Michele Malagola1, Raffaella Greco2, Stella Santarone3, Annalisa Natale3, Anna Paola Iori4, Luisa Quatrocchi4, Walter Barbieri4, Antonella Bruzzese4, Salvatore Leotta5, Alessandra Carotti6, Antonio Pierini6, Simona Bernardi1, Enrico Morello1, Nicola Polverelli1, Alessandro Turra1, Federica Cattina1, Lisa Gandolfi1, Benedetta Rambaldi1, Francesca Lorentino2, Francesca Serio2, Giuseppe Milone5, Andrea Velardi6, Robin Foà4, Fabio Ciceri2, Domenico Russo1 and Jacopo Peccatori2.
1 Chair of
Hematology, Dept of Clinical and Experimental Sciences, University of
Brescia, Bone Marrow Transplant Unit, ASST-Spedali Civili of Brescia.
2 IRCCS San Raffaele Scientific Institute, Milano, Italy, Hematology and Bone Marrow Transplantation Unit.
3 Santo Spirito Hospital, Pescara, Department of Hematology, Bone Marrow Transplant Center, Pescara.
4 Haematology, Department of Translational and Precision Medicine, Policlinico Umberto I, “Sapienza” University, Rome.
5 Department of Medical and Surgical specialties, Hematology Section , University of Catania, Catania.
6
Hematopoietic Stem Cell Transplantation Program, Hematology and
Clinical Immunology Section, Department of Medicine, University of
Perugia.
Correspondence to: Michele Malagola, MD. Chair of Hematology,
Department of Clinical and Surgical Sciences, University of Brescia.
Brescia – Italy. E-mail:
michele.malagola@unibs.it
Published: September 1, 2019
Received: April 12, 2019
Accepted: July 6, 2019
Mediterr J Hematol Infect Dis 2019, 11(1): e2019048 DOI
10.4084/MJHID.2019.048
This is an Open Access article distributed
under the terms of the Creative Commons Attribution License
(https://creativecommons.org/licenses/by-nc/4.0),
which permits unrestricted use, distribution, and reproduction in any
medium, provided the original work is properly cited.
|
|
Abstract
CMV
represents one of the most severe life-threatening complications of
allogeneic stem cell transplantation (allo-SCT). Pre-emptive treatment
is highly effective, but toxicity and repetitive reactivation of CMV
represent a significant challenge in the clinical practice. The use of
anti-CMV specific immunoglobulins (Megalotect) is controversial.
We
retrospectively collected data on 92 patients submitted to allo-SCT for
hematological malignancies, in whom Megalotect was used either for
prophylaxis (n=14) or with pre-emptive therapy, together with an
anti-CMV specific drug (n=78). All the patients were considered at
high-risk, due to the presence of at least one risk factor for CMV
reactivation.
The treatment was well tolerated, with no reported
infusion reactions, nor other adverse events, none of the 14 cases
treated with Megalotect as prophylaxis developed CMV reactivation.
51/78 (65%) patients who received Megalotect during pre-emptive
treatment achieved complete clearance of CMV viremia, and 14/51
patients (29%) developed a breakthrough CMV infection. 7/78 patients
(9%) developed CMV disease. The projected 1-year OS, 1-year TRM, and
1-year RR is 74%, 15%, and 19%, respectively. No differences were
observed in terms of OS, TRM, and RR by comparing patients who achieved
a complete response after treatment versus those who did not.
These
retrospective data suggest that Megalotect is safe and well-tolerated.
When used as prophylaxis, no CMV reactivation was recorded. Further
prospective trials are warranted to identify the best set of patients
who can benefit from Megalotect alone or in addition to anti-CMV
specific drugs.
|
Introduction
Cytomegalovirus
(CMV) infection still represent one of the major complications in the
setting of allogeneic stem cell transplantation (allo-SCT),[1,2]
particularly when the immunological reconstitution is delayed or
incomplete like in haploidentical or cord-blood transplantation.[3,4]
It can cause multi-organ disease in recipients of SCT, including
pneumonia, hepatitis, gastroenteritis, retinitis, and encephalitis, and
the disease can develop both early and later after the transplant
procedure.[3,4,5]
Reactivation of CMV can be
observed in about 30 to 50% of the patients, depending on risk factors
such as donor/recipient serology, development of graft versus host
disease (GVHD), type of donor, level of donor/recipient matching and
recipient's age.[1,2] Moreover, any level of viremia is associated with impaired outcome after allo-SCT,[6] mainly if infections develops early after transplant.[7]
Considering the increase of allo-SCT with post-transplant
cyclophosphamide as GVHD prophylaxis in the last decade, this scenario
is changing: various groups registered a high rate of viral infections
in the early period, with a satisfactory infectious profile in
long-term follow-up thanks to a rapid and robust immune-reconstitution.[8,9]
In
the past years, several trials explored the role of prophylaxis in
reducing the incidence of CMV infection in allotransplanted patients.[10]
Gancyclovir has been demonstrated to be effective in reducing the
incidence of CMV reactivation, CMV disease, and the use of pre-emptive
therapy, but not overall mortality. Moreover, the toxic profile of
gancyclovir, namely represented by severe neutropenia, hampered the
extensive use of this drug for prophylaxis. Recently, letermovir has
been demonstrated to be highly effective in reducing the incidence of
clinically significant CMV infection and overall mortality, together
with a very safe profile.[11]
Gancyclovir, valganciclovir, foscarnet, and cidofovir have been widely used for pre-emptive therapy,[12,13] guided by the monitoring of CMV DNA-emia in plasma and, more recently, whole blood.[14]
This approach induces complete viral clearance in up to 70% of the
cases, and this has dramatically reduced the incidence of one of the
most dangerous complications after transplant, represented by CMV
disease, that now can be seen in less than 10% of allotransplanted
patients.[1,2] Nevertheless, the routinely use of
pre-emptive therapy is associated with evident toxicity in terms of
neutropenia for gancyclovir and valganciclovir and renal impairment for
foscarnet and cidofovir[13] and, moreover, with the emergence of gancyclovir-resistant strains.[15]
As a consequence, each Clinician who manages CMV after allo-SCT aims to
reduce the cumulative dose of anti-CMV specific drugs, in order to
limit their toxicity.
Intravenous immunoglobulins (IV-Ig) have
been proposed as potentially useful either in prophylaxis or in the
pre-emptive setting against CMV. Even though some recently data in the
pediatric population showed that IV-Ig significantly reduced the
incidence of CMV infections,[16] and a recently published meta-analysis showed that the prophylactic use of IV-Ig reduced CMV disease,[17] the results of historical meta-analysis did not lead to similar conclusions,[18] and currently the routinely use of IV-Ig for CMV prophylaxis is not recommended.[19-22]
Anti-CMV Ig (Megalotect) is a specific Ig, which inhibits the entrance
of CMV in the host cells. Moreover, it can neutralize viral particles,
aid in complement-mediated lysis of viral particles, promote
opsonization and phagocytosis, enhance antibody-dependent cellular
cytotoxicity (ADCC), and enhance complement-mediated cytolysis.[23-25]
Even though these mechanisms of action are well established, few data
are available concerning the role of Megalotect in CMV management, and
published data are mainly on solid organ transplantation.[23-25] Moreover, in the setting of allo-SCT, most of the published data come from the old single-center trial[26] or recently published retrospective small series of patients.[27]
Thus,
we planned this retrospective multi-center study and collected the data
on 92 allotransplanted patients, who received at least one dose of
Megalotect either for prophylaxis or during pre-emptive therapy
together with an anti-CMV specific drug.
Materials and Methods
We
retrospectively collected the data on 92 patients submitted to allo-SCT
in 6 Italian Bone Marrow Transplant Units between 2016 and 2017, who
received at least one dose of Megalotect, either for prophylaxis or
during pre-emptive therapy. In the two years of data collection, 539
patients have been consecutively allotransplanted in those Centers, and
242 (45%) developed at least one CMV reactivation.
Local databases
and clinical charts were used for data collection, and selected queries
were addressed on missing data. The allo-SCT platforms, in terms of
conditioning regimens, GVHD prophylaxis and antimicrobial prophylaxis,
were based on local guidelines and protocols, upon written informed
consent for transplant procedures and the use of medical records for
research. This study is retrospective. No Ethical Committee approval
has been requested. All the transplanted patients for whom data have
been collected have regularly signed the EBMT informed consent for
transplant data collection which is requested for European PROMISE
database. The clinical and biological data collected for this paper are
those routinely assessed for every transplanted patient.
CMV
DNA-emia was monitored by RT-qPCR on either plasma or whole blood,
according to single Center policy. In the vast majority of patients
(90%), quantification of CMV DNA was made using the Q-CMV Real-Time
Complete Kit (ELITechGroup S.p.A) as previously published.[12]
The response after pre-emptive treatment has been retrospectively
evaluated at the time of the first CMV negative PCR from the start of
pre-emptive therapy.
Statistical Analysis.
Categorical variables were described as frequencies and continuous
variables as median value. Overall survival (OS) was defined as the
interval from allo-SCT to death, whatever the cause, and patients were
censored at the date of the last contact if alive. Cumulative
incidences were estimated for acute GVHD, transplant-related mortality
(TRM), and relapse to accommodate competing risks.[28]
Relapse or progression was a competing risk for TRM; death from any
cause was a competing risk for relapse. Relapse/progression and death
from any causes were competing for risks for GVHD. The probabilities of
overall survival (OS), progression-free survival (PFS) and GVHD and
relapse-free survival (GRFS) were estimated using the Kaplan-Meyer
estimator.[29] All statistical analyses were performed with R (R Development Core Team, Vienna, Austria) software package.
Results
This
report focuses on a series of 92 allotransplanted patients who received
Megalotect either for prophylaxis (n=14 - 15%) or during first-line
pre-emptive therapy, together with an anti-CMV specific drug (n=78 -
85%).
The clinical and transplant characteristics of the 14 patients who received Megalotect in prophylaxis are reported in Table 1a.
It should be noticed that 2/14 cases (14%) were CMV negative. These
cases received Megalotect in prophylaxis because of the haploidentical
donor. The clinical and transplant characteristics of the 78 patients
who received Megalotect with an anti-CM specific drug (pre-emptive
setting) are reported in Table 1b.
Briefly, the median age of our patients' population was 47 years (range
0 – 69). 6/78 patients (8%) were below the age of 14 years. The great
majority of the patients were transplanted for acute leukemia (64/78 –
82%), in complete remission (60/78 cases – 77%), with a myeloablative
conditioning regimen (56/78 – 72%) and from a matched unrelated donor
(36/78 – 46%). The donor was haploidentical for 30/78 patients (39%).
Interestingly, 74/78 patients (95%) were CMV IgG positive before
allo-SCT. Four patients were CMV negative, and they all received a
haploidentical donor. The rationale for Megalotect use in these cases
was related to the high risk of developing CMV infection and disease
because of the nonidentical donor. All but nine patients received an
un-manipulated T-cell replete graft. Conventional anti-thymocyte
globulin in combination with cyclosporine and a short course of
methotrexate with or without mycophenolate was the most commonly used
prophylaxis (50/78 cases; 64%).
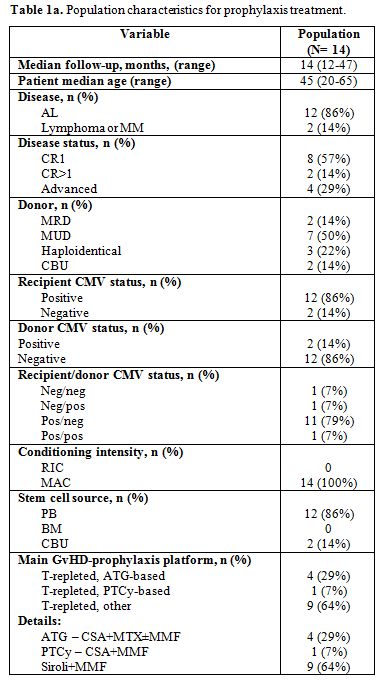 |
Table 1a. Population characteristics for prophylaxis treatment. |
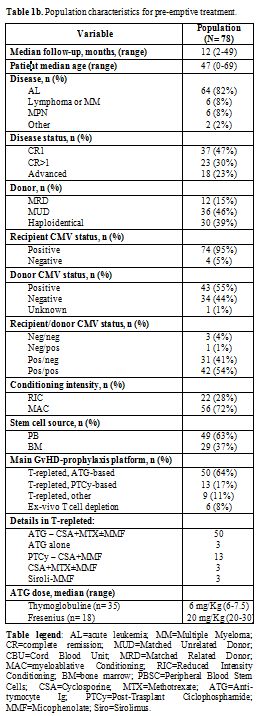 |
Table 1b. Population characteristics for pre-emptive treatment. |
Megalotect
was well tolerated, and no infusion-related adverse reactions were
observed. The details on Megalotect dose and schedule and CMV
reactivation in the two settings of patients are reported in Table 2a and 2b.
Briefly,
focusing on the 14 patients (15%) who received Megalotect as
prophylaxis, the median dose of Megalotect was 50 UI/Kg (range 50-100).
Prophylaxis started at day -7 until engraftment. Respectively, 21%
(n=3), 36% (n=5) and 43% (n=6) of these patients received Megalotect on
a weekly, every two weeks, and every three weeks schedule. The median
number of administrations was 2 (range 1-9). None of these patients
developed CMV reactivation by day +100 (Table 2a).
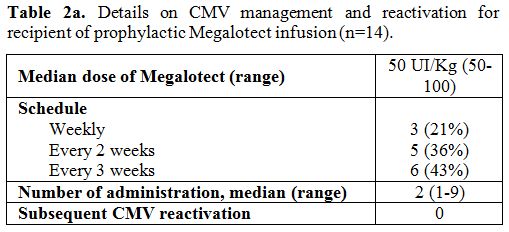 |
Table 2a. Details on CMV management and reactivation for recipient of prophylactic Megalotect infusion (n=14). |
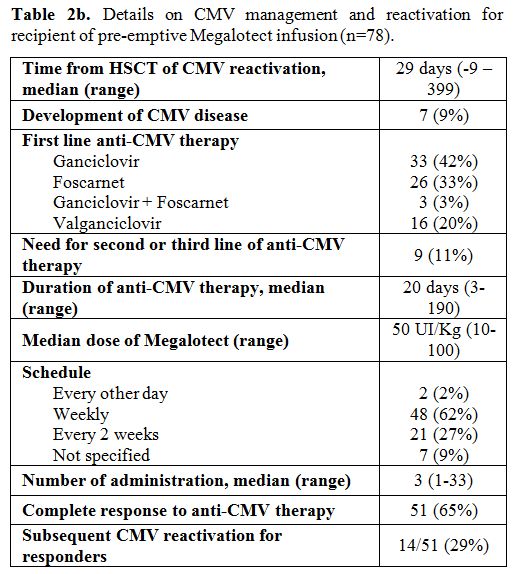 |
Table 2b. Details on CMV management and reactivation for recipient of pre-emptive Megalotect infusion (n=78). |
Moving
to the 78 patients (85%) who received Megalotect during first-line
pre-emptive therapy, the median time from allo-SCT to CMV reactivation
was 29 days (range -9 - +399), 73/78 patients (94%) reactivated CMV
from day 0 to day +100 from allo-SCT. The median dose of Megalotect was
50 UI/Kg (range 10-100). Respectively, 62% (n=48) and 27% (n=21) of
these patients received Megalotect on a weekly and every two weeks
schedule. The median number of administrations was 3 (range 1-33). The
first dose of Megalotect was administered within five days from the
start of pre-emptive treatment. The anti-CMV specific drug used as
pre-emptive therapy was gancyclovir in 33 cases (42%), foscarnet in 26
cases (33%), valganciclovir in 16 cases (20%) and two-drugs combination
in 3 cases (3%). After a median of 20 days of therapy (range 3 – 190),
51 out of 78 patients (65%) achieved complete clearance of CMV viremia
with Megalotect and first-line standard anti-CMV drug. 16/78 patients
(20%) received pre-emptive therapy for more than four weeks, as
maintenance. In 14/51 patients (29%), a breakthrough CMV infection was
observed, and this was treated with second-line anti-CMV drugs only,
without Megalotect. More detailed data on the breakthrough infection
have been obtained in 12/14 cases. In these cases, the breakthrough CMV
infection occurred after a median of 30 days (range 7 – 60) from CMV
negativity obtained with first-line pre-emptive therapy with anti-CMV
specific drug and Megalotect. In all the cases the breakthrough CMV
infection occurred after Megalotect discontinuation. Seven out of 78
patients (9%) developed CMV disease,
with gut and lung localization in 5 and 2 cases,
respectively. In 2/7 cases (40%), CMV disease was recorded after the
failure of first-line anti-CMV treatment. Thus, 7% of the patients
(2/27) who did not achieve CR after first-line pre-emptive therapy
developed CMV disease. The median time from allo-SCT to CMV disease was
35 days (range 9 – 281), the median time from first CMV reactivation to
CMV disease was 31 days (range 2 – 270), and 4/7 cases (57%) developed
CMV disease early during the first CMV reactivation. All these cases of
CMV disease were managed with anti-CMV specific drugs (gancyclovir in 2
cases, foscarnet in 3 cases and combination of the two drugs in 2
cases) with IV-Ig as suggested by data from metanalysis.[19]
Overall,
the cumulative incidence of grades II-IV and III-IV aGVHD at 100 days
was 38% (95% CI 28-48) and 10% (95% CI 5-17), respectively (Table 3).
The incidence of moderate-severe chronic GVHD was 10% (9/92 cases). The
projected 1-year OS, 1-year TRM and 1-year relapse rate
(RR) was 74% (95% CI 63-82), 15% (95% CI 8-24) and 19% (95%
CI 11-28), respectively (Table 3).
No differences were observed in terms of OS, TRM, and RR by comparing
patients who achieved a complete response after treatment versus those
who did not (data not showed).
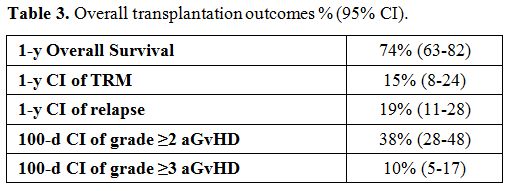 |
Table
3. Overall transplantation outcomes % (95% CI). |
Discussion
Although
the mortality for CMV in allotransplanted patients has decreased
significantly because of pre-emptive therapy, CMV still reactivates in
30% - 50% of allo-SCT recipients.[1,2] CMV treatment
has been optimized in allo-SCT recipients over the past decade, mainly
when used preemptively, but several questions remain. Moreover, new
treatment options for CMV are urgently needed because the currently
available drugs have significant limitations.[13]
In
this paper, we report the outcome of 92 hematological patients treated
with allo-SCT in 6 Italian Transplant Centers, who received at least
one dose of Megalotect either for prophylaxis (n=14) or during
pre-emptive treatment (n=78). Even though these results derive from a
retrospective analysis, we observed that Megalotect was safe with no
reported adverse reactions. In the prophylaxis setting, no CMV
infections were observed. This result is of particular interest and,
although it should be confirmed in prospective trials, it suggests that
Megalotect by itself may help to control CMV infection. In fact, some
in vitro studies suggest that the binding of Megalotect to the viral
antigens may prevent the CMV binding to target cells, thus modulating
CMV infection and disease, until anti-CMV CD8+ T-cells are present.[30]
It should be noticed that the dose, the schedule, and the number of
administrations of Megalotect in the prophylaxis setting is widely
variable in this series. This heterogeneity is due to the lack of
published data and reflects the different Centers' policy and internal
guidelines for CMV management. Even though the introduction of
letermovir for CMV prophylaxis in the first 100 days after allo-SCT is
rapidly changing the scenario of CMV management, we think that 100
UI/Kg i.v. every two weeks from -7 to engraftment or eventually day +90
after allo-SCT could be the object of further prospective trials
exploring the role of anti-CMV Ig in this setting.
Moreover, in
the pre-emptive setting, 65% of the patients achieved complete
CMV-clearance with first-line therapy and Megalotect after a median of
20 days (range 3 – 190). As observed for the prophylaxis setting, the
wide range of anti-CMV pre-emptive treatment duration is atypical, and
this reflects the different policies of the different centers in this
field. 16/78 patients (20%) received pre-emptive therapy for more than
four weeks, as maintenance. Moreover, it should be noticed that the
time-point of CMV reactivation in these 78 cases varies widely
concerning allo-SCT (from the day -9 to day +399). Most of the patients
(73/78, 94%) reactivated CMV between day 0 and 100 days from allo-SCT.
We decided to include in this report also the five patients who
received Megalotect with an anti-CMV specific drug for a late CMV
reactivation (mostly during GVHD), in order to have a "real-life"
picture of the CMV management in the transplant Centers that
participated to the study. We are aware that our results are in line
with the response rate reported with conventional pre-emptive therapy
with anti-CMV specific drugs alone, but it should be noticed that our
patients represent a highly negatively selected cohort, in terms of
risk of CMV reactivation. Thus, we can speculate that Megalotect may
have played a role in inducing a fast and complete viral clearance in
the majority of patients. We compared our cohort of patients with a
historical cohort of 122 patients transplanted from 2010 to 2017 in 2
of the six transplant Centers, who received pre-emptive therapy for CMV
reactivation without Megalotect. We did not find any statistically
significant difference in terms of response rate, duration of
pre-emptive treatment, and breakthrough CMV infections. It should be
noticed that, due to the evolution of the transplant approach in the
last 20 years, these two populations were not well balanced with
respect to the clinical and transplant characteristics and this is an
extreme bias for drawing any conclusion (data not shown). Therefore, we
believe that there is an urgent need for a prospective trial to better
explore the role of Megalotect in CMV prevention and treatment.
Only
9% of the patients of the present series developed CMV disease, and
none of the 24 deaths were related to CMV. We think that these data are
of interest, considering that all the patients were at high risk of CMV
infection and disease, mainly for unfavorable serology (R+) or
haploidentical transplant or acute GVHD requiring treatment.
The
role of anti-CMV Ig in the management of CMV in allotransplanted
patients has been poorly explored in clinical trials, and currently,
its use is not recommended in clinical practice. In 1998 Bacigalupo and
Colleagues published the data of a randomized trial on 128 patients who
received Megalotect versus conventional IV-Ig weekly from the day -7 to
day +100.[26] Antigenemia was used for CMV
monitoring, and they found a trend for a reduced incidence of 1-year
cumulative incidence of CMV antigenemia and grades II-IV acute GVHD in
patients treated with hyper-immune anti-CMV Ig. Very recently Alsuliman
and Colleagues published the results of a retrospective analysis on 23
patients who received Megalotect with or without anti-CMV specific
drugs, mainly as salvage treatment. They observed a response rate of
87% after a median of 15 days of therapy, and the incidence of
subsequent CMV reactivation was 22%.[27]
The
optimal dose of anti-CMV Ig in these patients has to be better
investigated. Some of the published papers report dosages much higher
(100 – 200 UI/Kg/dose) than the ones reported in this analysis and
usually administered for more than the median doses reported in our
series.[26,27] It should be noticed that the optimal
dose and schedule of anti-CMV Ig has not been established yet, and the
high variability reported in the few published papers probably reflects
the different Centers' policy of CMV management. Of note, the dose of
50 UI/Kg administered in our patients was high enough to maintain a
level of peripheral blood IgG greater than 500 mg/dl, which is
considered associated with relatively high efficacy of the humoral
immune system in controlling infections after allo-SCT. Further studies
are warranted in order to address the optimal dose and schedule of
Megalotect.
As previously stated, our data are retrospective, with
several limitations that can derive from many aspects, including the
changing of transplant scenario, the evolution of pre-emptive strategy
and CMV monitoring and the possible bias of positive or negative
selection by Clinicians in the choice to administer Megalotect. As a
consequence, prospective trials to explore the role of Megalotect in
prophylaxis and pre-emptive settings are strongly warranted in
high-risk patients. In this latter group, the major issue is to assess
if a combination of anti-CMV specific drugs and Megalotect may reduce
the days of pre-emptive therapy and thus the toxicity, and to verify if
the combination can reduce the incidence of breakthrough CMV infection.
The
future management of CMV infection is expected to change rapidly, due
to the availability in clinical practice of the new anti-CMV drugs,
namely letermovir, recently licensed in the United States and Europe
for the prophylaxis of CMV in the first 100 days after transplant.[11,13]
The use of this drug will probably reduce the incidence of early CMV
reactivation, but we will have to manage late-onset CMV reactivations,
which are expected in about one-third of the patients who will receive
letermovir for prophylaxis. It may be interesting to prospectively
explore the role of Megalotect in preventing this event too.
References
- Styczynski J. Who Is the Patient at Risk of CMV
Recurrence: A Review of the Current Scientific Evidence with a Focus on
Hematopoietic Cell Transplantation. Infect Dis Ther. 2018;7(1):1-16. https://doi.org/10.1007/s40121-017-0180-z PMid:29204910 PMCid:PMC5840099
- Pollack
M, Heugel J, Xie H, Leisenring W, Storek J, Young JA, Kukreja M, Gress
R, Tomblyn M, Boeckh M. An international comparison of current
strategies to prevent herpesvirus and fungal infections in
hematopoietic cell transplant recipients. Biol Blood Marrow Transplant.
2011;17:664-673. https://doi.org/10.1016/j.bbmt.2010.07.026 PMid:20699126 PMCid:PMC3358229
- Aversa
F, Prezioso L, Manfra I, Galaverna F, Spolzino A, Monti A. Immunity to
Infections after Haploidentical Hematopoietic Stem Cell
Transplantation. Mediterr J Hematol Infect Dis. 2016 Oct
25;8(1):e2016057. eCollection 2016. https://doi.org/10.4084/mjhid.2016.057 PMid:27872737 PMCid:PMC5111540
- Montoro,
J., & Sanz, J. (2016). Infectious Complications After Umbilical
Cord-Blood Transplantation From Unrelated Donors. Mediterr J Hematol
Infect Dis, 8, e2016051. https://doi.org/10.4084/mjhid.2016.051 PMid:27872731 PMCid:PMC5111514
- Boeckh
M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D,
Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late
cytomegalovirus disease and mortality in recipients of allogeneic
hematopoietic stem cell transplants: importance of viral load and
T-cell immunity. Blood. 2003;101:407-414. https://doi.org/10.1182/blood-2002-03-0993 PMid:12393659
- Green
ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, Sorror ML, Goyal
S, Özkök S, Yi J, Sahoo F, Kimball LE, Jerome KR, Marks MA, Boeckh M.
Cytomegalovirus viral load and mortality after haemopoietic stem cell
transplantation in the era of pre-emptive therapy: a retrospective
cohort study. Lancet Haematol. 2016;3:e119-27. https://doi.org/10.1016/S2352-3026(15)00289-6
- Teira
P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS,
Saad A, Antin JH, Savani BN, Lazarus HM, Seftel M, Saber W, Marks D,
Aljurf M, Norkin M, Wingard JR, Lindemans CA, Boeckh M, Riches ML,
Auletta JJ. Early cytomegalovirus reactivation remains associated with
increased transplant-related mortality in the current era: a CIBMTR
analysis. Blood. 2016;127(20):2427-38. https://doi.org/10.1182/blood-2015-11-679639 PMid:26884374 PMCid:PMC4874224
- Crocchiolo
R, Bramanti S, Vai A, Sarina B, Mineri R, Casari E, Tordato F, Mauro E,
Timofeeva I, Lugli E, Mavilio D, Carlo-Stella C, Santoro A, Castagna L.
Infections after T-replete haploidentical transplantation and high-dose
cyclophosphamide as graft-versus-host disease prophylaxis. Transpl
Infect Dis. 2015;17:242-249. https://doi.org/10.1111/tid.12365 PMid:25648539
- Cieri
N, Oliveira G, Greco R, Forcato M, Taccioli C, Cianciotti B, Valtolina
V, Noviello M, Vago L, Bondanza A, Lunghi F, Marktel S, Bellio L,
Bordignon C, Bicciato S, Peccatori J, Ciceri F, Bonini C. Generation of
human memory stem T cells after haploidentical Trepletehematopoietic
stem cell transplantation. Blood. 2015;125:2865-2874. https://doi.org/10.1182/blood-2014-11-608539 PMid:25736310
- Gagelmann
N, Ljungman P, Styczynski J, Kröger N. Comparative Efficacy and Safety
of Different Antiviral Agents for Cytomegalovirus Prophylaxis in
Allogeneic Hematopoietic Cell Transplantation: A Systematic Review and
Meta-Analysis. Biol Blood Marrow Transplant. 2018;24(10):2101-2109 https://doi.org/10.1016/j.bbmt.2018.05.017 PMid:29777868
- Marty
FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S,
Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA,
Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y,
Kartsonis NA, Leavitt RY, Badshah C. Letermovir Prophylaxis for
Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med.
2017;377(25):2433-2444. https://doi.org/10.1056/NEJMoa1706640 PMid:29211658
- Greco
R, Crucitti L, Noviello M, Racca S, Mannina D, Forcina A, Lorentino F,
Valtolina V, Rolla S, Dvir R, Morelli M, Giglio F, Barbanti MC, Lupo
Stanghellini MT, Oltolini C, Vago L, Scarpellini P, Assanelli A,
Carrabba MG, Marktel S, Bernardi M, Corti C, Clementi M, Peccatori J,
Bonini C, Ciceri F. Human Herpesvirus 6 Infection Following
Haploidentical Transplantation: Immune Recovery and Outcome. Biol Blood
Marrow Transplant. 2016;22(12):2250-2255 https://doi.org/10.1016/j.bbmt.2016.09.018 PMid:27697585
- Meesing
A, Razonable RR. New Developments in the Management of Cytomegalovirus
Infection After Transplantation. Drugs. 2018;78(11):1085-1103. https://doi.org/10.1007/s40265-018-0943-1 PMid:29961185
- Lazzarotto
T, Chiereghin A, Piralla A, Piccirilli G, Girello A, Campanini G,
Gabrielli L, Costa C, Prete A, Bonifazi F, Busca A, Cairoli R, Colombo
AA, Zecca M, Sidoti F, Bianco G, Paba P, Perno CF, Cavallo R, Baldanti
F; AMCLI-GlaIT working group. Cytomegalovirus and Epstein-Barr Virus
DNA Kinetics in Whole Blood and Plasma of Allogeneic Hematopoietic Stem
Cell Transplantation Recipients. Biol Blood Marrow Transplant.
2018;24(8):1699-1706. https://doi.org/10.1016/j.bbmt.2018.03.005 PMid:29545186
- Chemaly
RF, Chou S, Einsele H, Griffiths P, Avery R, Razonable RR, Mullane KM,
Kotton C, Lundgren J, Komatsu TE, Lischka P, Josephson F, Douglas CM,
Umeh O, Miller V, Ljungman P; Resistant Definitions Working Group of
the Cytomegalovirus Drug Development Forum. Definitions of Resistant
and Refractory Cytomegalovirus Infection and Disease in Transplant
Recipients for Use in Clinical Trials. Clin Infect Dis.
2019;68(8):1420-1426. https://doi.org/10.1093/cid/ciy696 PMid:30137245
- Goldstein
G, Rutenberg TF, Mendelovich SL, Hutt D, Oikawa MT, Toren A, Bielorai
B. The role of immunoglobulin prophylaxis for prevention of
cytomegalovirus infection in pediatric hematopoietic stem cell
transplantation recipients. Pediatr Blood Cancer. 2017;64(7). https://doi.org/10.1002/pbc.26420 PMid:28087884
- Ahn
H, Tay J, Shea B, Hutton B, Shorr R, Knoll GA, Cameron DW, Cowan J.
Effectiveness of immunoglobulin prophylaxis in reducing clinical
complications of hematopoietic stem cell transplantation: a systematic
review and meta-analysis. Transfusion. 2018;58: 2437-2452. https://doi.org/10.1111/trf.14656 PMid:29770447
- Raanani
P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O.
Immunoglobulin prophylaxis in hematopoietic stem cell transplantation:
systematic review and meta-analysis. J Clin Oncol. 2009;27(5):770-81. https://doi.org/10.1200/JCO.2008.16.8450 PMid:19114702
- Emery
V, Zuckerman M, Jackson G, Aitken C, Osman H, Pagliuca A, Potter M,
Peggs K, Clark A; British Committee for Standards in Haematology;
British Society of Blood and Marrow Transplantation; UK Virology
Network. Management of cytomegalovirus infection in haemopoietic stem
cell transplantation. Br J Haematol. 2013;162(1):25-39. https://doi.org/10.1111/bjh.12363 PMid:23647436
- Tomblyn
M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR,
Young JA, Boeckh MJ; Center for International Blood and Marrow
Research; National Marrow Donor program; European Blood and
MarrowTransplant Group; American Society of Blood and Marrow
Transplantation; Canadian Blood and Marrow Transplant Group; Infectious
Diseases Society of America; Society for Healthcare Epidemiology of
America; Association of Medical Microbiology and Infectious Disease
Canada; Centers for Disease Control and Prevention. Guidelines for
preventing infectious complications among hematopoietic cell
transplantation recipients: a global perspective. Biol Blood Marrow
Transplant. 2009;15(10):1143-238. https://doi.org/10.1016/j.bbmt.2009.06.019 PMid:19747629 PMCid:PMC3103296
- Ljungman
P, de la Camara R, Cordonnier C, Einsele H, Engelhard D, Reusser P,
Styczynski J, Ward K; European Conference on Infections in Leukemia.
Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8)
infections in patients with hematological malignancies and after SCT.
Bone Marrow Transplant. 2008;42(4):227-40. https://doi.org/10.1038/bmt.2008.162 PMid:18587440
- Ljungman
P, de la camara R, Crocchiolo R, Einsele H, Hubacek P, Hill J, et al.
Guidelines For Management Of Cmv Infections In Patients With
Hematological Malignancies And After Stem Cell Transplantation From The
2017 European Conference On Infections In Leukemia (ECIL-7) 2017
(Available from: http://www.ecil-leukaemia.com)
- Bonaros
NE, Kocher A, Dunkler D, Grimm M, Zuckermann A, Ankersmit J, Ehrlich M,
Wolner E, Laufer G. Comparison of combined prophylaxis of
cytomegalovirus hyperimmune globulin plus ganciclovir versus
cytomegalovirus hyperimmune globulin alone in high-risk heart
transplant recipients. Transplantation. 2004;77(6):890-7. https://doi.org/10.1097/01.TP.0000119722.37337.DC PMid:15077033
- Kotton
CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A;
Transplantation Society International CMV Consensus Group. Updated
international consensus guidelines on the management of cytomegalovirus
in solid-organ transplantation. Transplantation. 2013;96(4):333-60. https://doi.org/10.1097/TP.0b013e31829df29d PMid:23896556
- Grossi
P, Mohacsi P, Szabolcs Z, Potena L. Cytomegalovirus Immunoglobulin
After Thoracic Transplantation: An Overview. Transplantation. 2016;100
Suppl 3:S1-4 https://doi.org/10.1097/TP.0000000000001094 PMid:26900989 PMCid:PMC4764015
- Zikos
P, Van Lint MT, Lamparelli T, Gualandi F, Occhini D, Mordini N, Berisso
G, Bregante S, Bacigalupo A. A randomized trial of high dose polyvalent
intravenous immunoglobulin (HDIgG) vs. Cytomegalovirus (CMV)
hyperimmune IgG in allogeneic hemopoietic stem cell transplants (HSCT).
Haematol. 1998;83(2):132-7
- Alsuliman
T, Kitel C, Dulery R, Guillaume T, Larosa F, Cornillon J,
Labussière-Wallet H, Médiavilla C, Belaiche S, Delage J, Alain S,
Yakoub-Agha I. Cytotect®CP as salvage therapy in patients with CMV
infection following allogeneic hematopoietic cell transplantation: a
multicenter retrospective study. Bone Marrow Transplant.
2018;53(10):1328-1335. https://doi.org/10.1038/s41409-018-0166-9 PMid:29654288
- Gooley
TA, Leisenring W, Crowley J, Storer BE. Estimation of failure
probabilities in the presence of competing risks: new representation of
old estimators. Stat Med. 1999;18(6):695-706. https://doi.org/10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.0.CO;2-O
- Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958; 53: 457-481. https://doi.org/10.1080/01621459.1958.10501452
- Carbone
J. The Immunology of Posttransplant CMV Infection: Potential Effect of
CMV Immunoglobulins on Distinct Components of the Immune Response to
CMV. Transplantation. 2016;100 Suppl 3:S11-8.https://doi.org/10.1097/TP.0000000000001095 PMid:26900990 PMCid:PMC4764014




