with high-grade fever and fatigue, without rash or
meningismus. He denied any recent travel, sick contacts, or blood
transfusion. Despite wide-spectrum antibiotics, his mental status and
weakness rapidly worsened, also developing dizziness and ataxia. White
blood cells count was 4500/μL, with moderate lymphopenia (600/μL).
Analysis of lymphocyte subpopulation displayed an almost total absence
of B cells and a reduced number of total T (560/μL) and NK cells
(16/μL), with marked reduced T CD4/CD8 ratio (0.16, normal range
1.15-2.84). There was hypogammaglobulinemia (IgG 3.0 g/L) due to
previous treatments. His cerebrospinal fluid (CSF) was clear with
normal opening pressure, hyperprotidorrachia (65 mg/dL), average
glucose, mild pleocytosis (10 leukocytes/μL) and negative bacterial and
fungal cultures. Flow cytometric analysis did not show leukemic
involvement. Magnetic resonance imaging (MRI) returned negative for
both focal and diffuse signal alterations, whereas
electroencephalography (EEG) showed diffuse slow wave activity without
paroxysm. Within 3 days, he became poorly responsive to verbal stimuli
and diffusely hyporeflexic. Because of the clinical suspect of viral
encephalitis, Acyclovir treatment and immunoglobulins supplementation
were promptly started without improvement in his neurological
condition. Polymerase chain reaction (PCR) for WNV returned positive in
both serum and urine, with no detectable specific serum antibodies
likely due to hypogammaglobulinemia. All the other virological tests
concerning neurotropic viruses, performed on CSF and serum, were
negative. On hospital day 16, the patient’s cognitive performance began
to improve, and he gradually recovered his motor functions as well as
osteo-tendon reflexes. Finally, he was discharged and transferred to a
rehabilitation clinic for a full recovery. We searched for anomalies in
CCR5. Sequencing of Ccr5 gene led to identifying CCR5 delta-32 mutation in heterozygosis.
(b)
An 85-year-old woman was diagnosed with Rai III/Binet C CLL in June
2014. For progressive and symptomatic anemia due to bone marrow
failure, the patient underwent treatment with Chlorambucil and
Rituximab in 2016, achieving a temporary improvement in her peripheral
blood counts. In June 2018 she experienced a new worsening of anemia
with high need of transfusion support. FISH and TP53 mutational status
were negative, but cytogenetic analysis using B-cell mitogens showed
del(3) (p13p21) and del(14) (q24) in 8 metaphases out of 20. Because of
progressive disease, the patient started the second line Ibrutinib
treatment. However, she autonomously discontinued drug assumption due
to poor gastrointestinal tolerance as soon as thirty days after
beginning. At the end of July 2018, she was admitted to the emergence
department with fever, myalgias, anorexia and worsening fatigue. Her
blood cell counts showed moderate lymphocytosis (24000/μL) with mild
anemia and thrombocytopenia, whereas C-reactive protein and
procalcitonin were in the normal range. Empirical antibiotic therapy
was initially administered. However, her neurological conditions
rapidly worsened over the following days, developing psychomotor
agitation with stereotyped afinalistic movements of the lower limbs and
altered mental status. Cranial computed tomography scan was
unremarkable, and EEG showed diffuse slowing of background activity
without focal spikes. Diagnostic lumbar puncture was performed, with
normal opening pressure, clear CSF, pleocytosis of 44 leukocytes/μL
(mononuclear prevalent), hyperglycorrhachia (66 mmol/L) and
hyperprotidorrachia (67 mg/dL). As there was the clinical suspicion of
viral encephalitis, intravenous Acyclovir treatment was initially
administered. Serum and CSF WNV IgM returned positive, whereas all the
other microbiological tests performed on serum and CSF did not. Also,
serum WNV PCR was positive. Despite supportive measures, the patient’s
state of consciousness progressively declined, becoming unresponsive to
any external stimuli. She finally died sixteen days after her hospital
admission. Sequencing of Ccr5 gene revealed no alterations.
Sequencing method.
Genomic DNA was extracted from 200 μL of whole blood by use of QIAamp
DNA mini kit (Hilden, DE), according to with manufacturer’s
instructions. DNA was amplified by Platinum Taq DNA polymerase High
Fidelity (Invitrogen, Carlsbad, CA) using primers flanking the site of
the 32 base pair deletion: 5’-CGCATCAAGTGTCAAGTCCAATC-3’ and 5’-
TGTAAACTGAGCTTGCTCGCT-3’ (M-Medical, Cornaredo, IT). PCR products were
purified with FastGene extraction kit (Nippon Genetics, Tokyo, JP). The
reverse PCR primer was then used for Sanger sequencing with GenomeLab
DTCS quick start kit (Sciex, Framingham, MA) and CEQ 8000 Genetic
Analysis System (Sciex) following the manufacturer instructions.
Discussion
Since
2008, Italy contribution to European WNV infections increased
substantially, with peaks of cases reported in 2013 and 2015.[7]
WNV is maintained in a continuous mosquito-bird cycle, wherein
mosquitos are the vectors, and the birds are the reservoir. Humans,
just like horses and other mammals, represent dead-end hosts and do not
contribute to further spread of the infection.[2] West
Nile and other viral zoonotic infections have long been identified as a
relevant cause of morbidity in people bearing genetic or acquired
immune deficiencies. In recent years, the rapid spread of WNV infection
have been overlapped with the rising number of immunocompromised hosts,
due to increased median life expectancy, more powerful anticancer
strategies and widespread usage of immunosuppressive and
immunomodulatory drugs, also for autoimmune and other non-malignant
conditions. In haematological setting, several cases of neuroinvasive
WNV disease have been reported (Table 1).[8-18]
They mainly comprise patients diagnosed with WNV encephalitis while on
treatment for lymphoid malignancies or recently undergone bone marrow
transplantation, even in paediatric age. Most of them were hospitalized
due to high-grade fever and neurological symptoms ranging from coarse
tremor and cerebellar ataxia to seizures and confusional state. About a
half of cases showed thalamic involvement at MRI, with hyperintensity
on T2-weighted imaging and altered FLAIR sequences.[8,10-13,15] Diagnosis of neuroinvasive disease was confirmed either by positive WNV PCR (in CSF or plasma)[8,9,10-14,16] or by positive serological tests (IgM),[12,13,15,18] in the presence of suggestive clinical features. Notably, all Rituximab-treated patients were diagnosed by PCR,[8,9,11,14]
as serological tests always returned negative due to treatment-related
B-cell impairment. However, clinicians may also be aware that viremic
phase is short in humans,[16] thus negative molecular
tests do not exclude the diagnosis. This makes even more challenging
the diagnostic process in immunocompromised subjects. In them, the
outcome was fatal for 8 out of 15 patients, and some survivors
experienced severe neurological sequelae,[13,14] which often parallel cortical thinning and regional atrophy detected by neuroimaging studies.[19] None of them was evaluated for Ccr5 gene status.
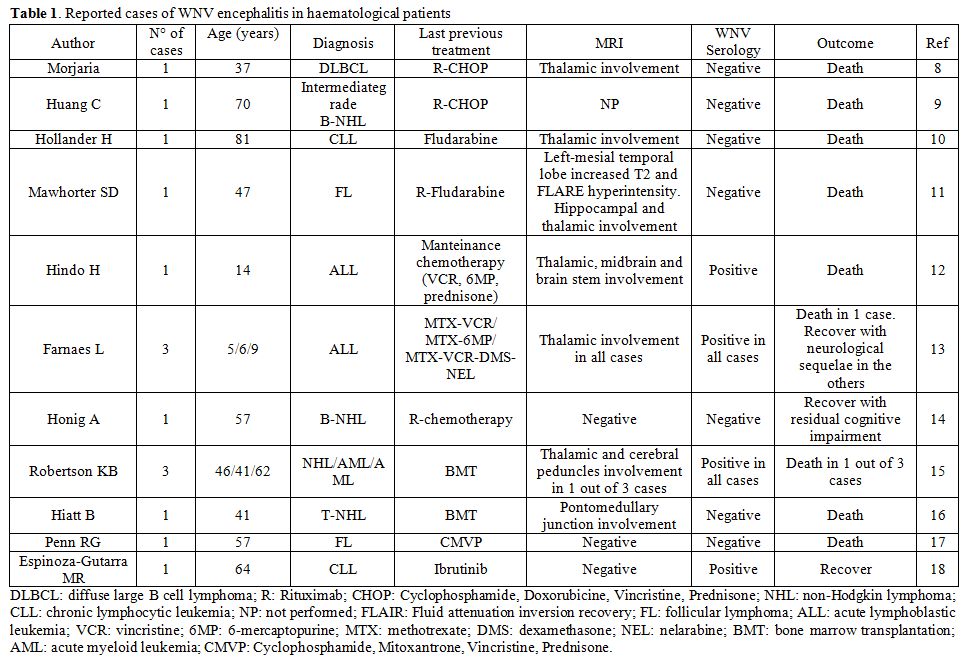 |
Table
1. Reported cases of WNV encephalitis in haematological patients. |
The
two cases we described well recapitulate the main clinical and
laboratoristic features of severe WNV infection. Both of them were
affected by CLL and were recently treated in an outpatient setting with
chemoimmunotherapy and Ibrutinib, respectively. To our knowledge, only
two patients affected by WNV encephalitis previously diagnosed with CLL
have been described in the literature.[10,18]
Our patients presented with fever and went through a rapid
deterioration of neurological conditions. C-reactive protein was not
elevated, CSF analysis showed alterations consistent with blood-brain
barrier (BBB) damage and neuroimaging was insignificant. This latter
occurs in about 50% of patients affected by WNV encephalitis, the other
half developing abnormal MRI findings, which commonly involve thalami,
basal ganglia, mesial temporal structures, brain stem, and cerebellum.[20]
In the first, Rituximab-treated, patient, the diagnosis was based on
positive WNV PCR, as serology returned negative. The fatal outcome of
the second patient was likely favoured by older age and female gender,
usually associated with worse recovery from coma.[21]
Since most neuroinvasive WNV disease in non-haematological setting occur in the elderly,[2] the development of such a severe clinical picture in the first, young patient prompted us to investigate the presence of Ccr5 delta-32 mutation as an additional risk factor for neuroinvasive infection.[22]
CCR5 is the G protein-coupled receptor binding to three CC chemokines,
namely CCL3, CCL4 and CCL5, involved in T lymphocyte trafficking
through the BBB. It is mainly expressed by T helper 1 subset upon
antigen recognition, whereby coupling the amplification of the
inflammatory response with the appropriate environmental context.[23,24] By sequencing the Ccr5
gene, we found the patient was heterozygous for the delta-32 mutation,
which generates a truncated form of CCR5 persistently retained within
the endoplasmic reticulum, thus lowering its surface expression. Of
note, delta-32 CCR5 has been reported to act as a dominant negative
mutant, further reducing the cell sensing for CCR5 cognate ligands.[25]
Therefore, impairment of CCR5-based chemotactic system, along with low
T CD4 cell count due to previous chemotherapy, may have played a
non-negligible role in the pathogenesis of his neuroinvasive WNV
infection. We speculate that Ccr5
genotyping might be of some importance to identify immunocompromised
patients particularly at risk for life-threatening neurological
complications. Effective preventive strategies, mostly still to be
uncovered, might be primarily directed to them.
Conclusions
The
two cases we reported highlight some general considerations. First, the
rapid spread of WNV infection should induce clinicians to take it into
account within the diagnostic workup of immunocompromised patients with
fever and neurological symptoms, especially in summer months. In
haematological setting, outpatient regimens are even likely to increase
the risk of neuroinvasive disease, and diagnosis may be difficult in
those patients treated with monoclonal antibodies blunting humoral
response. Then, assessment of genetic risk factors for severe WNV
infection, such as Ccr5
delta-32 mutation, may be useful to direct future preventive or
pre-emptive strategies. However, supportive measures remain the only
way to face the disease so far, and fatal outcome is still
frequent.
References
- Campbell GL, Ceianu CS, Savage HM. Epidemic West
Nile encephalitis in Romania: waiting for history to repeat itself. Ann
N Y Acad Sci. 2001;951:94-101. https://doi.org/10.1111/j.1749-6632.2001.tb02688.x PMid:11797808
- Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013;310:308-15. https://doi.org/10.1001/jama.2013.8042 PMid:23860989 PMCid:PMC4563989
- World Health Organization. Regional Office for Europe. http://www.euro.who.int/en/countries/italy/news/news/2018/8/west-nile-virus-infections-spike-in-southern-and-central-europe .
- Montgomery RR. Age-related alterations in immune responses to West Nile virus infection. Clin Exp Immunol. 2017;187:26-34. https://doi.org/10.1111/cei.12863 PMid:27612657 PMCid:PMC5167051
- Montgomery
RR, Murray KO. Risk factors for West Nile virus infection and disease
in populations and individuals. Expert Rev Anti Infect Ther.
2015;13:317-325. https://doi.org/10.1586/14787210.2015.1007043 PMid:25637260 PMCid:PMC4939899
- Palma
M, Gentilcore G, Heimersson K, et al. T cells in chronic lymphocytic
leukemia display dysregulated expression of immune checkpoints and
activation markers. Haematologica. 2017;102:562-572. https://doi.org/10.3324/haematol.2016.151100 PMid:27927767 PMCid:PMC5394965
- Rizzo
C, Napoli C, Venturi G, et al. West Nile virus transmission: results
from the integrated surveillance system in Italy, 2008 to 2015. Euro
Surveill. 2016;21. doi: 10.2807/1560-7917.ES.2016.21.37.30340. https://doi.org/10.2807/1560-7917.ES.2016.21.37.30340
- Morjaria
S, Arguello E, Taur Y, et al. West Nile Virus Central Nervous System
Infection in Patients Treated With Rituximab: Implications for
Diagnosis and Prognosis, With a Review of Literature. Open Forum Infect
Dis. 2015;2:ofv136. doi: 10.1093/ofid/ofv136. https://doi.org/10.1093/ofid/ofv136
- Huang
C, Slater B, Rudd R, et al. First isolation of West Nile virus from a
patient with encephalitis in the United States. Emerg Infect Dis.
2002;8:1367-1371. https://doi.org/10.3201/eid0812.020532 PMid:12498649 PMCid:PMC2738499
- Hollander
H, Schaefer PW, Hedley-Whyte ET. Case records of the Massachusetts
General Hospital. Case 22-2005. An 81-year-old man with cough, fever,
and altered mental status. N Engl J Med. 2005;353:287-295. https://doi.org/10.1056/NEJMcpc059017 PMid:16034015
- Mawhorter
SD, Sierk A, Staugaitis SM, et al. Fatal West Nile Virus infection
after rituximab/fludarabine--induced remission for non-Hodgkin's
lymphoma. Clin Lymphoma Myeloma. 2005;6:248-250. https://doi.org/10.3816/CLM.2005.n.053 PMid:16354331
- Hindo
H, Buescher ES, Frank LM, Pettit D, Dory C, Byrd R. West Nile virus
infection in a teenage boy with acute lymphocytic leukemia in
remission. J Pediatr Hematol Oncol. 2005;27:659-662. https://doi.org/10.1097/01.mph.0000188111.04459.6f PMid:16344671
- Farnaes
L, Schiff D, McElroy AK, Coufal NG, Crawford JR, Cannavino C.
Encephalitis and Thalamic Injury From Neuroinvasive West Nile Virus in
Children on Treatment for Acute Lymphoblastic Leukemia. Pediatr Neurol.
2018;80:84-87. https://doi.org/10.1016/j.pediatrneurol.2017.11.013 PMid:29398166
- Honig
A, Karussis D. Delayed-onset flaccid paralysis related to west Nile
virus reactivation following treatment with rituximab: a case report.
BMC Res Notes. 2014;7:852. https://doi.org/10.1186/1756-0500-7-852 PMid:25427863 PMCid:PMC4289184
- Robertson
KB, Barron MA, Nieto Y. West Nile virus infection in bone marrow
transplant patients. Bone Marrow Transplant. 2004;34:823-824. https://doi.org/10.1038/sj.bmt.1704684 PMid:15361905
- Hiatt
B, DesJardin L, Carter T, Gingrich R, Thompson C, de
Magalhaes-Silverman M. A fatal case of West Nile virus infection in a
bone marrow transplant recipient. Clin Infect Dis. 2003;37:e129-131. https://doi.org/10.1086/378891
- Penn
RG, Guarner J, Sejvar JJ, et al. Persistent neuroinvasive West Nile
virus infection in an immunocompromised patient. Clin Infect Dis.
2006;42:680-683. https://doi.org/10.1086/500216 PMid:16447115
- Espinoza-Gutarra
MR, Cervantez SL, Nooruddin Z. West Nile Encephalitis, an Unusual
Infection in a Chronic Lymphocytic Leukemia Patient. Case Rep Hematol.
2018;2018:3270348. https://doi.org/10.1155/2018/3270348
- Murray
KO, Nolan MS, Ronca SE, et al. The Neurocognitive and MRI Outcomes of
West Nile Virus Infection: Preliminary Analysis Using an External
Control Group. Front Neurol. 2018;9:111. https://doi.org/10.3389/fneur.2018.00111 PMid:29636722 PMCid:PMC5880927
- Petropoulou
KA, Gordon SM, Prayson RA, Ruggierri PM. West Nile virus
meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol.
2005;26:1986-1995.
- Hart J Jr, Tillman G,
Kraut MA, et al. West Nile virus neuroinvasive disease: neurological
manifestations and prospective longitudinal outcomes. BMC Infect Dis.
2014;14:248. https://doi.org/10.1186/1471-2334-14-248 PMid:24884681 PMCid:PMC4020876
- Lim
JK, Louie CY, Glaser C, et al. Genetic deficiency of chemokine receptor
CCR5 is a strong risk factor for symptomatic West Nile virus infection:
a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis.
2008;197:262-265. https://doi.org/10.1086/524691 PMid:18179388
- Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal. 2004;16:1201-1210. https://doi.org/10.1016/j.cellsig.2004.04.007 PMid:15337520
- Glass
WG, Lim JK, Cholera R, Pletnev AG, Gao JL, Murphy PM. Chemokine
receptor CCR5 promotes leukocyte trafficking to the brain and survival
in West Nile virus infection. J Exp Med. 2005;202:1087-1098. https://doi.org/10.1084/jem.20042530 PMid:16230476 PMCid:PMC2213214
- Chelli
M, Alizon M. Determinants of the trans-dominant negative effect of
truncated forms of the CCR5 chemokine receptor. J Biol Chem.
2001;276:46975-46982. https://doi.org/10.1074/jbc.M106432200 PMid:11600494
[TOP]
